Properties and Performance of TiAlSiN and AlCrN Monolayer and Multilayer Coatings for Turning Ti-6Al-4V
Abstract
:1. Introduction
2. Materials and Methods
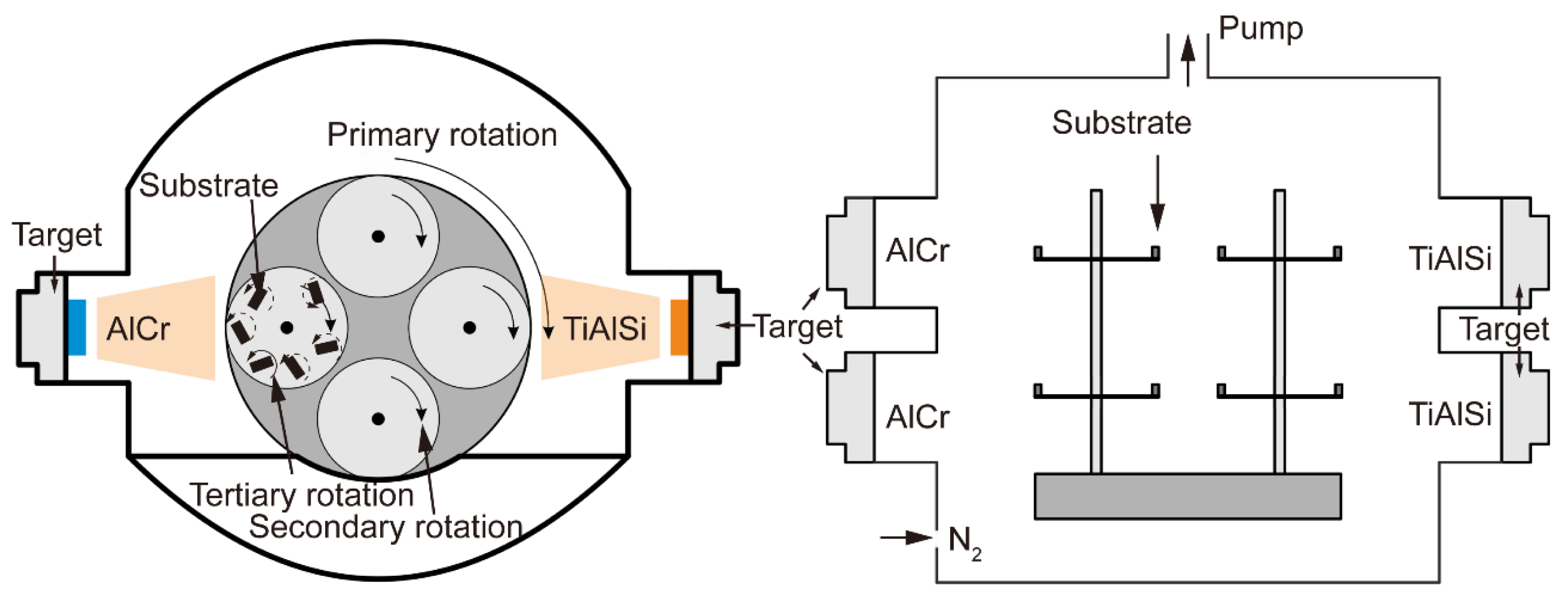
2.1. Coating Deposition
2.2. Coating Characterisation
