Enhanced Corrosion Resistance and Surface Wettability of PVDF/ZnO and PVDF/TiO2 Composite Coatings: A Comparative Study
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Starting Materials
2.2. Samples Preparation
2.3. Characterization Techniques
3. Results and Discussion
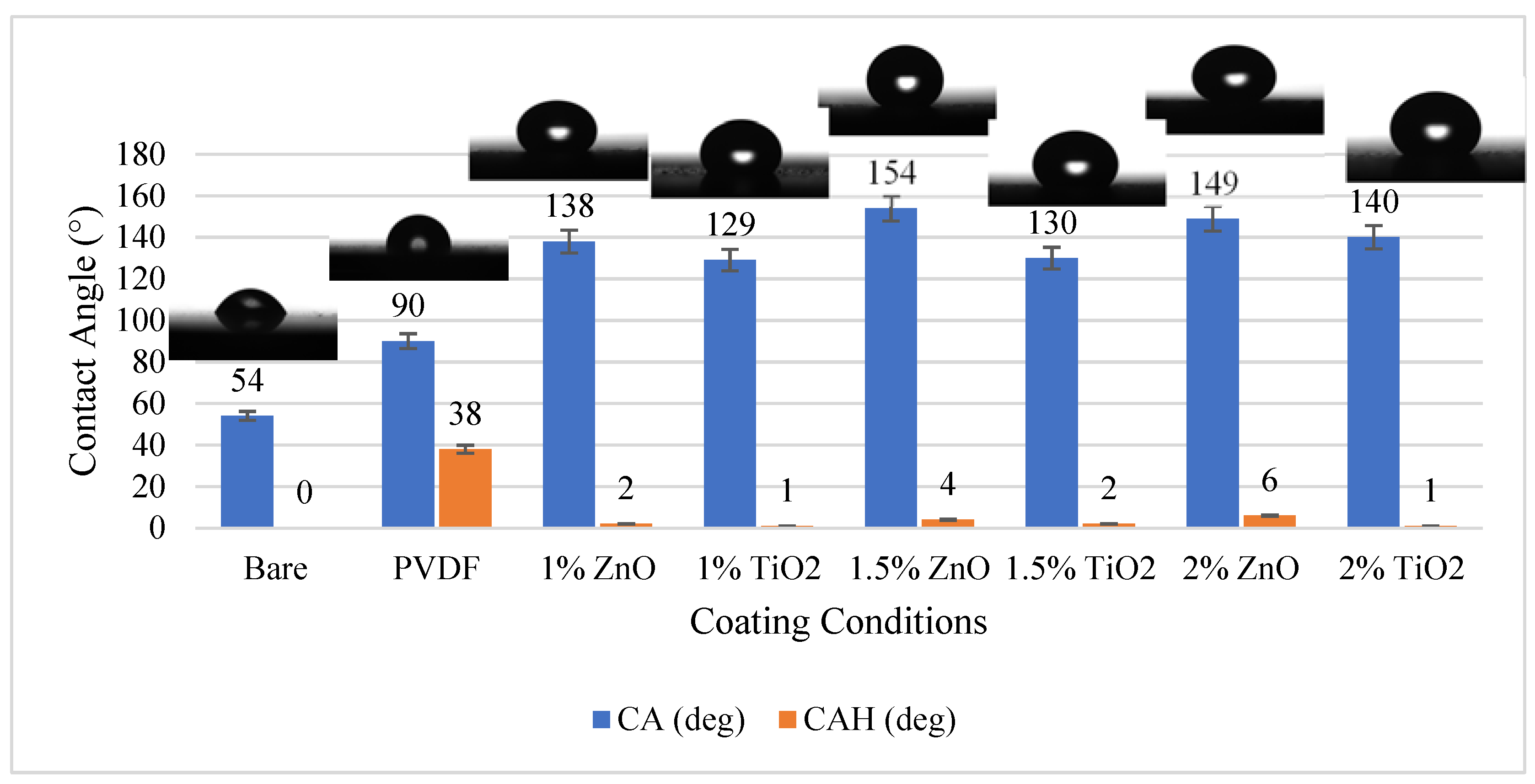
3.1. Analysis of the Wettability Properties of Nanoparticle-Coated Surfaces
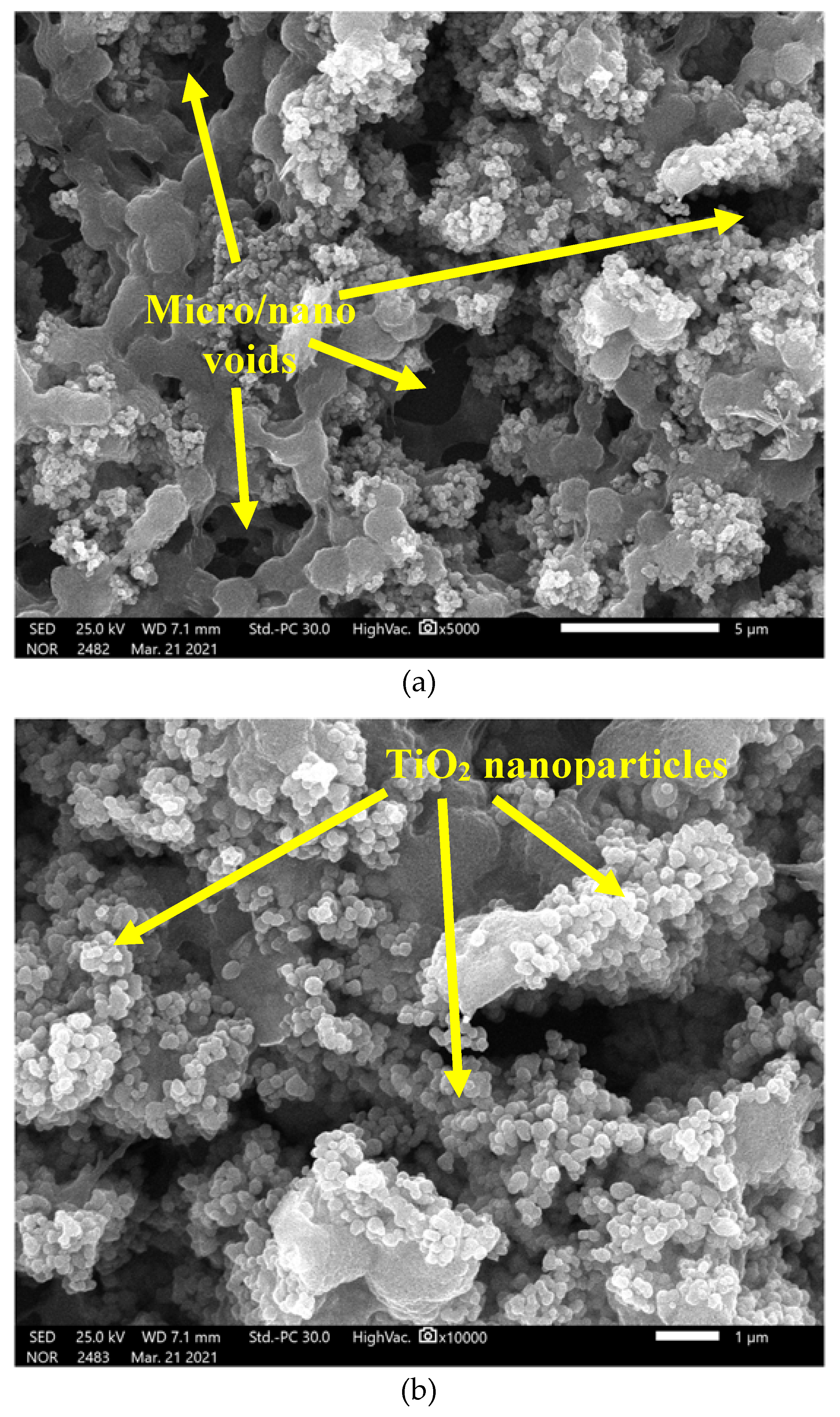
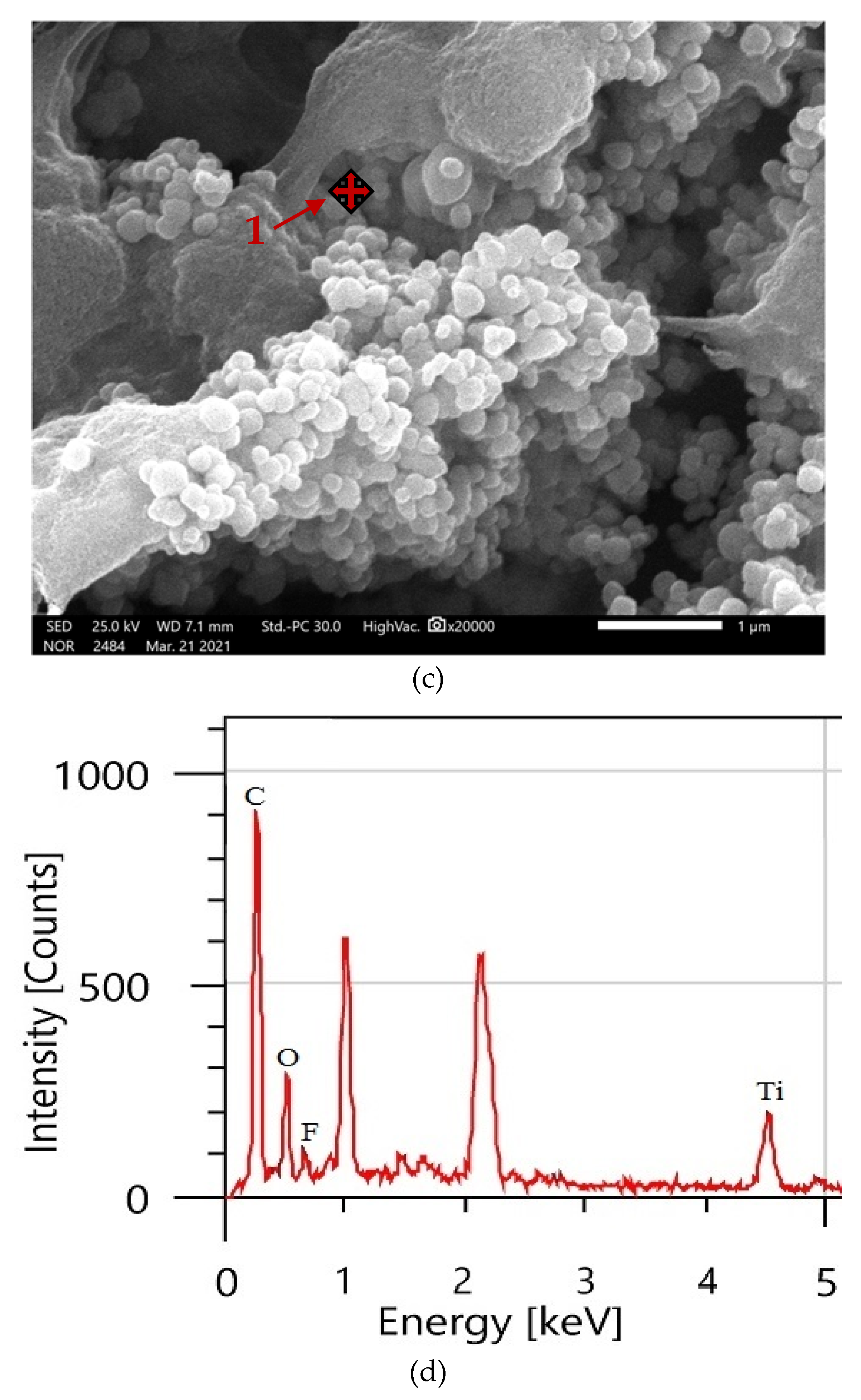
