Influence of Flow and Pressure of Carburising Mixture on Low-Pressure Carburising Process Efficiency
Abstract
:1. Introduction
2. Low-Pressure Carburizing
3. Materials and Methods
4. Results
5. Discussion
5.1. Effect of Carboniferous Gas Type on Carburisation Intensity
5.2. Effect of Pressure and Flow Rate on Carburisation Intensity
6. Summary
- Carburising in pure acetylene is significantly different from carburising in acetylene mixtures, even on mixtures containing other carbon-bearing gases (ethylene, ethene) with carbon in the same amount.
- It is possible to determine, experimentally, a range of pressures in which increasing gas flow increases the kinetics of the diffusion of carbon into the steel.
- Only pressure and flow calculations performed with computer methods make it possible to credibly estimate the surface demand for carbon-bearing gas in low-pressure processes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yada, K.; Watanabe, O. Reactive Flow simulation of vacuum carburizing by acetylene gas. Comput. Fluids 2013, 79, 65–76. [Google Scholar] [CrossRef]
- Sawicki, J.; Kruszyński, B.; Wójcik, R. The Influence of grinding conditions on the distribution of residual stress in the surface layer of 17CrNi6-6 steel after carburizing. Adv. Sci. Technol. Res. J. 2017, 11, 17–22. [Google Scholar]
- Stachurski, W.; Zgórniak, P.; Sawicki, J.; Przybysz, M. Hardening-related deformations of gear wheels after vacuum carburizing and quenching in gas. Adv. Sci. Technol. Res. J. 2017, 11, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Kula, P.; Dybowski, K.; Lipa, S.; Pietrasik, R.; Atraszkiewicz, R.; Klimek, L.; Januszewicz, B.; Wolowiec, E. Investigating fatigue strength of vacuum carburized 17CrNi6-6 steel using a resonance high frequency method. Solid State Phenom. 2015, 225, 45–52. [Google Scholar]