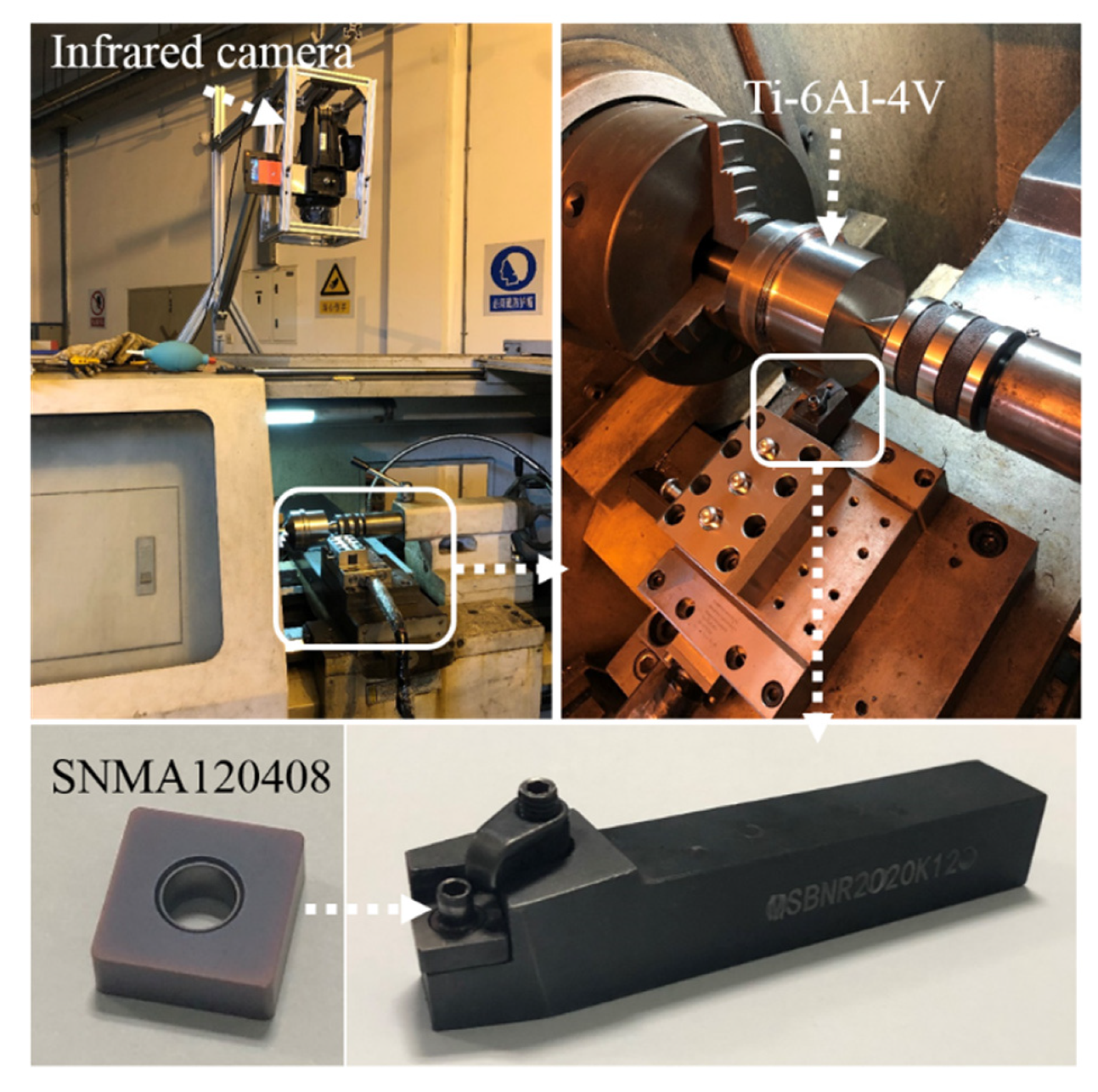
2.3. Cutting Experiment Planning
3. Results and Discussion
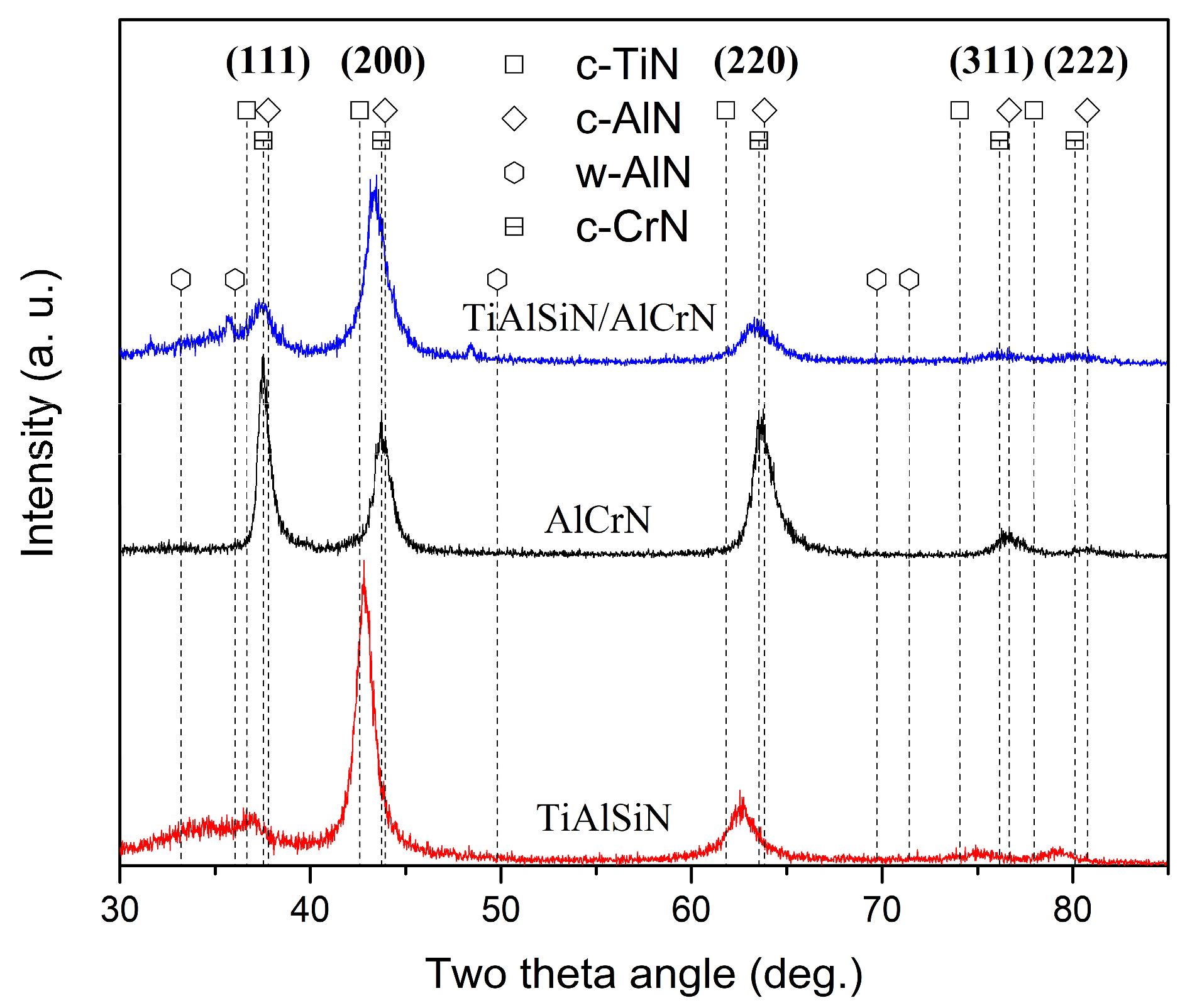
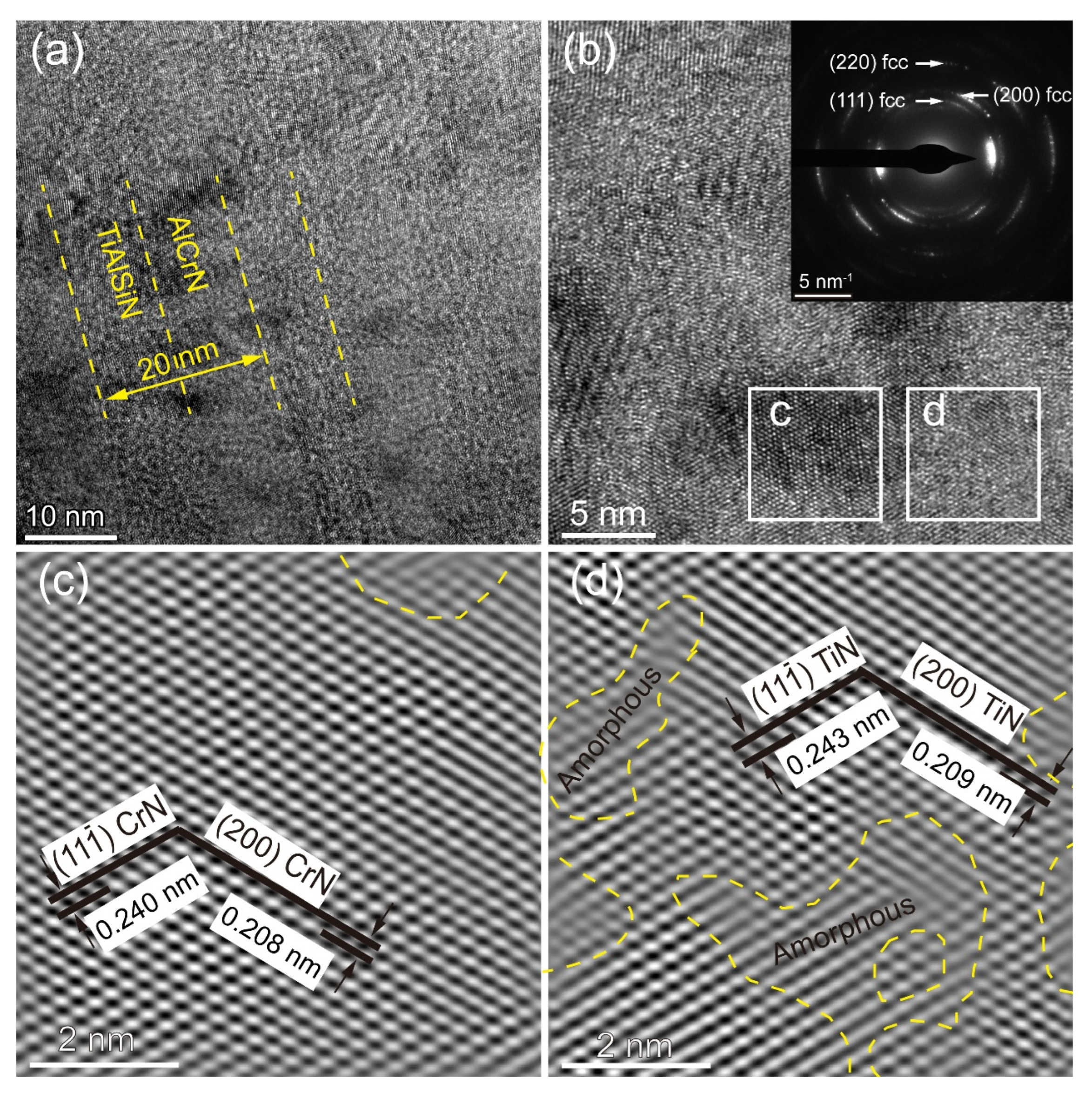
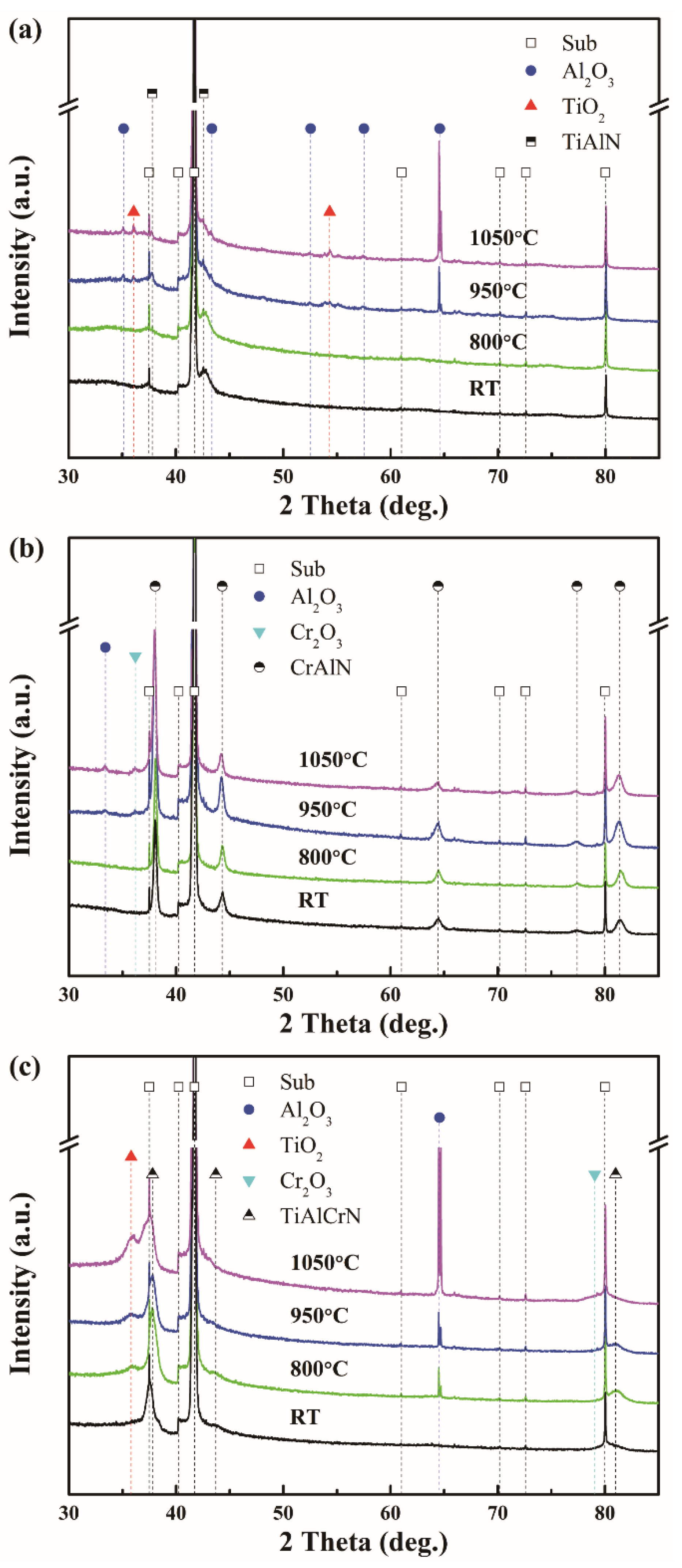
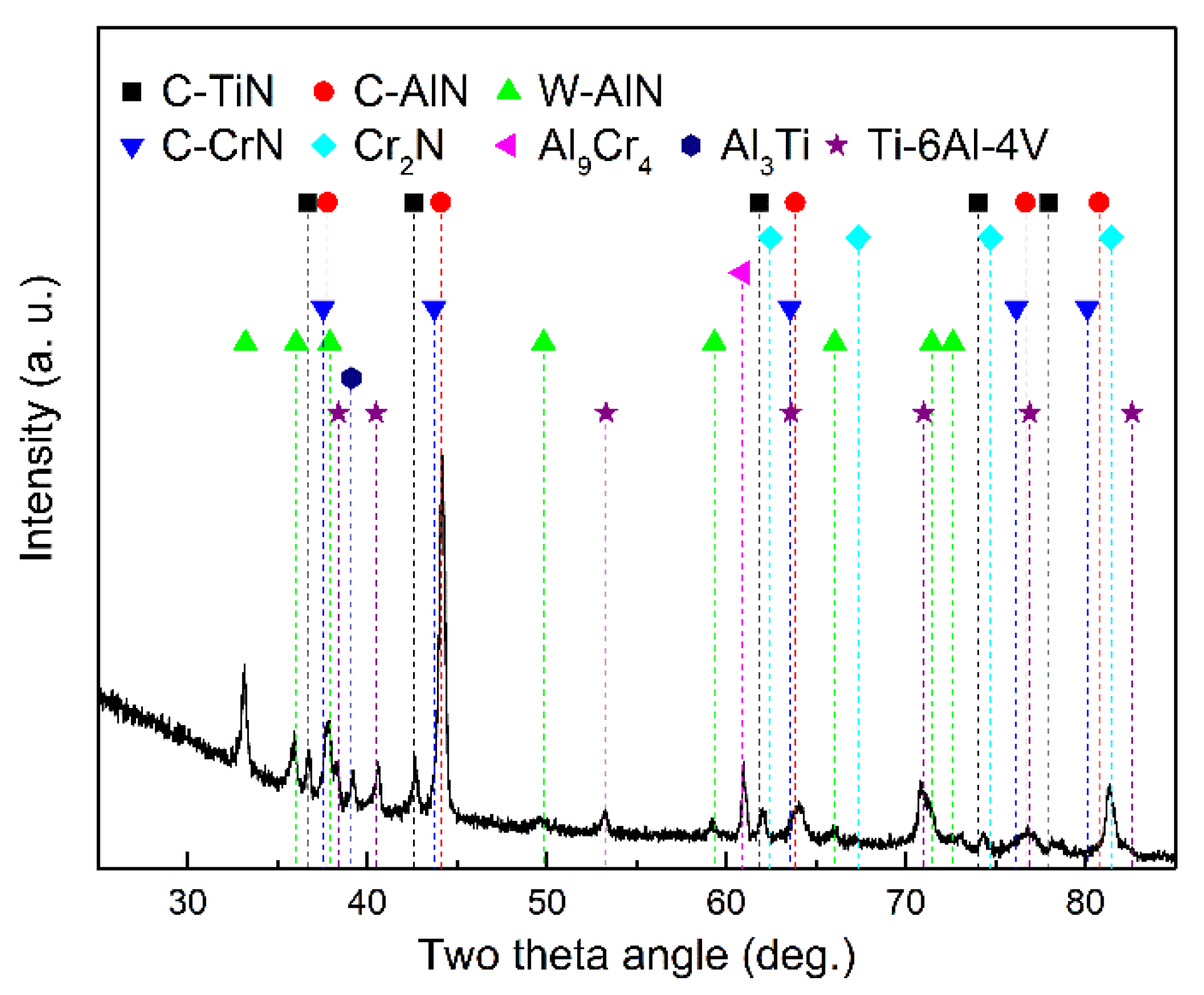
