Vascularization Reconstruction Strategies in Craniofacial Bone Regeneration
Abstract
:1. Introduction
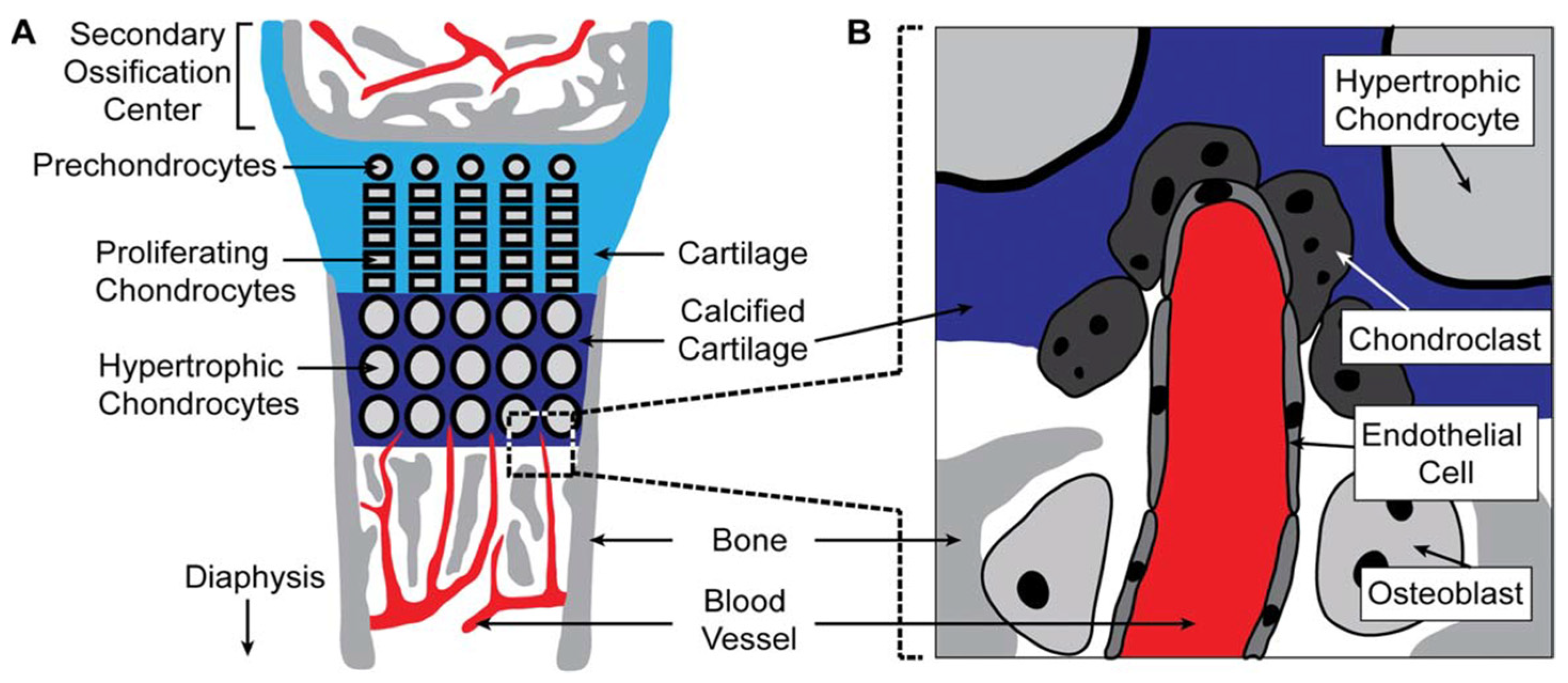
2. Challenges of Vascularization in Craniofacial Bone Regeneration
3. Various Vascularization Strategies in Craniofacial Bone Regeneration
3.1. Cell Sources for Craniofacial Bone Vascularization
3.2. Cell Signaling and Angiogenic Growth Factors
3.3. Co-Culture Systems with Different Cell Types or Growth Factors
3.4. Biological Requirements for Biomimetic Scaffolds Used for Craniofacial Bone Vascularization
3.4.1. Surface Morphology
3.4.2. Porous Characters
3.4.3. Angiogenic GF Release Property
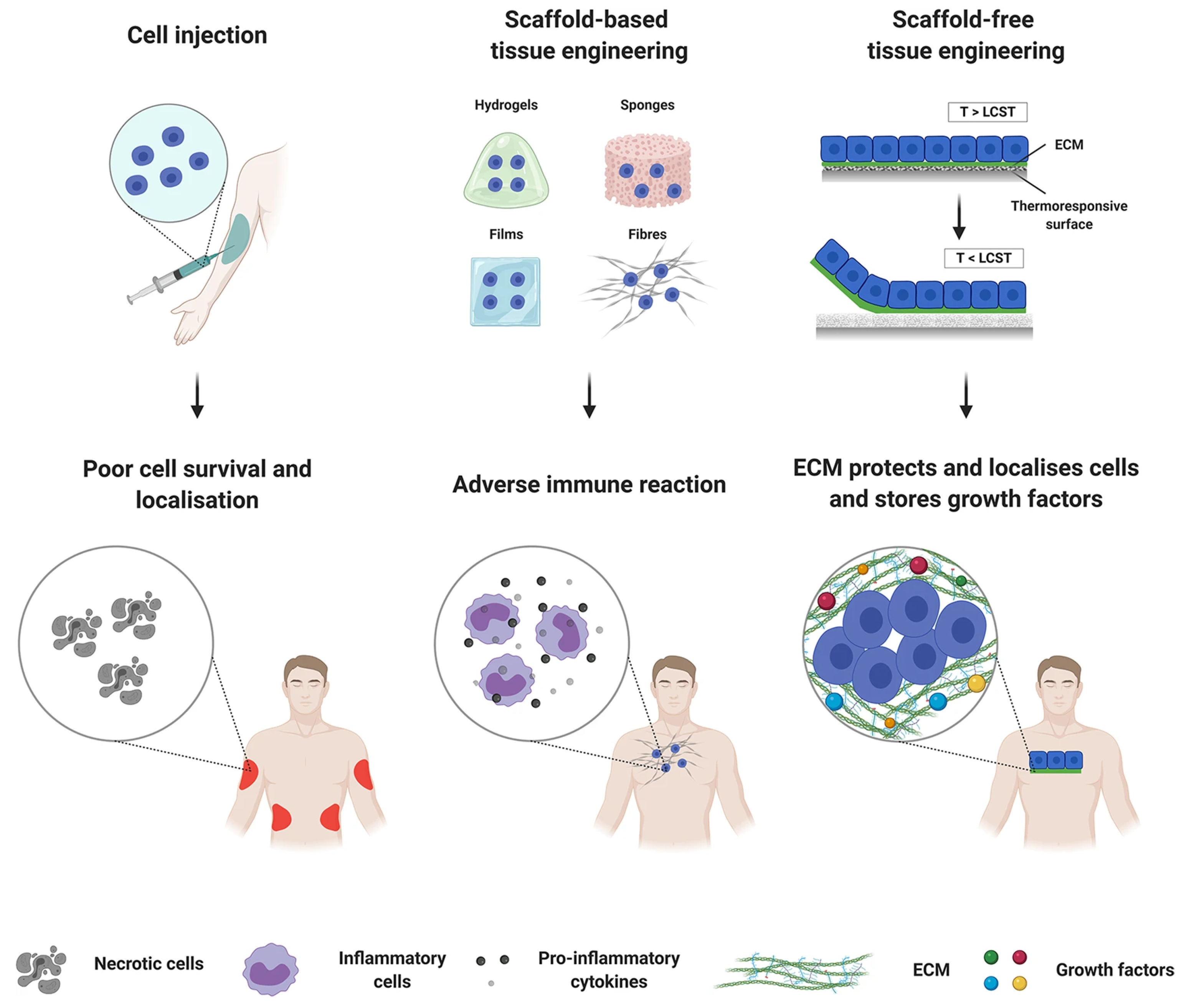
3.5. Scaffold-Free Technique
4. Conclusions and Further Expectations
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, W.; Yelick, P.C. Craniofacial Tissue Engineering. Cold Spring Harb. Perspect. Med. 2018, 8, a025775. [Google Scholar] [CrossRef] [PubMed]
- Hollý, D.; Klein, M.; Mazreku, M.; Zamborský, R.; Polák, Š.; Danišovič, Ľ.; Csöbönyeiová, M. Stem Cells and Their Derivatives-Implications for Alveolar Bone Regeneration: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 11746. [Google Scholar] [CrossRef] [PubMed]
- Aghali, A. Craniofacial Bone Tissue Engineering: Current Approaches and Potential Therapy. Cells 2021, 10, 2993. [Google Scholar] [CrossRef] [PubMed]
