Fabrication of Multifunctional SERS Platform Based on Ag NPs Self-Assembly Ag-AAO Nanoarray for Direct Determination of Pesticide Residues and Baicalein in Real Samples
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Materials
2.2. Sample Preparation
2.2.1. Preparation of Porous AAO Template
2.2.2. Preparation of Ag-AAO Nanoarray
2.2.3. Preparation of Ag NPs-Ag-AAO Nanoarray by Oil-Water Separation Self-Assembly Method
2.3. Characterization and SERS Measurements
2.4. Electromagnetic Field Simulations
3. Results and Discussion
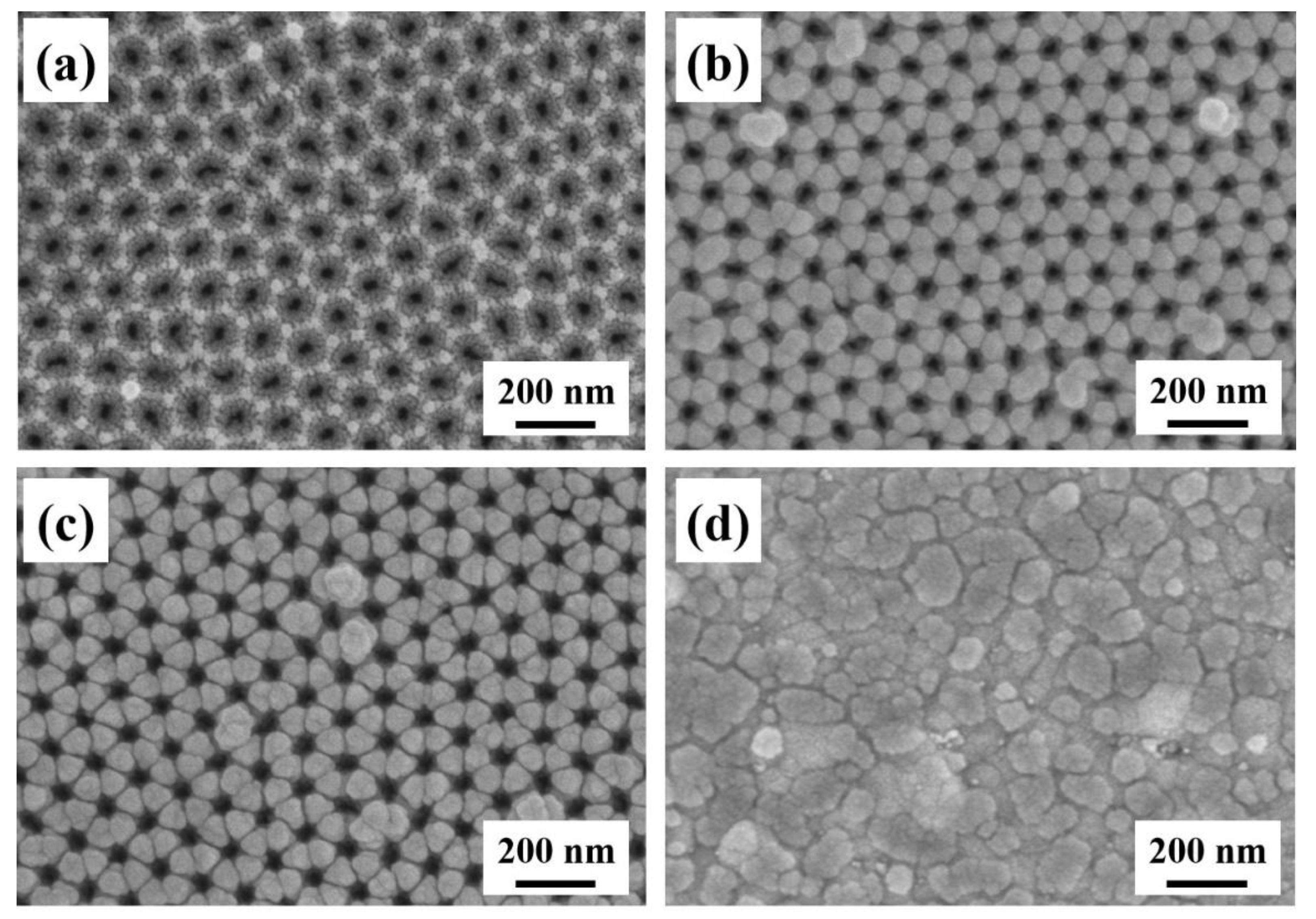
3.1. Morphology Characterization of the AAO Nanotemplate
3.2. Nanomorphology of the Ag-AAO Nanoarrays and 3D-FDTD Simulations
3.3. SERS Performances of the Ag NPs30-Ag20-AAO Substrate
3.4. Sensitivity of the Ag NPs30-Ag20-AAO Substrate
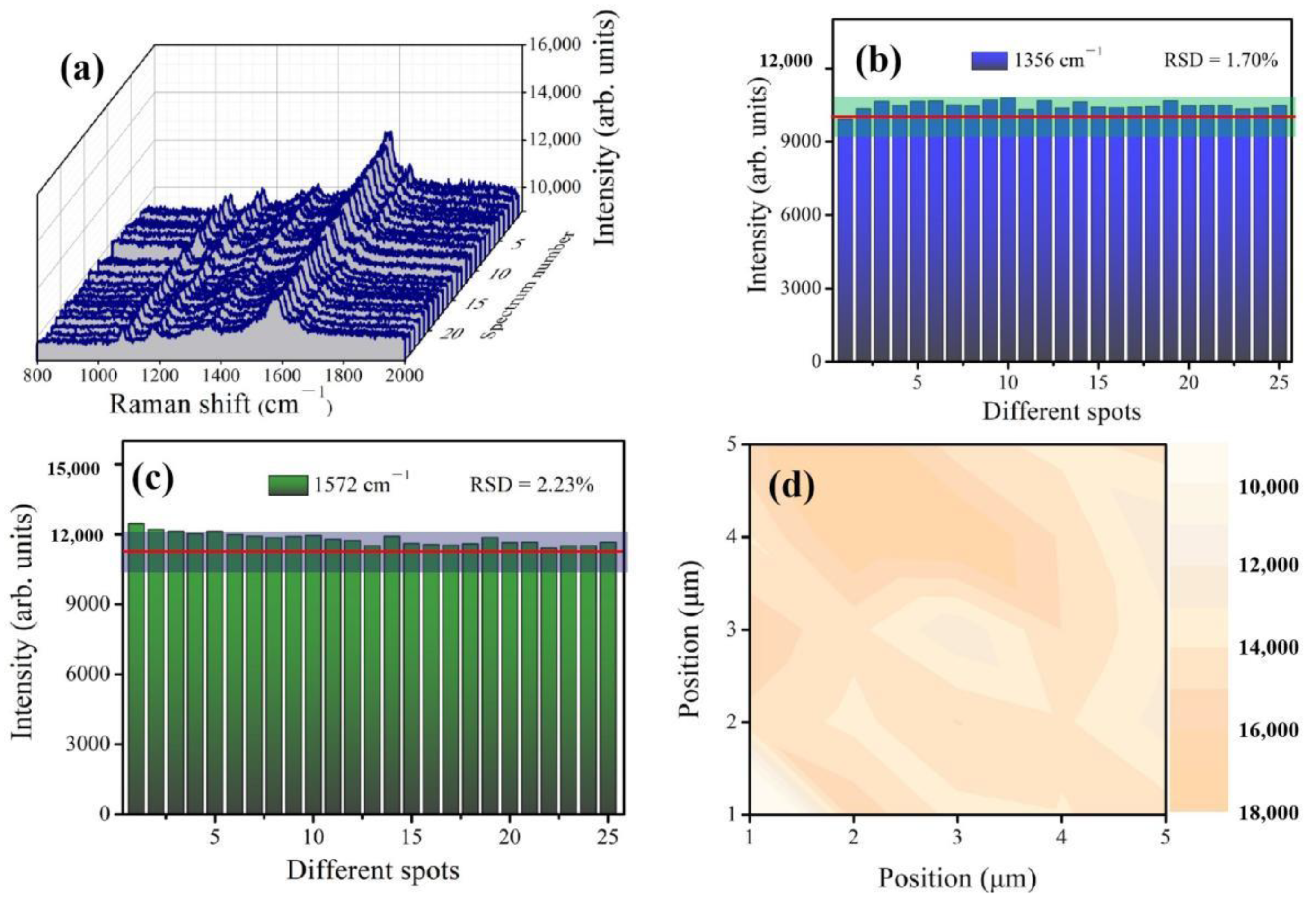
3.5. Reproducibility of the Ag NPs30-Ag20-AAO Substrate
3.6. Experimental Enhancement Factor (EEF) Calculation
3.7. Detection of the Baicalein by the Ag NPs30-Ag20-AAO Substrate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ru, E.C.L.; Etchegoin, P.G. Single-molecule surface-enhanced Raman spectroscopy. Annu. Rev. Phys. Chem. 2012, 63, 65–87. [Google Scholar] [PubMed] [Green Version]
- Zheng, X.S.; Jahn, I.J.; Weber, K.; Cialla-May, D.; Popp, J. Label-free SERS in biological and biomedical applications: Recent progress, current challenges and opportunities. Spectrochim. Acta A 2018, 197, 56–77. [Google Scholar] [CrossRef]
- Li, C.H.; Liu, A.H.; Zhang, C.; Wang, M.H.; Li, Z.; Xu, S.C.; Jing, S.Z.; Yu, J.; Yang, C.; Man, B.Y. Ag gyrus-nanostructure supported on graphene/Au film with nanometer gap for ideal surface enhanced Raman scattering. Opt. Express 2017, 25, 20631–20641. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, M.; Li, Z.; Liu, C.; Wei, Y.; Zhang, C.; Man, B.; Lei, F. Hierarchical particle-in-quasicavity architecture for ultratrace in situ Raman sensing and its application in real-time monitoring of toxic pollutants. Anal. Chem. 2020, 92, 14754–14761. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Qi, F.; Zhao, C.L.; Xie, C.X.; Wang, J.G.; Chang, C.T.; Li, Y.J.; Zhang, L.C. Facile fabrication of ultrathin freestanding nanoporous Cu and Cu-Ag films with high SERS sensitivity by dealloying Mg-Cu(Ag)-Gd metallic glasses. J. Mater. Sci. Technol. 2021, 70, 205–213. [Google Scholar] [CrossRef]