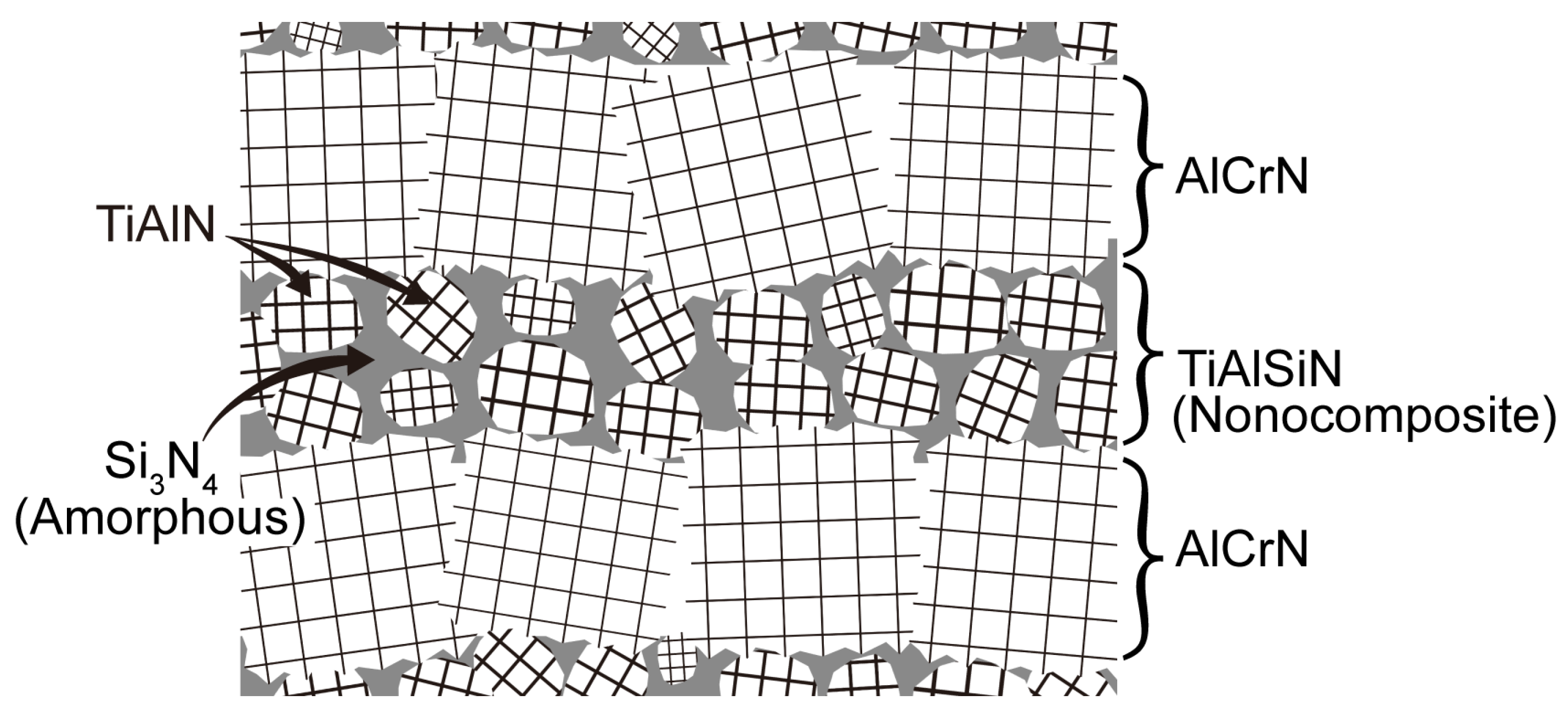
3.1. Coating Composition, Physical Phase, and Microstructure
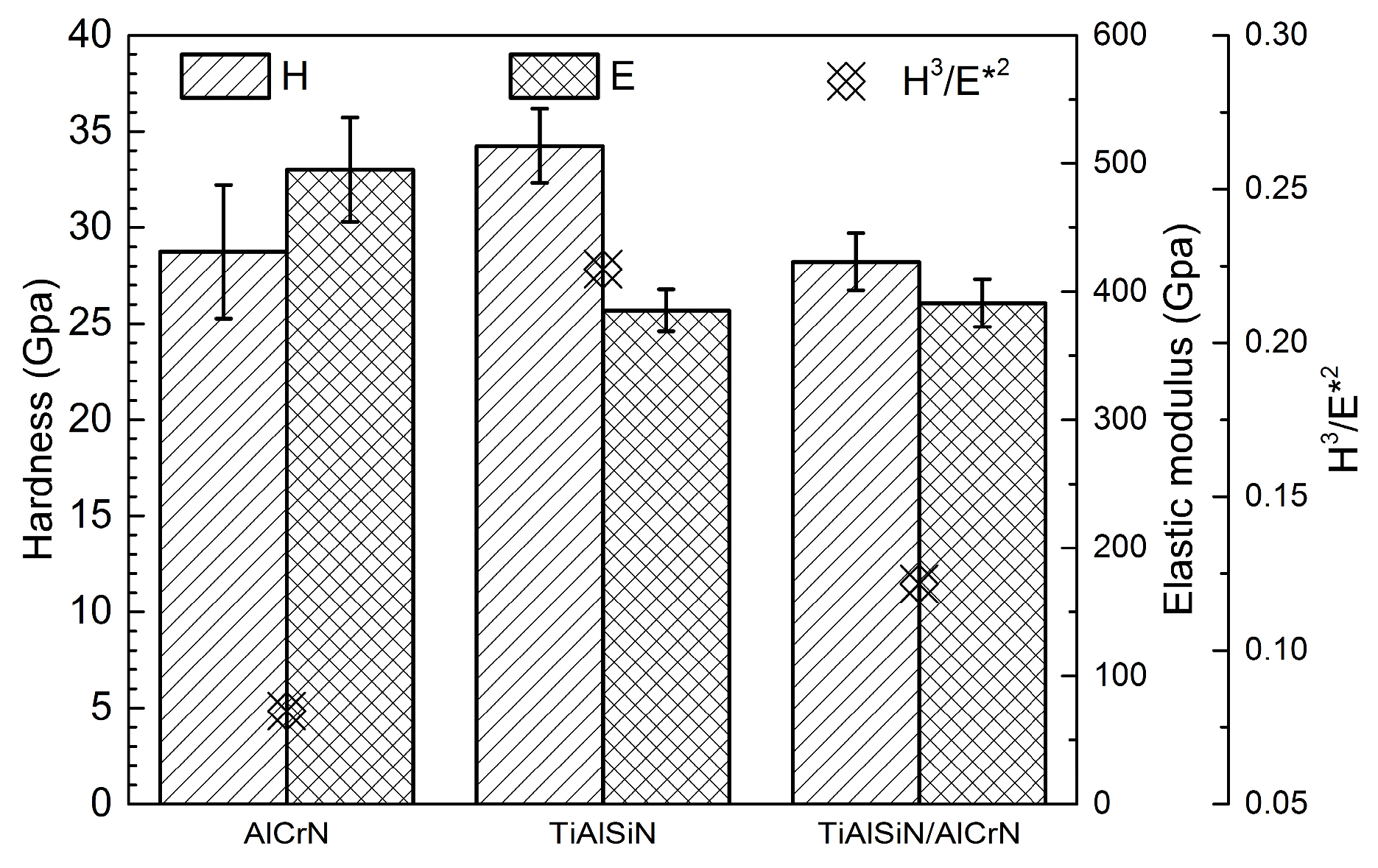
3.2. Mechanical Properties
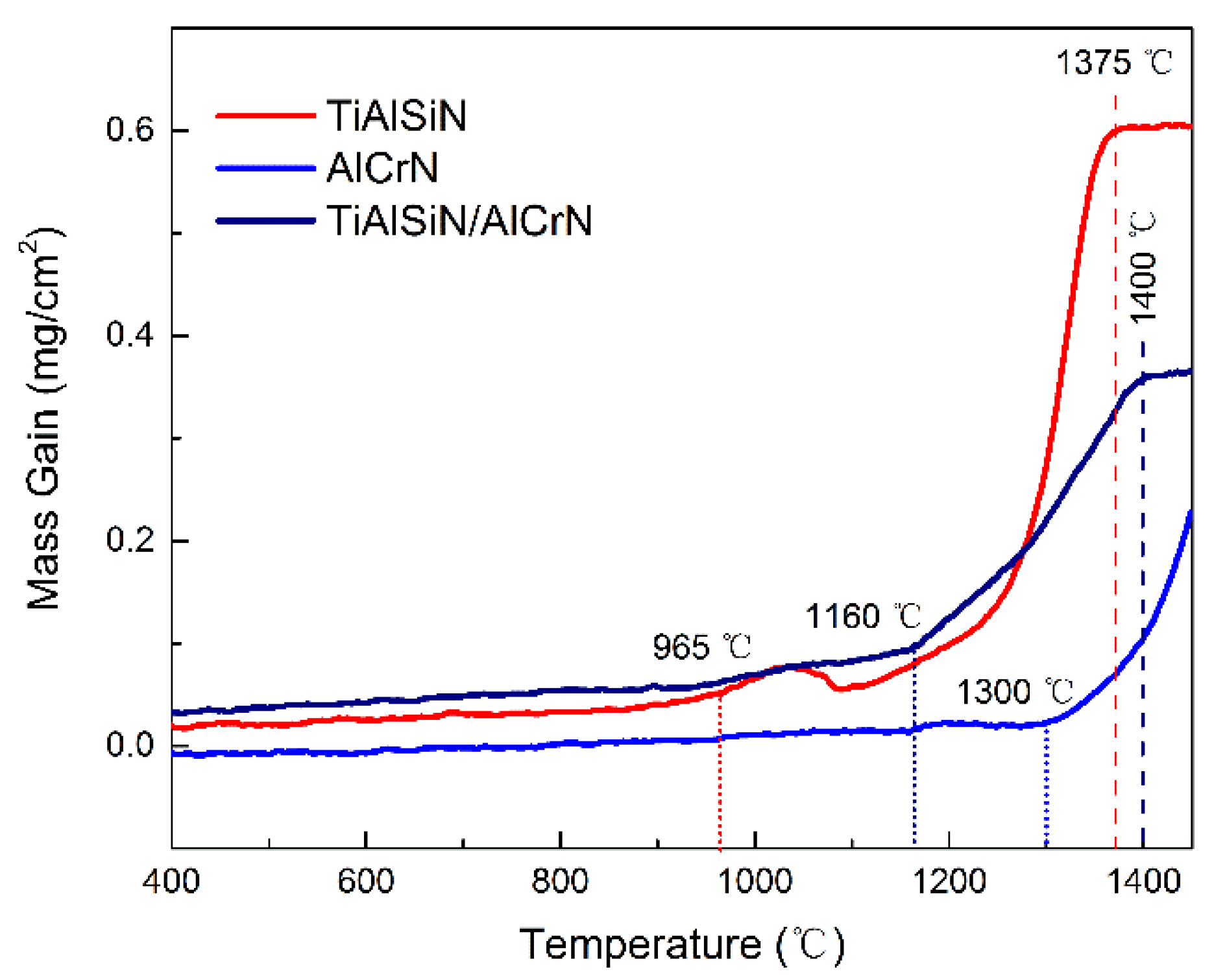
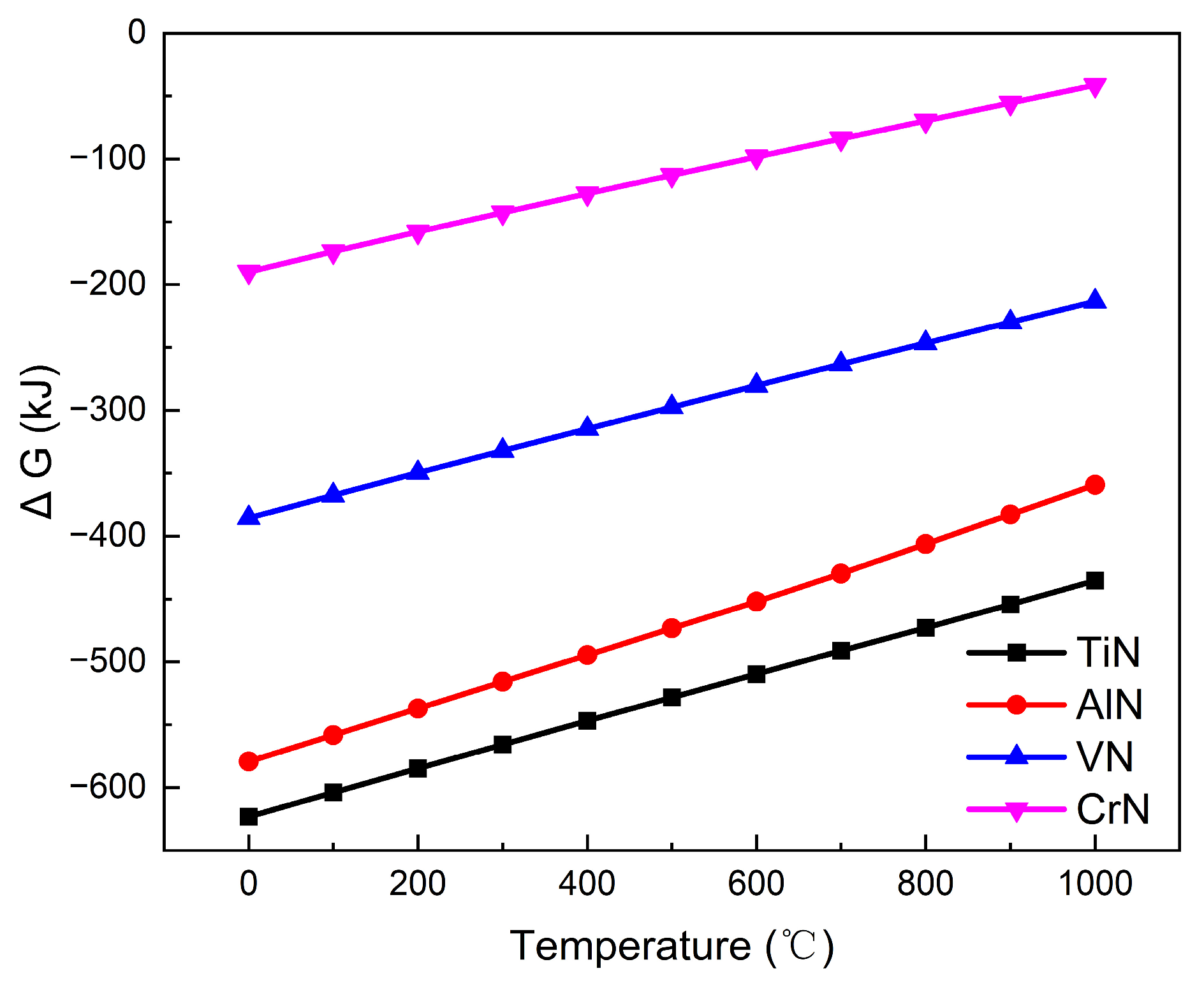