3.2. Surface Morphology Analysis
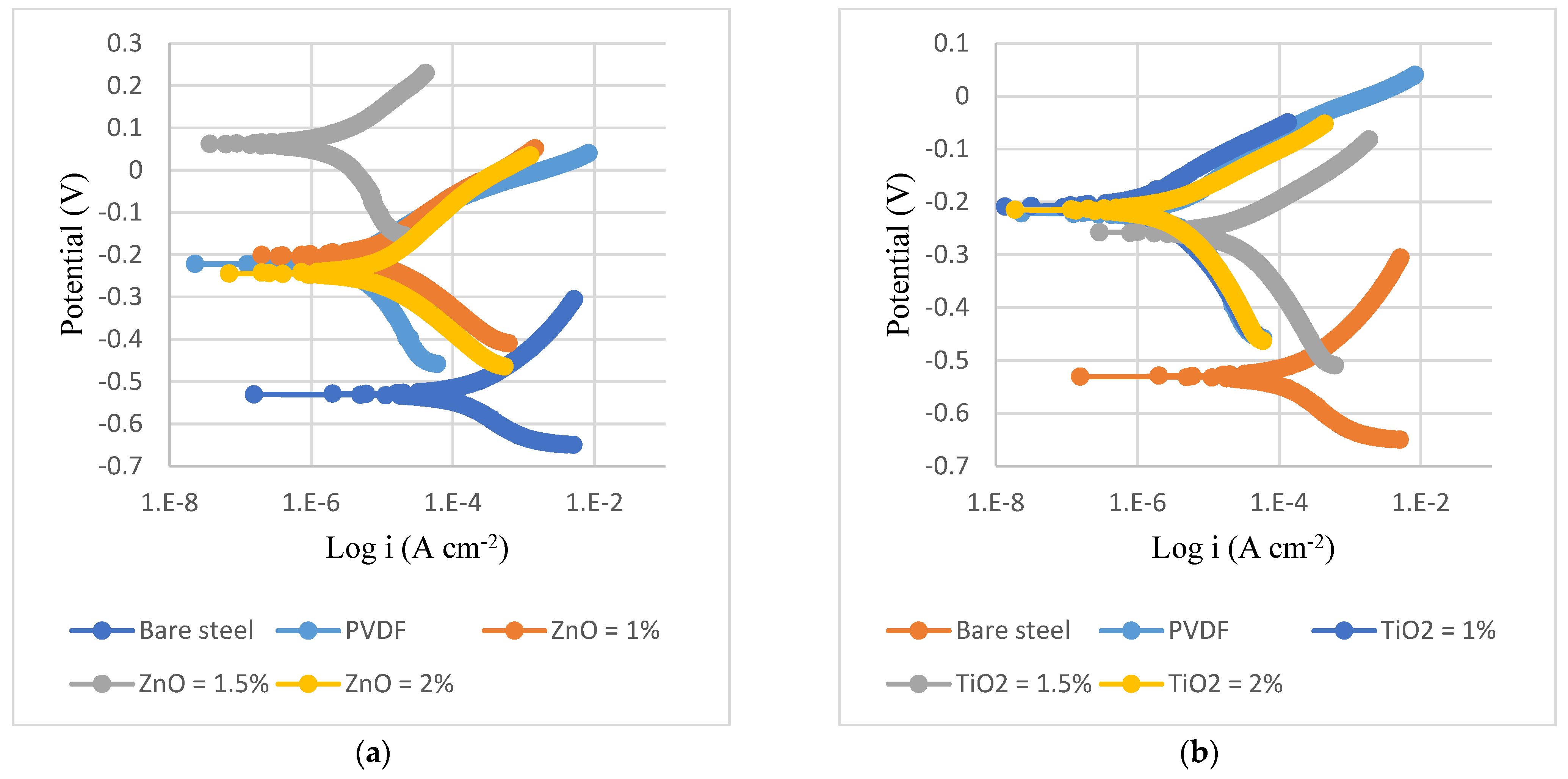
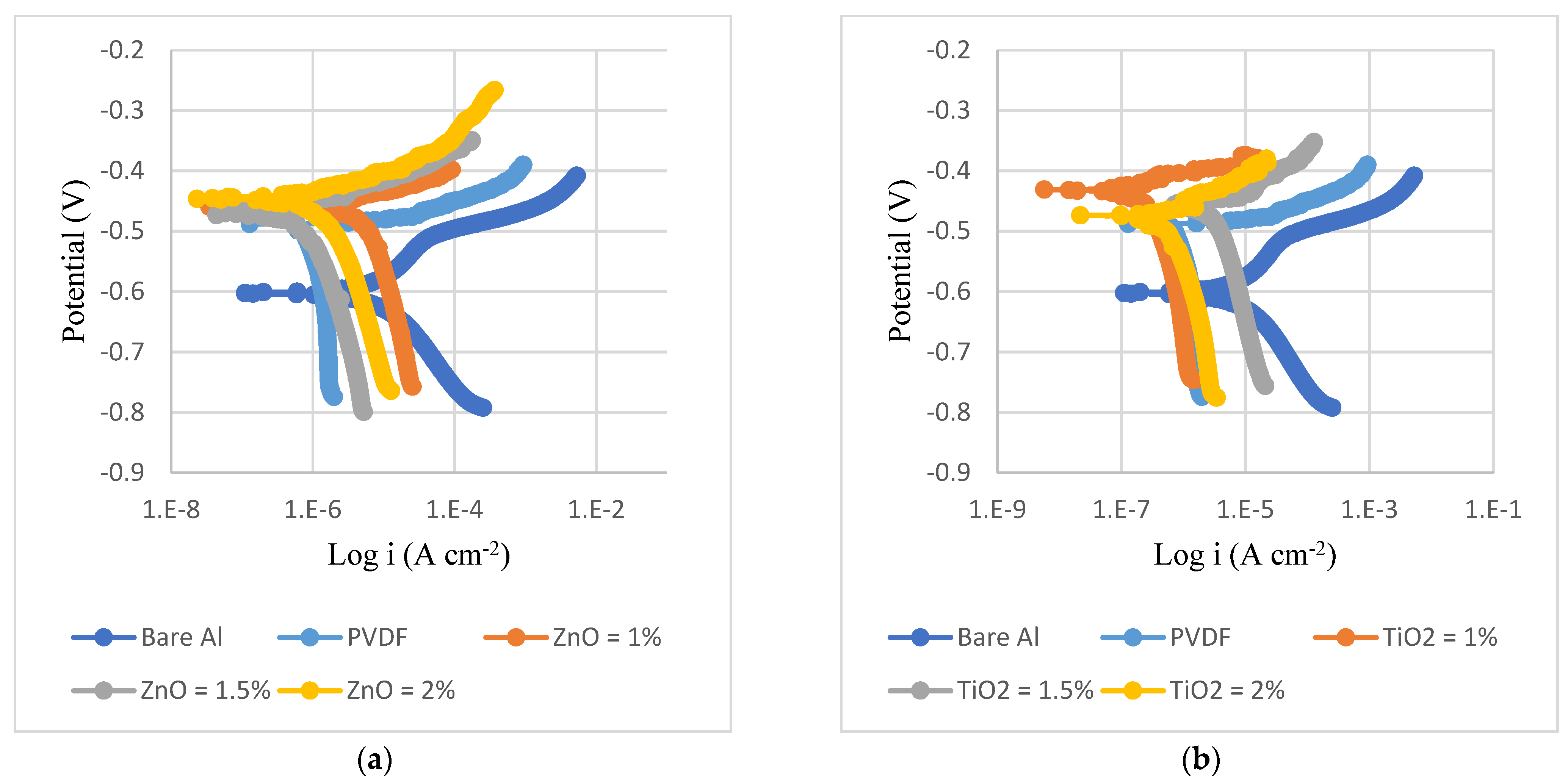
3.3. Corrosion Analysis
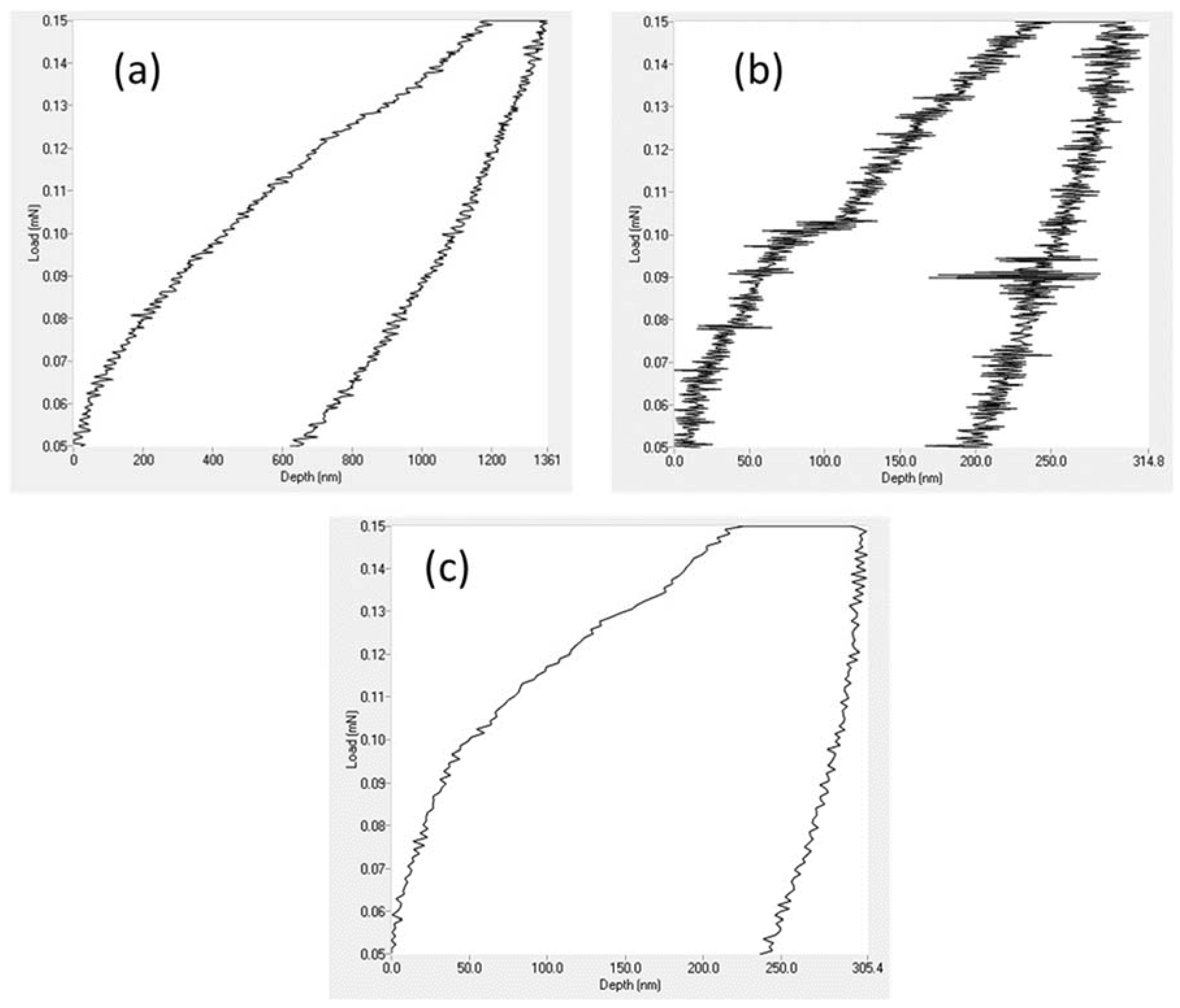
3.4. Nanohardness Analysis
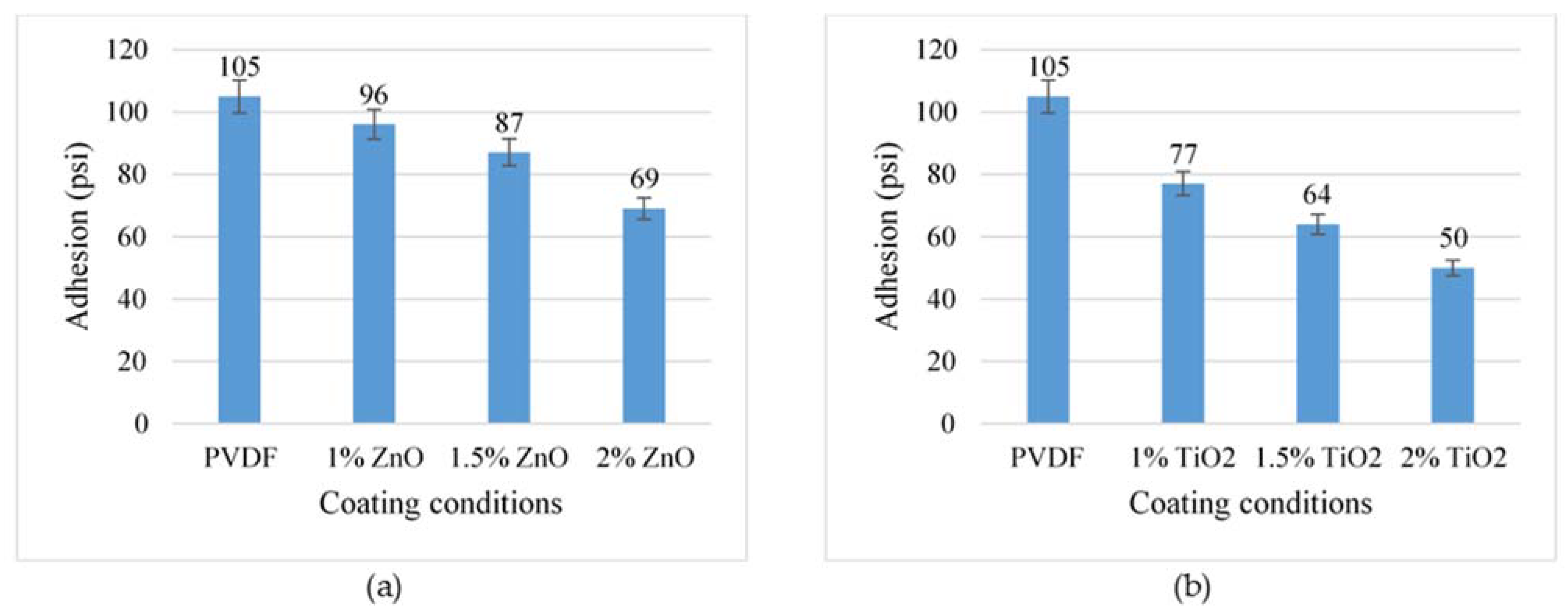
3.5. Adhesion Analysis
4. Conclusions
- Adding ZnO and TiO2 nanoparticles to PVDF polymer increases its hydrophobicity and surface energy distribution. ZnO has better wettability properties than TiO2 and is useful for self-cleaning surfaces, anti-fog coatings, and corrosion protection.
- Adding ZnO and TiO2 nanoparticles changes the morphology of PVDF coatings and helps achieve superhydrophobicity. The difference in morphology is due to the size and shape of the nanoparticles.