- Meng, S.; Chen, R.; Xie, J.; Li, J.; Cheng, J.; Xu, Y.; Cao, H.; Wu, X.; Zhang, Q.; Wang, H. Surface-enhanced Raman scattering holography chip for rapid, sensitive and multiplexed detection of human breast cancer-associated MicroRNAs in clinical samples. Biosens. Bioelectron. 2021, 190, 1113470–1113479. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Cong, S.; Yuan, Y.; Chen, Z.; Hou, J.; Yang, M.; Su, Y.; Zhang, Y.; Li, L.; Li, Q.; Geng, F.; et al. Noble metal-comparable SERS enhancement from semiconducting metal oxides by making oxygen vacancies. Nat. Commun. 2015, 6, 7800–7806. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.; Bassou, G.; Aourag, H.; Dufour, J.P.; De Fornel, F.; Carcenac, F.; Zayats, A.V. Local excitation of surface plasmon polaritons at discontinuities of a metal film: Theoretical analysis and optical near-field measurements. Phys. Rev. B 2002, 65, 125409. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [Green Version]
- Otto, A. Surface-enhanced Raman scattering: “Classical” and “Classical” Origins. In Light Scattering in Solids IV. Topics in Applied Physics; Springer: Berlin, Germany, 1984; Volume 54, pp. 289–418. [Google Scholar]
- Campion, A.; Kambhampati, P. Surface enhanced Raman scattering. Chem. Soc. Rev. 1998, 27, 241–250. [Google Scholar] [CrossRef]
- Wang, Y.X.; Liu, S.S.; Gao, W.T.; Zhang, Y.J.; Yang, J.H. Surface-enhanced Raman spectroscopy based on ordered nanocap arrays. Superlattice. Microst. 2012, 52, 750–758. [Google Scholar] [CrossRef]
- Xu, H.; Jian, Q.; Xu, L.; Gao, S. Wireless battery-free generation of electric fields on one-dimensional asymmetric Au/ZnO nanorods for enhanced Raman sensing. Anal. Chem. 2021, 93, 9286−9295. [Google Scholar] [CrossRef]