3.3. High-Temperature Oxidation Resistance
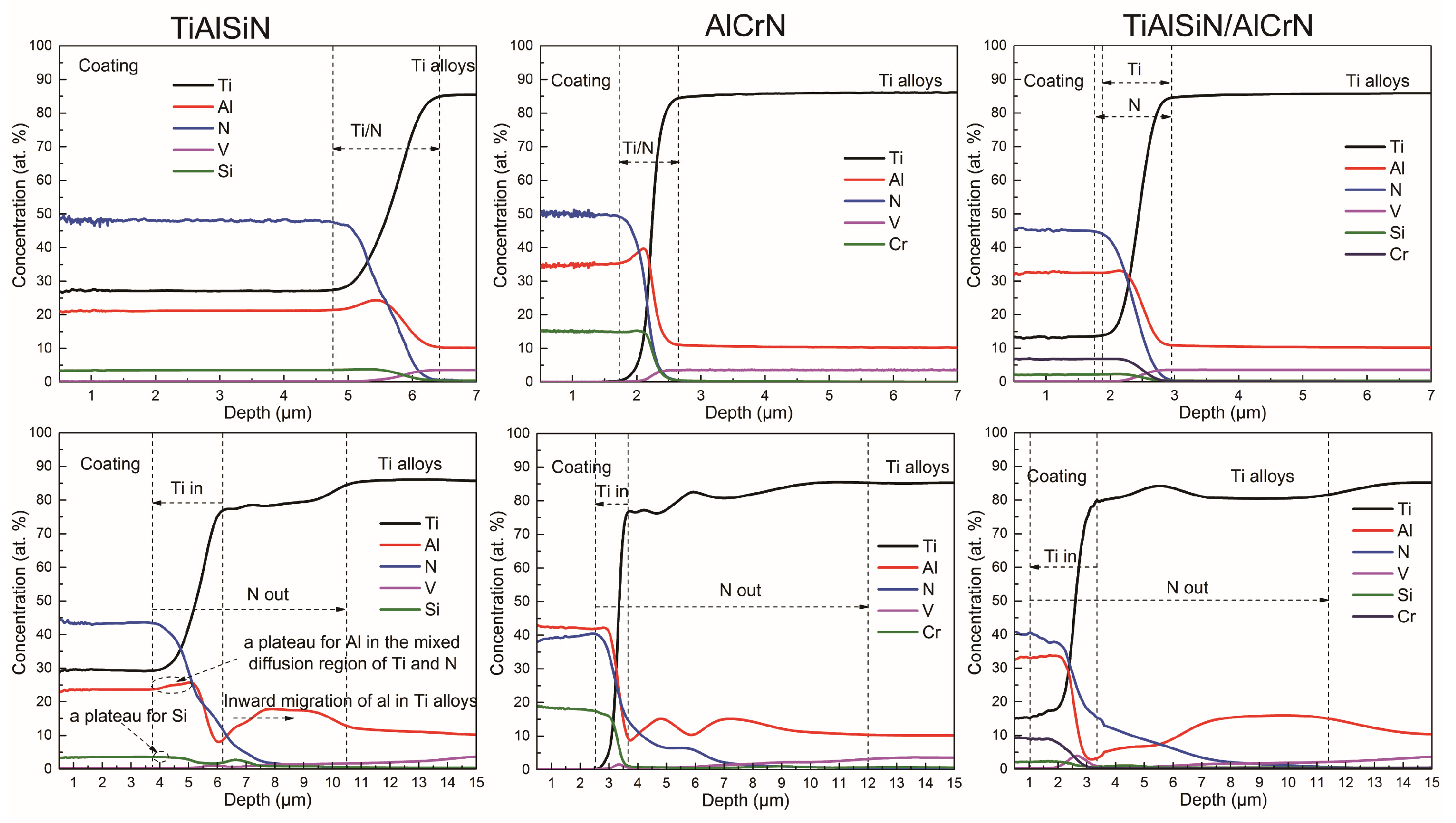
3.4. Thermal Diffusion between the Coating and the Titanium Alloy
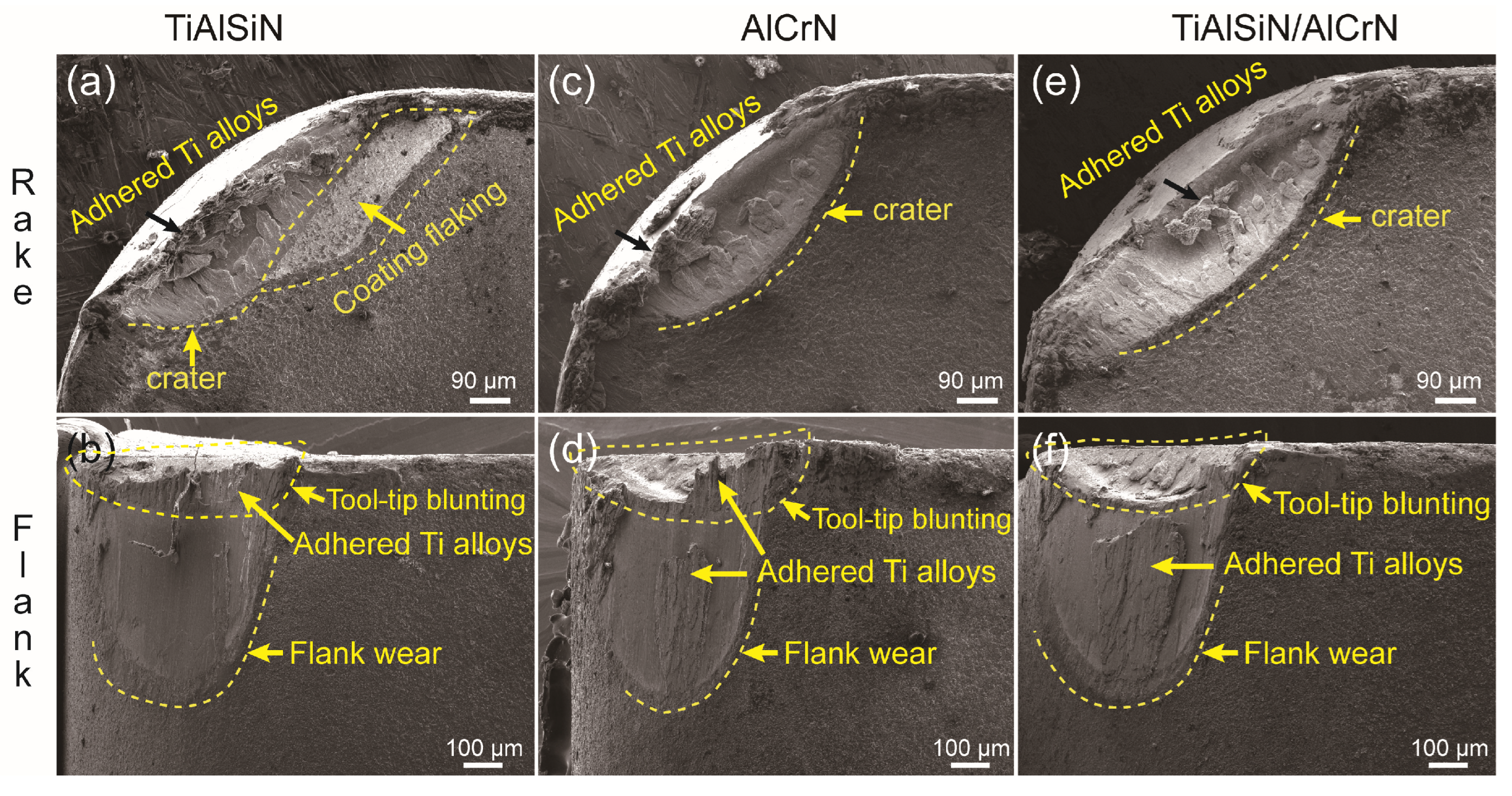
3.5. Cutting Performance of Coated Tools
4. Conclusions
- Among the many factors affecting coating performance, adhesion strength is the primary condition for cutting applications. When the adhesion strength is above a threshold value, the tool life starts to depend on other factors besides the adhesion strength. TiAlSiN coating has the lowest adhesion strength, leading to the lowest cutting life, despite its high H3/E*2 value and low titanium alloy affinity; AlCrN coating has the highest adhesion strength among the three coatings, but not the highest cutting life.