- Kula, P.; Olejnik, J.; Heilman, P. Method for Under-Pressure Carburizing of Steel Workpieces. U.S. Patent No. 7,550,049, 23 June 2006. [Google Scholar]
- Poor, R.P. Vacuum Carburizing with Unsaturated Aromatic Hydrocarbons. U.S. Patent No. 7,033,446, 25 April 2006. [Google Scholar]
- Collin, R.; Gunnarson, S.; Thulin, D. A mathematical model for predicting carbon concentration profiles of gas-carburized steel. J. Iron Steel Inst. 1972, 210, 785–789. [Google Scholar]
- Turpin, T.; Dulcy, J.; Gantois, M. Carbon diffusion and phase transformations during gas carburizing of high-alloyed stainless steels: Experimental study and theoretical modeling. Metall. Mater. Trans. A 2005, 36, 2751–2760. [Google Scholar] [CrossRef]
- Antes, H.W. Calculating the gas flow rate for vacuum carburization. Heat Treating Progress 2005, 8, 51–53. [Google Scholar]
- Herring, D.H. A case for acetylene based low pressure carburizing of gears. Thermal Proc. Gear Solut. 2012, 9, 40–45. [Google Scholar]
- Bensabath, T.; Monnier, H.; Glaude, P.-A. Detailed kinetic modeling of the formation of toxic polycyclic aromatic hydrocarbons (PAHs) coming from pyrolysis in low-pressure gas carburizing conditions. J. Anal. Appl. Pyrolysis 2016, 122, 342–354. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.U.; Bajohr, S.; Buchholz, D.; Reimert, R.; Minh, H.D.; Norinaga, K.; Janardhanan, V.M.; Tischer, S.; Deutschmann, O. Pyrolysis of propane under vacuum carburizing conditions: An experimental and modeling study. J. Anal. Appl. Pyrolysis 2008, 81, 148–156. [Google Scholar] [CrossRef]
- Mendiara, T.; Domene, M.P.; Millera, A.; Bilbao, R.; Alzueta, M.U. An experimental study of the soot formed in the pyrolysis of acetylene. J. Anal. Appl. Pyrolysis 2005, 74, 486–493. [Google Scholar] [CrossRef]
- Tsepov, S.N.; Krishtal, M.A. Characteristics of structure formation in the surface layers of metal during vacuum carburizing. Metal. Sci. Heat Treat. 1983, 25, 358–359. [Google Scholar]