- Tang, L.; Tian, C.; Gu, W.; Jiang, Z. Surface-enhanced Raman scattering-based lateral flow immunoassay mediated by hydrophilic-hydrophobic Ag-modified PMMA substrate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 262, 120092. [Google Scholar] [CrossRef]
- Caldwell, J.; Taladriz-Blanco, P.; Rothen-Rutishauser, B.; Petri-Fink, A. Detection of sub-micro- and nanoplastic particles on gold nanoparticle-based substrates through surface-enhanced Raman scattering (SERS) Spectroscopy. Nanomaterials 2021, 11, 1149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, G.Q.; Feng, S.J.; Zhao, X. Engineering of flexible granular Au nanocap ordered array and its surface enhanced Raman spectroscopy effect. Nanotechnology 2020, 31, 035303–035312. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.Q.; Kang, Y.L.; Li, W.L.; Sun, G.F.; Liu, H.; Li, M.; Ye, Z.R.; Zhou, M.; Zhou, J.G.; Yang, S.K. Bioinspired brochosomes as broadband and omnidirectional surface-enhanced Raman scattering substrates. J. Phys. Chem. Lett. 2019, 10, 6484–6491. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Luo, C.L.; Liu, Z.Q.; Chen, Y.Y.; Dong, S.L.; Jiang, C.Z.; Yang, S.K.; Wang, F.B.; Xiao, X.H. Volume-enhanced Raman scattering detection of viruses. Small 2019, 15, 1805516–1805523. [Google Scholar] [CrossRef]
- Politano, G.G.; Cazzanelli, E.; Versace, C.; Vena, C.; De Santo, M.P.; Castriota, M.; Ciuchi, F.; Bartolino, R. Graphene oxide on magnetron sputtered silver thin films for SERS and metamaterial applications. Appl. Surf. Sci. 2018, 427, 927–933. [Google Scholar] [CrossRef]
- Yilmaz, M. Silver-nanoparticle-decorated gold nanorod arrays via bioinspired polydopamine coating as surface-enhanced Raman spectroscopy (SERS) platforms. Coatings 2019, 9, 198. [Google Scholar] [CrossRef] [Green Version]
- Demirci, G.; Muszynska, J.; Cetinkaya, O.; Filipczak, P.; Zhang, Y.M.; Nowaczyk, G.; Halagan, K.; Ulanski, J.; Matyjaszewski, K.; Pietrasik, J.; et al. Effective SERS materials by loading Ag nanoparticles into poly(acrylic acid-stat-acrylamide)-block-polystyrene nano-objects prepared by PISA. Polymer 2021, 224, 123747–123756. [Google Scholar] [CrossRef]