- All elements within the coating and the titanium alloy will inter-diffuse at high temperatures, but the Ti and N elements are the most significant. The intrusion of N in the coating into the titanium alloy side is much greater than the intrusion of Ti in the titanium alloy into the coating side. The nitride coating containing Cr aggravates the loss of N in contact with the titanium alloy. In addition, the multilayer structure of the coating does not prevent diffusion; on the contrary, the interlayer defects inherent in the multilayer structure can lead to more severe diffusion than in a monolayer coating.
- Compared to static diffusion experiments between the coating and the titanium alloy, the main factor affecting the life of the coated tool is not the two-party diffusion, but still the H3/E*2 value of the coating, given the shorter contact time and lower cutting temperature in the cutting application. That is why although the TiAlSiN/AlCrN multilayer coating has more severe diffusion than the AlCrN monolayer, the cutting life is instead higher owing to its higher H3/E*2 value.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, R.D.; Bhavsar, S.N.; Patel, A.K. Experimental investigation on cutting force during end milling of AISI D2 tool steel using AlCrN coated tool. Mater. Today Proc. 2023, 80, 1397–1402. [Google Scholar] [CrossRef]
- Sousa, V.F.; Silva, F.J.; Alexandre, R.; Fecheira, J.S.; Pinto, G.; Baptista, A. Experimental study on the wear evolution of different PVD coated tools under milling operations of LDX2101 duplex stainless steel. Adv. Manuf. 2022, 11, 158–179. [Google Scholar] [CrossRef]
- Azim, S.; Gangopadhyay, S.; Mahapatra, S.S.; Mittal, R.K. Performance evaluation of CrAlN and TiAlN coatings deposited by HiPIMS in micro drilling of a Ni-based superalloy. Surf. Coat. Technol. 2022, 449, 128980. [Google Scholar] [CrossRef]
- Varghese, V.; Akhil, K.; Ramesh, M.; Chakradhar, D. Investigation on the performance of AlCrN and AlTiN coated cemented carbide inserts during end milling of maraging steel under dry, wet and cryogenic environments. J. Manuf. Process. 2019, 43, 136–144. [Google Scholar] [CrossRef]
- Veprek, S.; Veprek-Heijman, M.G.J. Concept for the Design of Superhard Nanocomposites with High Thermal Stability: Their Preparation, Properties, and Industrial Applications. In Nanostructured Coatings; Cavaleiro, A., De Hosson, J.T.M., Eds.; Springer: New York, NY, USA, 2006; pp. 347–406. [Google Scholar]
- Veprek, S.; Jilek, M. Super and ultrahard nanacomposite coatings: Generic concept for their preparation, properties and industrial applications. Vacuum 2002, 67, 443–449. [Google Scholar] [CrossRef]
- Veprek, S.; Veprek-Heijman, M.J.G. Industrial applications of superhard nanocomposite coatings. Surf. Coat. Technol. 2008, 202, 5063–5073. [Google Scholar] [CrossRef]
- Das, C.R.; Rangwala, M.; Ghosh, A. Influence of substrate bias voltage on microstructure and mechanical characteristics of TiAlSiN coating deposited by High Power Impulse Magnetron Sputtering (HiPIMS). Surf. Coat. Technol. 2023, 458, 129351. [Google Scholar] [CrossRef]
- Zhang, K.; Xin, L.; Ma, T.; Chang, H.; Lu, Y.; Feng, C.; Zhu, S.; Wang, F. Investigation of the role of silicon in TiAlSiN coating deposited on TiAl alloys during long-term oxidation. Corros. Sci. 2022, 204, 110394. [Google Scholar] [CrossRef]
- Liu, Z.R.; Pei, F.; Chen, L.; Mayrhofer, P.H. Effect of Si-addition on structure and thermal stability of Ti-Al-N coatings. J. Alloys. Compd. 2022, 917, 165483. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Ghosh, M.; Ramakrishna, R.; Rajam, K. Deposition and characterization of TiAlSiN nanocomposite coatings prepared by reactive pulsed direct current unbalanced magnetron sputtering. Appl. Surf. Sci. 2010, 256, 6420–6426. [Google Scholar] [CrossRef]
- Rodríguez-Barrero, S.; Fernández-Larrinoa, J.; Azkona, I.; López de Lacalle, L.; Polvorosa, R. Enhanced performance of nanostructured coatings for drilling by droplet elimination. Mater. Manuf. Process. 2016, 31, 593–602. [Google Scholar] [CrossRef]
- Vardanyan, E.; Ramazanov, K.; Nagimov, R.S.; Nazarov, A.Y. Properties of intermetallic TiAl based coatings deposited on ultrafine grained martensitic steel. Surf. Coat. Technol. 2020, 389, 125657. [Google Scholar] [CrossRef]
- Migranov, M.S.; Migranov, A.; Minigaleev, S.; Shehtman, S. Tribological properties of multilayer coatings for cutting tool. J. Frict. Wear. 2018, 39, 245–250. [Google Scholar] [CrossRef]
- Wieciński, P.; Smolik, J.; Garbacz, H.; Kurzydłowski, K. Failure and deformation mechanisms during indentation in nanostructured Cr/CrN multilayer coatings. Surf. Coat. Technol. 2014, 240, 23–31. [Google Scholar] [CrossRef]
- Vorontsov, A.; Filippov, A.; Shamarin, N.; Moskvichev, E.; Novitskaya, O.; Knyazhev, E.; Denisova, Y.; Leonov, A.; Denisov, V.; Tarasov, S. High-Temperature Oxidation of CrN/ZrN Multilayer Coatings. Metals 2022, 12, 1746. [Google Scholar] [CrossRef]
- Li, W.; Liu, P.; Zhu, X.; Pan, D.; Zhang, K.; Ma, F.; Liu, X. Effect of Si content on microstructural evolution and superhardness effect of TiN/CrAlSiN nanomultilayered films. J. Alloys Compd. 2015, 650, 592–597. [Google Scholar] [CrossRef]