- Herring, D.H.; Peters, R.V. New-formula acetylene cool for heat treatment. Gear Technol. 2013, 9, 90–96. [Google Scholar]
- Kubota, K. Vacuum Carburizing Method and Device, and Carburized Products; JH Corporation: Hanam-si, Korea, 1997. [Google Scholar]
- Jordan, D. Versatile High Velocity Integral Vacuum Furnace. U.S. Patent No. 7,514,035, 7 April 2009. [Google Scholar]
- Buchholz, D.; Khan, R.U.; Bajohr, S.; Reimert, R. Computational fluid dynamics modeling of acetylene pyrolysis for vacuum carburizing of steel. Ind. Eng. Chem. Res. 2010, 49, 1130–1137. [Google Scholar] [CrossRef]
- Norinaga, K.; Deutschmann, O.; Saegusa, N.; Hayashi, J. Analysis of pyrolysis products from light hydrocarbons and kinetic modeling for growth of polycyclic aromatic hydrocarbons with detailed chemistry. J. Anal. Appl. Pyrolysis 2009, 86, 148–160. [Google Scholar] [CrossRef]
- Saggese, C.; Sanchez, N.E.; Frassoldati, A.; Cuoci, A.; Faravelli, T.; Alzueta, M.U.; Ranzi, E. Kinetic modeling study of polycyclic aromatic hydrocarbons and soot formation in acetylene pyrolysis. Energy Fuels 2014, 28, 1489–1501. [Google Scholar] [CrossRef]
- Sanchez, N.E.; Callejas, A.; Millera, A.; Bilbao, R.; Alzueta, M.U. Polycyclic aromatic hydrocarbon (PAH) and soot formation in the pyrolysis of acetylene and ethylene: Effect of the reaction temperature. Energy Fuels 2012, 26, 4823–4829. [Google Scholar] [CrossRef]
- Slavinskaya, N.A.; Riedel, U.; Dworkin, S.B.; Thomson, M.J. Detailed numerical modeling of PAH formation and growth in non-premixed ethylene and ethane flames. Combust. Flame 2012, 159, 979–995. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, S.; Ishigami, I.; Yamanaka, K. Vacuum carburizing of low carbon steel with methane. Trans. Jpn. Inst. Metals 1987, 28, 48–56. [Google Scholar]
- Xu, C.; Al Shoaibi, A.S.; Wang, C.; Carstensen, H.; Dean, A.M. Kinetic modeling of ethane pyrolysis at high conversion. J. Phys. Chem. A 2011, 115, 10470–10490. [Google Scholar] [CrossRef]