- Guo, L.T.; Cao, H.W.; Cao, L.P.; Li, N.; Zhang, A.Q.; Shang, Z.B.; Jiao, T.F.; Liu, H.L.; Wang, M.L. Improve optical properties by modifying Ag nanoparticles on a razor clam SERS substrate. Opt. Express 2021, 29, 5152–5165. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.C.; Wang, M.L.; Zhu, Y.Y.; Wang, Y.H.; Xu, H.J. A novel natural SERS system for crystal violet detection based on graphene oxide wrapped Ag micro-islands substrate fabricated from Lotus leaf as a template. Appl. Surf. Sci. 2018, 459, 802–811. [Google Scholar] [CrossRef]
- Yang, W.; Li, Z.; Lu, Z.Y.; Yu, J.; Huo, Y.Y.; Man, B.Y.; Pan, J.; Si, H.P.; Jiang, S.Z.; Zhang, C. Graphene-Ag nanoparticles-cicada wings hybrid system for obvious SERS performance and DNA molecular detection. Opt. Express 2019, 27, 3000–3013. [Google Scholar] [CrossRef]
- Fang, Y.; Seong, N.H.; Dlott, D.D. Measurement of the distribution of site enhancements in surfaceenhanced Raman scattering. Science 2008, 321, 388–392. [Google Scholar] [CrossRef]
- Yan, X.Y.; Wang, M.Y.; Sun, X.; Wang, Y.H.; Shi, G.C.; Ma, W.L.; Hou, P. Sandwich-like Ag@Cu@CW SERS substrate with tunable nanogaps and component based on the Plasmonic nanonodule structures for sensitive detection crystal violet and 4-aminothiophenol. Appl. Surf. Sci. 2019, 479, 879–886. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D.J. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. B 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Fuchs, R. Theory of the optical properties of ionic crystal cubes. Phys. Rev. B 1975, 11, 1732–1740. [Google Scholar] [CrossRef]
- Gai, H.F.; Wang, J.; Tian, Q. Modified Debye model parameters of metals applicable for broadband calculations. Appl. Optics 2007, 46, 2229–2233. [Google Scholar] [CrossRef]
- Wang, M.L.; Yan, X.Y.; Shi, G.C.; Shang, Z.B.; Zhang, A.Q.; Ma, W.L. Optical properties of Ag@cicada wing substrate deposited by Ag nanoparticles. Curr. Appl. Phys. 2020, 20, 1253–1262. [Google Scholar] [CrossRef]
- García-Vidal, F.J.; Pendry, J.B. Collective theory for surface enhanced Raman scattering. Phys. Rev. Lett. 1996, 77, 1163–1166. [Google Scholar] [CrossRef]