- ZnO nanoparticles fill gaps at the surface of PVDF coatings, which hinders the entry of aggressive ions and improves corrosion resistance. Nanoparticles act as a barrier and enhance the adhesion, mechanical properties, density, and porosity of coatings.
- The addition of both ZnO and TiO2 nanoparticles significantly enhances the mechanical properties of PVDF coatings. Nanoindentation tests showed that the coatings containing 1.5% ZnO or 2% TiO2 exhibited increased hardness and elastic modulus compared to coatings with PVDF alone.
- Adding TiO2 nanoparticles has a more significant impact on the adhesion force of PVDF coatings than ZnO nanoparticles. Changes in surface properties, such as surface roughness and energy, cause a decrease in adhesion force with increasing nanoparticle concentration.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic materials for biomedical applications. Biomaterials 2017, 104, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.M.A.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, W.; Mou, J.; Zheng, S.; Jiang, L.; Sun, Z.; Wang, E. Research progress of biomimetic superhydrophobic surface characteristics, fabrication, and application. Adv. Mech. Eng. 2017, 9, 1687814017746859. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, L.; Qian, H.; Li, X. Superhydrophobic surfaces for corrosion protection: A review of recent progresses and future directions. J. Coat. Technol. Res. 2016, 13, 11–29. [Google Scholar] [CrossRef]
- Gu, J.; An, Q.; Li, J.; Ge, P.; Wu, Y.; Li, Y. Preparation of Superhydrophobic Materials and Establishment of Anticorrosive Coatings on the Tinplate Substrate by Alkylation of Graphene Oxide. Polymers 2023, 15, 1280. [Google Scholar] [CrossRef]
- Pathreeker, S.; Chando, P.; Chen, F.H.; Biria, S.; Li, H.; Finkelstein, E.B. Superhydrophobic Polymer Composite Surfaces Developed via Photopolymerization. ACS Appl. Polym. Mater. 2021, 3, 4661–4672. [Google Scholar] [CrossRef]
- Chatterjee, S.; Das, P.; Tripathy, U.; Singh, B.P.; Besra, L. Development of polymer-based superhydrophobic coating on cloth. Bull. Mater. Sci. 2020, 43, 130. [Google Scholar] [CrossRef]
- Liu, M.; Luo, Y.; Jia, D. Synthesis of mechanically durable superhydrophobic polymer materials with roughness-regeneration performance. c polymer materials with roughness-regeneration performance. Compos. Part A Appl. Sci. Manuf. 2019, 133, 105861. [Google Scholar] [CrossRef]
- Lee, E.; Lee, K.H. Facile fabrication of superhydrophobic surfaces with hierarchical structures. Sci. Rep. 2018, 8, 4101. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Manoudis, P.N.; Savva, A.; Panayiotou, C. Superhydrophobic polymer-particle composite films produced using various particle sizes. Surf. Interface Anal. 2012, 44, 870–875. [Google Scholar] [CrossRef]
- Radwan, A.B.; Mohamed, A.M.A.; Abdullah, A.M.; Al-Maadeed, M.A. Corrosion protection of electrospun PVDF-ZnO superhydrophobic coating. Surf. Coat. Technol. 2016, 289, 136–143. [Google Scholar] [CrossRef]
- Liu, H.; Gao, S.W.; Cai, J.S.; He, C.L.; Mao, J.J.; Zhu, T.X. Recent progress in fabrication and applications of superhydrophobic coating on cellulose-based substrates. Materials 2016, 9, 124. [Google Scholar] [CrossRef]
- Bayer, I.S.; Steele, A.; Martorana, P.J.; Loth, E. Fabrication of superhydrophobic polyurethane/organoclay nano-structured composites from cyclomethicone-in-water emulsions. Appl. Surf. Sci. 2010, 257, 823–826. [Google Scholar] [CrossRef]
- Pawar, P.G.; Xing, R.; Kambale, R.C.; Kumar, A.M.; Liu, S.; Latthe, S.S. Polystyrene assisted superhydrophobic silica coatings with surface protection and self-cleaning approach. Prog. Org. Coat. 2017, 105, 235–244. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, B.; Ma, X.; Lu, S.; Xu, W. Facile preparation of high density polyethylene superhydrophobic/superoleophilic coatings on glass, copper and polyurethane sponge for self-cleaning, corrosion resistance and efficient oil/water separation. J. Colloid Interface Sci. 2018, 525, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Ghashghaee, M.; Fallah, M.; Rabiee, A. Superhydrophobic nanocomposite coatings of poly(methyl methacrylate) and stearic acid grafted CuO nanoparticles with photocatalytic activity. Prog. Org. Coat. 2019, 136, 105270. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Fei, X.; Sun, M.; Zhang, C.; Li, Y. Preparation of a durable superhydrophobic membrane by electrospinning poly (vinylidene fluoride) (PVDF) mixed with epoxy-siloxane modified SiO2 nanoparticles: A possible route to superhydrophobic surfaces with low water sliding angle and high water contac. J. Colloid Interface Sci. 2011, 359, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Ji, Y.; Yu, G.; Zhai, Y. Preparation and properties of polyvinylidene fluoride nanocomposited membranes based on Poly(N-Isopropylacrylamide) modified graphene oxide nanosheets. Polymers 2019, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Yang, C.; Yu, N.; Shang, Y.; Sun, Y.; Wang, L. Superhydrophobic TiO2/polyvinylidene fluoride composite surface with reversible wettability switching and corrosion resistance. Chem. Eng. J. 2016, 290, 37–44. [Google Scholar] [CrossRef]
- Cengiz, U.; Cansoy, C.E. Applicability of Cassie-Baxter equation for superhydrophobic fluoropolymer-silica composite films. Appl. Surf. Sci. 2015, 335, 99–106. [Google Scholar] [CrossRef]
- Saleh, S.M.; Alminderej, F.M.; Mohamed, A.M.A. Superhydrophobic and Corrosion Behaviour of PVDF-CeO2 Composite Coatings. Materials 2022, 15, 8647. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xu, X.; Chen, J.; Yang, F. Optimization of preparation conditions of poly(vinylidene fluoride)/graphene oxide microfiltration membranes by the Taguchi experimental design. Desalination 2014, 334, 17–22. [Google Scholar] [CrossRef]
- Liu, F.; Abed, M.R.M.; Li, K. Preparation and characterization of poly(vinylidene fluoride) (PVDF) based ultrafiltration membranes using nano γ-Al2O3. J. Membr. Sci. 2011, 366, 97–103. [Google Scholar] [CrossRef]
- Wang, S.; Alenius, H.; El-Nezami, H.; Karisola, P. A New Look at the Effects of Engineered ZnO and TiO2 Nanoparticles: Evidence from Transcriptomics Studies. Nanomaterials 2022, 12, 1247. [Google Scholar] [CrossRef]
- Mardosaitė, R.; Tė, A.J.; Račkauskas, S. Superhydrophobic ZnO Nanowires: Wettability Mechanisms and Functional Applications. Cryst. Growth Des. 2021, 21, 4765–4779. [Google Scholar] [CrossRef]
- Velayi, E.; Norouzbeigi, R. Synthesis of hierarchical superhydrophobic zinc oxide nano-structures for oil/water separation. Ceram. Int. 2018, 44, 14202–14208. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, S.; Jiao, S.; Lv, Z.; Liu, E. Fabrication of superhydrophobic TiO 2 quadrangular nanorod film with self-cleaning, anti-icing properties. Ceram. Int. 2019, 45, 11508–11516. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Wu, Z.; Bao, Y.; Xiao, R.; Yu, H. Durable, self-cleaning and superhydrophobic bamboo timber surfaces based on TiO2 films combined with fluoroalkylsilane. Ceram. Int. 2016, 42, 9621–9629. [Google Scholar] [CrossRef]
- Kang, M.; Jung, R.; Kim, H.S.; Jin, H.J. Preparation of superhydrophobic polystyrene membranes by electrospinning. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313, 411–414. [Google Scholar] [CrossRef]
- Li, B.J.; Huang, L.J.; Zhou, M.; Ren, N.F. Morphology and wettability of ZnO nanostructures prepared by hydrothermal method on various buffer layers. Appl. Surf. Sci. 2013, 286, 391–396. [Google Scholar] [CrossRef]
- Choi, W.; Tuteja, A.; Mabry, J.M.; Cohen, R.E.; McKinley, G.H. A modified Cassie-Baxter relationship to explain contact angle hysteresis and anisotropy on non-wetting textured surfaces. J. Colloid Interface Sci. 2009, 339, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Bormashenko, E. Progress in understanding wetting transitions on rough surfaces. Adv. Colloid Interface Sci. 2015, 222, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; He, Y. Effects of nano sized zinc oxide on the performance of PVDF microfiltration membranes. Desalination 2012, 302, 71–79. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Hasan, H.; Seleman, M.M.E.-S.; Ahmed, E.; Saleh, S.M.; El-Maghraby, R.M. Performance of sprayed PVDF-Al2O3 composite coating for industrial and civil applications. Materials 2021, 14, 6358. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Z.; Wang, E.; Yuan, R.; Gao, D.; Zhang, X. A robust superhydrophobic PVDF composite coating with wear/corrosion-resistance properties. Appl. Surf. Sci. 2015, 332, 518–524. [Google Scholar] [CrossRef]