- Harris, F. Case depth–an attempt at a practical definition. Metal. Progress 1943, 44, 265–272. [Google Scholar]
- Wołowiec-Korecka, E.; Korecki, M.; Sut, M.; Brewka, A.; Kula, P. Calculation of the mixture flow in a low-pressure carburizing process. Metals 2019, 9, 439. [Google Scholar] [CrossRef] [Green Version]
- Kula, P.; Olejnik, J.; Heilman, P. Hydrocarbon Gas Mixture for the under Pressure Carburizing of Steel. U.S. Patent No. 7,513,958, 7 April 2007. [Google Scholar]
- Herring, D.H. Vacuum Heat Treatment. Principles|Practices|Applications; BNP Media II: Troy, MI, USA, 2012. [Google Scholar]
- Watanabe, T.; Hirata, T. New Concept and Practical Operation of Carburizing and Nitriding; Agune Technology Center: Tokyo, Japan, 2015. [Google Scholar]
- Kula, P.; Olejnik, J.; Kowalewski, J. Smart control system optimizes vacuum carburizing process. Ind. Heat. 2003, 9, 99–102. [Google Scholar]
- Osterman, V.M. Development experience in low-torr range vacuum carburizing. Ind. Heat. 2005, 9, 95–99. [Google Scholar]
- Gorockiewicz, R.; Łapinski, A. Structure of the carbon layer deposited on the steel surface after low pressure carburizing. Vacuum 2010, 85, 429–433. [Google Scholar]
| Family | Combinations |
|---|---|
| Acetylene and Aceylene mixtures | 100% Acetylene (C2H2) [16] |
| Acetylene + Nitrogen a | |
| Acetylene + Hydrogen b [17] | |
| Acetylene + Etylene (C2H4) + Hydrogen c [5] Acetylene + Cyclohexane | |
| Cyclohexane and Cyclohexane Mixtures | 100% Cycloxehane (C6H12) [6] |
| Cycloxehane + Acetylene | |
| Methane and Methane Mixtures | 100% Methane (CH4) |
| Methane + Propane | |
| Propane and Propane Mixtures | 100% Propane (C3H8) |
| Propane + Methane d | |
| Propane + Hydrogen | |
| Propane + Butane (C4H10) |
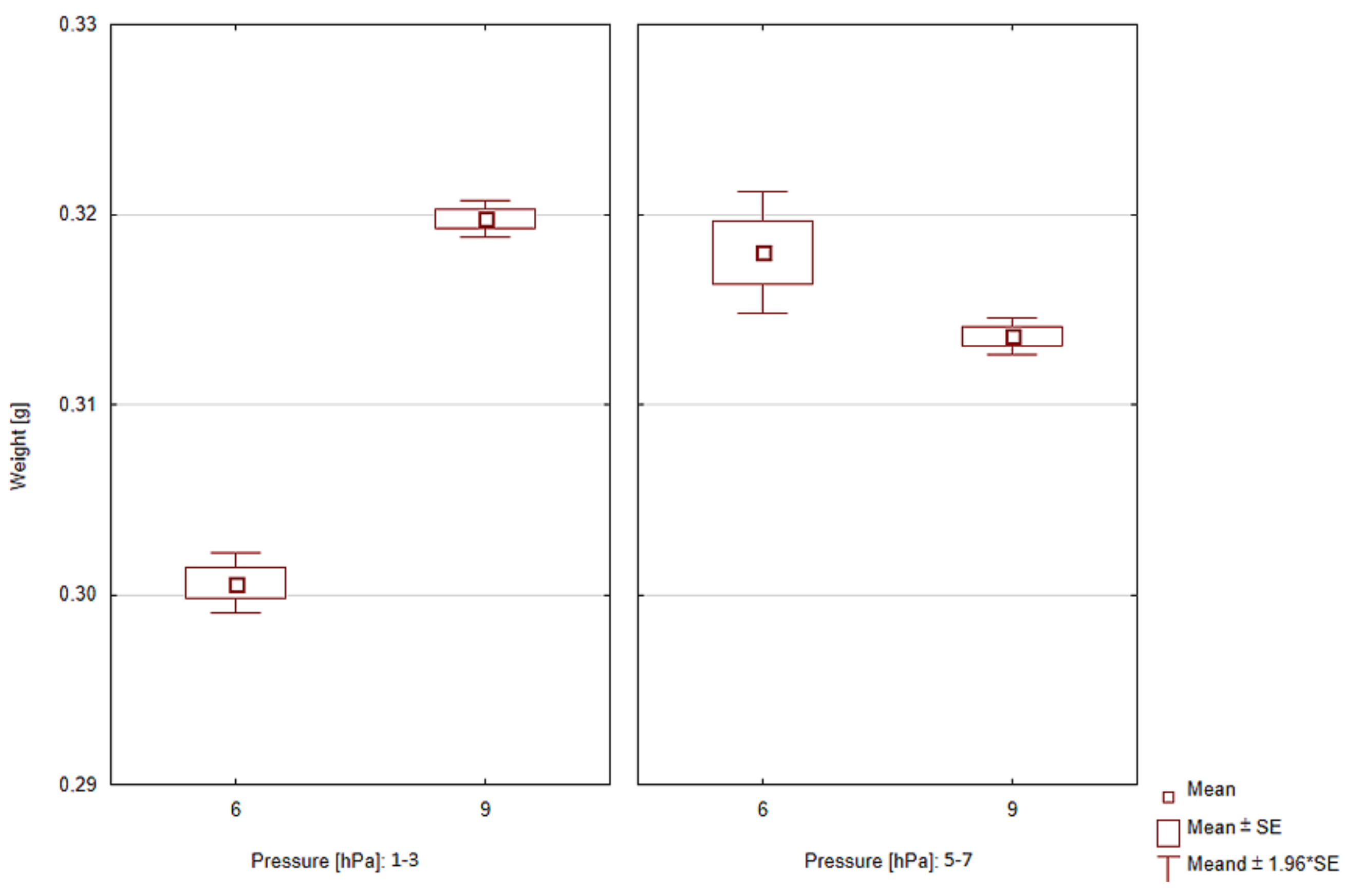