- Muhammad, M.; Shao, C.S.; Huang, Q. Label-free SERS diagnostics of radiation-induced injury via detecting the biomarker Raman signal in the serum and urine bio-samples based on Au-NPs array substrates. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 233, 117282–117291. [Google Scholar] [CrossRef]
- Wang, M.L.; Shang, Z.B.; Yan, X.Y.; Shi, G.C.; Cao, H.W.; Ma, W.L.; Jiao, T.F. Enhance fluorescence study of grating structure based on three kinds of optical disks. Opt. Commun. 2021, 481, 126522–126530. [Google Scholar] [CrossRef]
- Li, C.Y.; Huang, Y.Q.; Lai, K.Q.; Rasco, B.A.; Fan, Y.X. Analysis of trace methylene blue in fish muscles using ultra-sensitive surface-enhanced Raman spectroscopy. Food Control 2016, 65, 99–105. [Google Scholar] [CrossRef]
- Lin, S.; Lin, X.; Shang, Y.; Han, S.; Hasi, W.; Wang, L. Self-assembly of faceted gold nanocrystals for surface-enhanced Raman scattering application. J. Phys. Chem. C 2019, 123, 24714–24722. [Google Scholar] [CrossRef]
- Parsons, H.M.; Ekman, D.R.; Collette, T.W.; Viant, M.R. Spectral relative standard deviation: A practical benchmark in metabolomics. Analyst 2009, 134, 478–485. [Google Scholar] [CrossRef]
- Lv, M.Y.; Teng, H.Y.; Chen, Z.Y.; Zhao, Y.M.; Zhang, X.; Liu, L.; Wu, Z.L.; Liu, L.M.; Xu, H.J. Low-cost Au nanoparticle-decorated cicada wing as sensitive and recyclable substrates for surface enhanced Raman scattering. Sens. Actuators B. Chem. 2015, 209, 820–827. [Google Scholar] [CrossRef]
- Zhang, M.F.; Meng, J.T.; Wang, D.P.; Tang, Q.; Chen, T.; Rong, S.Z.; Liu, J.Q.; Wu, Y.C. Biomimetic synthesis of hierarchical 3D Ag butterfly wing scale arrays/graphene composites as ultrasensitive SERS substrates for efficient trace chemical detection. J. Mater. Chem. C 2018, 6, 1933–1943. [Google Scholar] [CrossRef]
- Chen, M.T.; Xiao, H.T.; Chen, B.; Bian, Z.X.; Kwan, H.Y. The advantages of using Scutellaria baicalensis and its flavonoids for the management of non-viral hepatocellular carcinoma. J. Funct. Foods 2021, 78, 104389–104401. [Google Scholar] [CrossRef]