- Liu, H.; Yang, F.-C.; Tsai, Y.-J.; Wang, X.; Li, W.; Chang, C.-L. Effect of modulation structure on the microstructural and mechanical properties of TiAlSiN/CrN thin films prepared by high power impulse magnetron sputtering. Surf. Coat. Technol. 2019, 358, 577–585. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Yang, Y.-J.; Weng, S.-Y. Effect of interlayer design on the mechanical properties of AlTiCrN and multilayered AlTiCrN/TiSiN hard coatings. Surf. Coat. Technol. 2020, 389, 125637. [Google Scholar] [CrossRef]
- Kameneva, A.; Kichigin, V. Corrosion, wear, and friction behavior of a number of multilayer two-, three-and multicomponent nitride coatings on different substrates, depending on the phase and elemental composition gradient. Appl. Surf. Sci. 2019, 489, 165–174. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, T.F.; Guo, Z.; Li, Z.; Fan, B.; Chen, G.; Xiong, Z.; Wang, Q. Mechanical, oxidation, and cutting properties of AlCrN/AlTiSiN nano-multilayer coatings. Surf. Coat. Technol. 2022, 433, 128094. [Google Scholar] [CrossRef]
- Fukumoto, N.; Ezura, H.; Suzuki, T. Synthesis and oxidation resistance of TiAlSiN and multilayer TiAlSiN/CrAlN coating. Surf. Coat. Technol. 2009, 204, 902–906. [Google Scholar] [CrossRef]
- Chen, W.; Lin, Y.; Zheng, J.; Zhang, S.; Liu, S.; Kwon, S. Preparation and characterization of CrAlN/TiAlSiN nano-multilayers by cathodic vacuum arc. Surf. Coat. Technol. 2015, 265, 205–211. [Google Scholar] [CrossRef]
- Hu, C.; Chen, L.; Lou, Y.; Zhao, N.; Yue, J. Influence of Si content on the microstructure, thermal stability and oxidation resistance of TiAlSiN/CrAlN multilayers. J. Alloys Compd. 2021, 855, 157441. [Google Scholar] [CrossRef]
- Xiao, B.; Liu, J.; Liu, F.; Zhong, X.; Xiao, X.; Zhang, T.F.; Wang, Q. Effects of microstructure evolution on the oxidation behavior and high-temperature tribological properties of AlCrN/TiAlSiN multilayer coatings. Ceram. Int. 2018, 44, 23150–23161. [Google Scholar] [CrossRef]
- Yue, Q.-B.; He, H.-B.; Li, H.-Y.; Zhang, J.; Li, Y.-M.; Ma, L. Research on friction characteristics of AlCrN and TiAlSiN coatings and properties of coated tools. Int. J. Precis. Eng. Manuf. 2019, 20, 1581–1589. [Google Scholar] [CrossRef]
- Rajguru, R.R.; Vasudevan, H. A study of micro hardness in the machining of Inconel 625 using TiAlSiN coated tools under dry cutting conditions. Adv. Mater. Process. Technol. 2022, 8, 1–11. [Google Scholar] [CrossRef]
- Silva, F.J.; Sousa, V.F.; Campilho, R.D.; Alexandre, R. Wear Behavior of Coated Tools When Milling S32101 Duplex Stainless Steel. Mater. Proc. 2022, 8, 45. [Google Scholar]
- Stoney, G.G. The tension of metallic films deposited by electrolysis. Proc. R. Soc. Lond. A 1909, 82, 172–175. [Google Scholar]
- Yousaf, M.; Pelenovich, V.; Yang, B.; Liu, C.; Fu, D. Effect of bilayer period on structural and mechanical properties of nanocomposite TiAlN/MoN multilayer films synthesized by cathodic arc ion-plating. Surf. Coat. Technol. 2015, 282, 94–102. [Google Scholar] [CrossRef]
- Mei, H.; Yan, K.; Wang, R.; Peng, W.; Huang, K.; Shi, J.; Zhang, D.; Gong, W.; Ren, F.; Wang, Q. Microstructure and mechanical properties of nanomultilayered AlTiN/Cu coatings prepared by a hybrid system of AIP and PDCMS. Ceram. Int. 2023, 49, 226–235. [Google Scholar] [CrossRef]
- Kimura, A.; Kawate, M.; Hasegawa, H.; Suzuki, T. Anisotropic lattice expansion and shrinkage of hexagonal TiAlN and CrAlN films. Surf. Coat. Technol. 2003, 169, 367–370. [Google Scholar] [CrossRef]
- Philippon, D.; Godinho, V.; Nagy, P.M.; Delplancke-Ogletree, M.P.; Fernández, A. Endurance of TiAlSiN coatings: Effect of Si and bias on wear and adhesion. Wear 2011, 270, 541–549. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Mitterer, C.; Musil, J. Structure–property relationships in single- and dual-phase nanocrystalline hard coatings. Surf. Coat. Technol. 2003, 174–175, 725–731. [Google Scholar] [CrossRef]
- Li, W.; Liu, P.; Zhao, Y.; Zhang, K.; Ma, F.; Liu, X.; Chen, X.; He, D. SiNx thickness dependent morphology and mechanical properties of CrAlN/SiNx nanomultilayers. Thin Solid Films 2013, 534, 367–372. [Google Scholar] [CrossRef]
- KONG, M.; YUE, J.-L.; LI, G.-Y. Research development of hard ceramic nano-multilayer films. J. Inorg. Mater. 2010, 25, 113–119. [Google Scholar] [CrossRef]
- Li, W.; Liu, P.; Zhu, X.; Zhang, K.; Ma, F.; Liu, X.; Chen, X.; He, D. Si content dependent microstructure and mechanical properties of CrN/TiSiN nanomultilayered films. Mater. Sci. Eng. A 2014, 610, 28–32. [Google Scholar] [CrossRef]





| Target | Coating | Thickness (μm) | Chemical Composition (at.%) | ||||
|---|---|---|---|---|---|---|---|
| Al | Cr | Ti | Si | N | |||
| Ti0.45Al0.45Si0.10 | TiAlSiN | 3.69 | 20.22 | − | 22.66 | 3.89 | 53.23 |
| Al0.7Cr0.3 | AlCrN | 3.63 | 33.81 | 18.14 | − | − | 48.05 |
| Ti0.45Al0.45Si0.10 and Al0.7Cr0.3 | TiAlSiN/AlCrN | 3.87 | 27.95 | 8.01 | 10.97 | 2.23 | 50.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Wang, Y.; Liu, G.; Hua, J.; Deng, X. Properties and Performance of TiAlSiN and AlCrN Monolayer and Multilayer Coatings for Turning Ti-6Al-4V. Coatings 2023, 13, 1229. https://doi.org/10.3390/coatings13071229
Liu J, Wang Y, Liu G, Hua J, Deng X. Properties and Performance of TiAlSiN and AlCrN Monolayer and Multilayer Coatings for Turning Ti-6Al-4V. Coatings. 2023; 13(7):1229. https://doi.org/10.3390/coatings13071229
Chicago/Turabian StyleLiu, Jie, Yongchao Wang, Guiqian Liu, Junfang Hua, and Xin Deng. 2023. "Properties and Performance of TiAlSiN and AlCrN Monolayer and Multilayer Coatings for Turning Ti-6Al-4V" Coatings 13, no. 7: 1229. https://doi.org/10.3390/coatings13071229