| Substrate | Condition | ZnO Nanoparticles (g/100 mL) | TiO2 Nanoparticles (g/100 mL) |
|---|---|---|---|
| Steel | Bare | - | - |
| PVDF | 1.0/1.5/2.0 | 1.0/1.5/2.0 | |
| Aluminum | Bare | - | - |
| PVDF | 1.0/1.5/2.0 | 1.0/1.5/2.0 | |
| Glass | Bare | - | - |
| PVDF | 1.0/1.5/2.0 | 1.0/1.5/2.0 |
| Steel | C | Mn | Cu | Si | W | Al | V | Nb | P | S | Fe |
| 0.105 | 0.78 | 0.084 | 0.042 | 0.040 | 0.038 | 0.038 | 0.036 | 0.033 | 0.018 | Bal. | |
| Al | Si | Fe | Mg | Mn | Ti | V | Cu | Cr | Al | ||
| 0.228 | 0.268 | 0.82 | 0.077 | 0.015 | 0.011 | 0.009 | 0.0005 | Bal. | |||
| Glass | SiO2 | Al2O3 | TiO2 | Cr2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | |
| 72.24 | 1.44 | 0.035 | 0.002 | 0.07 | 11.50 | 0.32 | 13.64 | 0.35 | 0.21 |
| Sample No. | Nanoceramic Particles | Amount (g) | Substrate | WCA (deg.) | WCAH (deg.) |
|---|---|---|---|---|---|
| 1 | Bare | - | Steel | 54 ± 3 | Pinned |
| 2 | Bare | - | Al | 71 ± 3 | Pinned |
| 3 | Bare | - | Glass | 51 ± 3 | Pinned |
| 4 | PVDF alone | 5 | Steel | 90 ± 2 | 38 ± 3 |
| 5 | PVDF alone | 5 | Al | 91 ± 2 | 35 ± 2 |
| 6 | PVDF alone | 5 | Glass | 93 ± 2 | 25 ± 2 |
| 7 | ZnO | 1 | Steel | 138 ± 2 | 2 ± 1 |
| 8 | ZnO | 1 | Al | 157 ± 2 | 4 ± 1 |
| 9 | ZnO | 1 | Glass | 144 ± 2 | 5 ± 2 |
| 10 | ZnO | 1.5 | Steel | 154 ± 1 | 4 ± 1 |
| 11 | ZnO | 1.5 | Al | 160 ± 1 | 5 ± 1 |
| 12 | ZnO | 1.5 | Glass | 156 ± 1 | 6 ± 1 |
| 13 | ZnO | 2 | Steel | 149 ± 1 | 6 ± 1 |
| 14 | ZnO | 2 | Al | 154 ± 3 | 5 ± 1 |
| 15 | ZnO | 2 | Glass | 161 ± 5 | 7 ± 2 |
| 25 | TiO2 | 1 | Steel | 131 ± 2 | 2 ± 1 |
| 26 | TiO2 | 1 | Al | 126 ± 1 | 3 ± 1 |
| 27 | TiO2 | 1 | Glass | 137 ± 2 | 4 ± 1 |
| 28 | TiO2 | 1.5 | Steel | 128 ± 1 | 3 ± 1 |
| 29 | TiO2 | 1.5 | Al | 126 ± 2 | 4 ± 1 |
| 30 | TiO2 | 1.5 | Glass | 127 ± 1 | 3 ± 1 |
| 31 | TiO2 | 2 | Steel | 140 ± 1 | 2 ± 1 |
| 32 | TiO2 | 2 | Al | 142 ± 1 | 7 ± 1 |
| 33 | TiO2 | 2 | Glass | 141 ± 2 | 3 ± 2 |
| Coating Type | C (Mass %) | F (Mass %) | O (Mass %) | Zn (Mass %) | Ti (Mass %) |
|---|---|---|---|---|---|
| PVDF alone | 47.65 | 52.35 | - | - | - |
| PVDF + 1.5% ZnO | 39.30 | 31.22 | 11.77 | 17.71 | - |
| PVDF + 2% TiO2 | 54.92 | 8.30 | 30.95 | - | 5.83 |
| Substrate | Coating | βa (mV) | βc (mV) | Rp (K Ω cm2) | CR Rate (mpy) | i_corr (µA/cm2) | η (%) |
|---|---|---|---|---|---|---|---|
| Steel | Bare | 117 | 139 | 0.7 | 17 | 37.75 | - |
| PVDF | 73 | 198 | 7 | 0.24 | 3.34 | 91 | |
| PVDF + 1.5% ZnO | 156 | 371 | 47 | 0.046 | 1.02 | 97 | |
| PVDF + 2% TiO2 | 401 | 589 | 36 | 0.21 | 2.91 | 92.3 | |
| Al | Bare | 54 | 122 | 2 | 3.54 | 8.26 | - |
| PVDF | 93 | 865 | 28 | 0.08 | 1.3 | 84 | |
| PVDF + 1.5% ZnO | 432 | 373 | 83 | 0.07 | 1.04 | 87 | |
| PVDF + 2% TiO2 | 510 | 163 | 82 | 0.05 | 0.66 | 92 |
| Sample | Hardness (MPa) | Elastic Modulus (MPa) | Maximum Depth (nm) |
|---|---|---|---|
| PVDF alone | 10.1 | 44.4 | 1360.6 |
| PVDF + 1.5% ZnO | 137.5 | 919.4 | 314.8 |
| PVDF + 2% TiO2 | 264.0 | 1036.1 | 305.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.M.A.; Alateyah, A.I.; Hasan, H.; Matli, P.R.; El-Sayed Seleman, M.M.; Ahmed, E.; El-Garaihy, W.H.; Golden, T.D. Enhanced Corrosion Resistance and Surface Wettability of PVDF/ZnO and PVDF/TiO2 Composite Coatings: A Comparative Study. Coatings 2023, 13, 1729. https://doi.org/10.3390/coatings13101729
Mohamed AMA, Alateyah AI, Hasan H, Matli PR, El-Sayed Seleman MM, Ahmed E, El-Garaihy WH, Golden TD. Enhanced Corrosion Resistance and Surface Wettability of PVDF/ZnO and PVDF/TiO2 Composite Coatings: A Comparative Study. Coatings. 2023; 13(10):1729. https://doi.org/10.3390/coatings13101729
Chicago/Turabian StyleMohamed, Adel M. A., Abdulrahman I. Alateyah, Hosam Hasan, Penchal Reddy Matli, Mohamed M. El-Sayed Seleman, Essam Ahmed, Waleed H. El-Garaihy, and Teresa D. Golden. 2023. "Enhanced Corrosion Resistance and Surface Wettability of PVDF/ZnO and PVDF/TiO2 Composite Coatings: A Comparative Study" Coatings 13, no. 10: 1729. https://doi.org/10.3390/coatings13101729