- Liao, H.F.; Ye, J.; Gao, L.L.; Liu, Y.L. The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: A comprehensive review. Biomed. Pharmacother. 2021, 133, 110917–110933. [Google Scholar] [CrossRef] [PubMed]









| Sputtering Time (min) | Peak Intensity I (cps) | Increase Factor (IAg NPs30-Ag20-AAO/IAgx-AAO) | |
|---|---|---|---|
| IAgx-AAO | IAg NPs30-Ag20-AAO | ||
| 10 | 182,101.97 ± 5480.78 | 1.037 × 106 ± 159.3 | 5.69 ± 0.029 |
| 15 | 296,142.98 ± 3581.30 | 1.037 × 106 ± 159.3 | 3.50 ± 0.044 |
| 20 | 407,642.04 ± 6139.09 | 1.037 × 106 ± 159.3 | 2.54 ± 0.026 |
| Raman (cm−1) | SERS (cm−1) | Vibrational Mode |
|---|---|---|
| 596 (w) | 595 (w) | Skeletal deformation of C–S–C |
| 671 (w) | 672 (w) | Out-of-plane bending of C–H |
| 770 (w) | 768 (w) | In-plane bending of C–H |
| 1154 (w) | 1155 (w) | In-plane bending of C–H |
| 1394 (m) | 1396 (m) | Symmetrical stretching of C–N |
| - | 1468 (w) | Asymmetrical stretching of C–N |
| 1623 (s) | 1626 (s) | Ring stretching of C–C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, G.; Li, K.; Gu, J.; Yuan, W.; Xu, S.; Han, W.; Gu, J.; Wang, L.; Zhang, Z.; Chen, C.; et al. Fabrication of Multifunctional SERS Platform Based on Ag NPs Self-Assembly Ag-AAO Nanoarray for Direct Determination of Pesticide Residues and Baicalein in Real Samples. Coatings 2021, 11, 1054. https://doi.org/10.3390/coatings11091054
Shi G, Li K, Gu J, Yuan W, Xu S, Han W, Gu J, Wang L, Zhang Z, Chen C, et al. Fabrication of Multifunctional SERS Platform Based on Ag NPs Self-Assembly Ag-AAO Nanoarray for Direct Determination of Pesticide Residues and Baicalein in Real Samples. Coatings. 2021; 11(9):1054. https://doi.org/10.3390/coatings11091054
Chicago/Turabian StyleShi, Guochao, Kuihua Li, Jungai Gu, Wenzhi Yuan, Shiqi Xu, Wei Han, Jianjun Gu, Liyong Wang, Zhibin Zhang, Congzhe Chen, and et al. 2021. "Fabrication of Multifunctional SERS Platform Based on Ag NPs Self-Assembly Ag-AAO Nanoarray for Direct Determination of Pesticide Residues and Baicalein in Real Samples" Coatings 11, no. 9: 1054. https://doi.org/10.3390/coatings11091054