| Process | Gas Type | Pressure [hPa] | Flow [dm3/min] | Mass Increasing Mean ± SD [g] |
|---|---|---|---|---|
| 1 | C2H2 | 1–3 | 6 | 0.301 ± 0.002 |
| 2 | C2H2 | 5–7 | 6 | 0.318 ± 0.004 |
| 3 | C2H2 | 5–7 | 9 | 0.314 ± 0.001 |
| 4 | C2H2 | 1–3 | 9 | 0.320 ± 0.001 |
| Total | - | - | - | 0.313 ± 0.008 |
| Statistical analysis | ||||
| Factor | Range | t | p | |
| Pressure | 1–3 hPa | −20.34 | <0.001 | |
| 5–7 hPa | 2.56 | <0.05 | ||
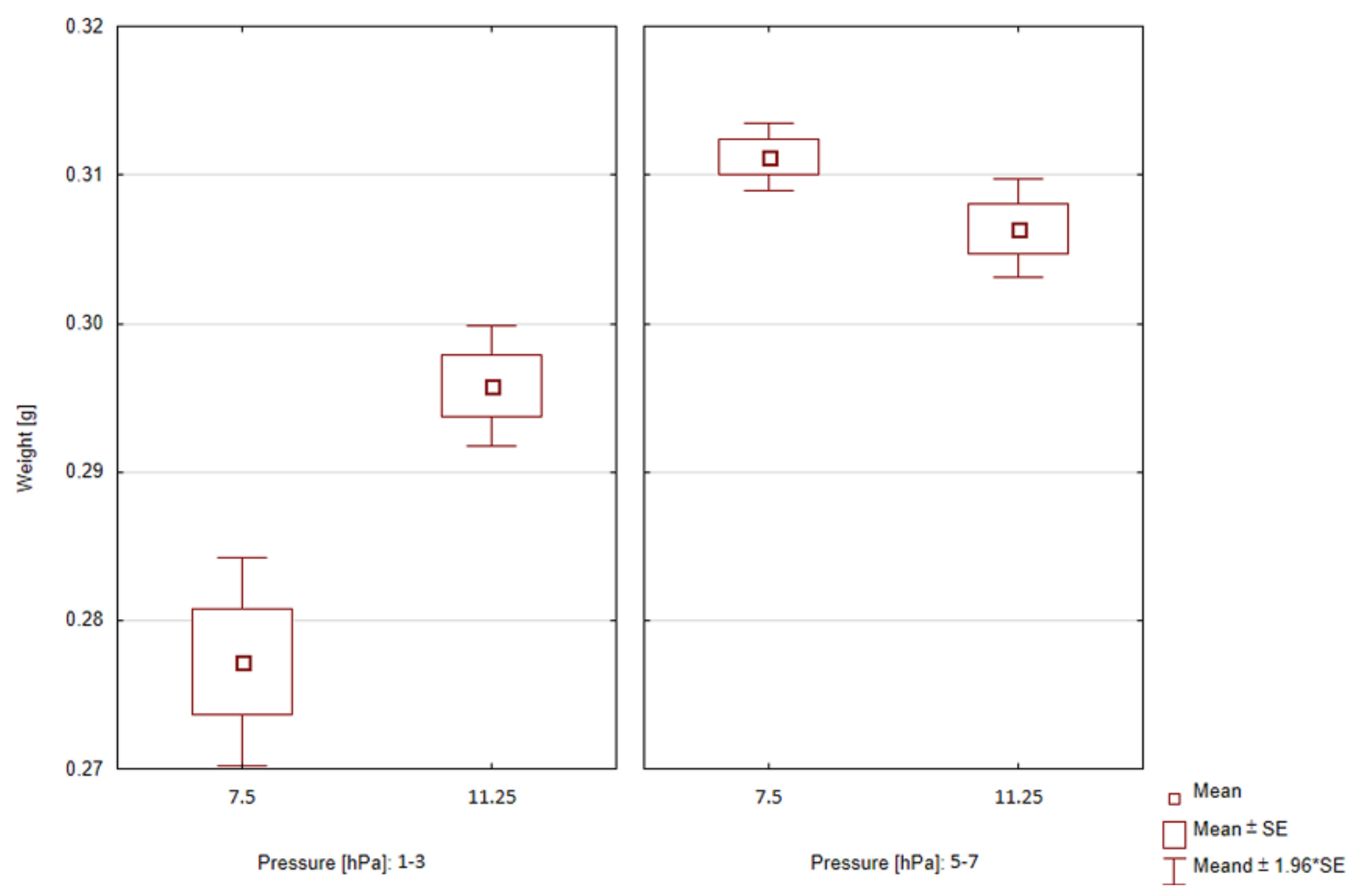
| Process | Gas Type | Pressure [hPa] | Flow [dm3/min] | Mass Increasing Mean ± SD [g] |
|---|---|---|---|---|
| 5 | C2H2:C2H4:H2 | 1–3 | 7.5 | 0.278 ± 0.008 |
| 6 | C2H2:C2H4:H2 | 5–7 | 7.5 | 0.311 ± 0.003 |
| 7 | C2H2:C2H4:H2 | 5–7 | 11.25 | 0.306 ± 0.004 |
| 8 | C2H2:C2H4:H2 | 1–3 | 11.25 | 0.296 ± 0.005 |
| Total | - | - | - | 0.298 ± 0.015 |
| Statistical analysis | ||||
| Factor | Range | t | p | |
| Pressure | 1–3 hPa | −4.49 | <0.01 | |
| 5–7 hPa | 2.34 | <0.05 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wołowiec-Korecka, E.; Korecki, M.; Klimek, L. Influence of Flow and Pressure of Carburising Mixture on Low-Pressure Carburising Process Efficiency. Coatings 2022, 12, 337. https://doi.org/10.3390/coatings12030337
Wołowiec-Korecka E, Korecki M, Klimek L. Influence of Flow and Pressure of Carburising Mixture on Low-Pressure Carburising Process Efficiency. Coatings. 2022; 12(3):337. https://doi.org/10.3390/coatings12030337
Chicago/Turabian StyleWołowiec-Korecka, Emilia, Maciej Korecki, and Leszek Klimek. 2022. "Influence of Flow and Pressure of Carburising Mixture on Low-Pressure Carburising Process Efficiency" Coatings 12, no. 3: 337. https://doi.org/10.3390/coatings12030337
APA StyleWołowiec-Korecka, E., Korecki, M., & Klimek, L. (2022). Influence of Flow and Pressure of Carburising Mixture on Low-Pressure Carburising Process Efficiency. Coatings, 12(3), 337. https://doi.org/10.3390/coatings12030337