- Martín-Del-Campo, M.; Rosales-Ibañez, R.; Rojo, L. Biomaterials for Cleft Lip and Palate Regeneration. Int. J. Mol. Sci. 2019, 20, 2176. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, Z.; Xu, H.H.K.; Dai, Z.; Yu, K.; Xiao, L.; Schneider, A.; Weir, M.D.; Oates, T.W.; Bai, Y.; et al. Effects of Metformin Delivery via Biomaterials on Bone and Dental Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 15905. [Google Scholar] [CrossRef]
- Meijer, E.M.; van Dijk, C.G.M.; Kramann, R.; Verhaar, M.C.; Cheng, C. Implementation of Pericytes in Vascular Regeneration Strategies. Tissue Eng. Part B Rev. 2022, 28, 1–12. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, T.; Lin, Y.; Cai, X. Vascularization in Craniofacial Bone Tissue Engineering. J. Dent. Res. 2018, 97, 969–976. [Google Scholar] [CrossRef]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Sarrion, P.; Pouraghaei, S.; Chau, Y.; Ansari, S.; Li, S.; Aghaloo, T.; Moshaverinia, A. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci. Transl. Med. 2020, 12, eaay6853. [Google Scholar] [CrossRef]
- Kasper, F.K.; Melville, J.; Shum, J.; Wong, M.; Young, S. Tissue Engineered Prevascularized Bone and Soft Tissue Flaps. Oral Maxillofac. Surg. Clin. N. Am. 2017, 29, 63–73. [Google Scholar] [CrossRef]
- Weiland, A.J.; Phillips, T.W.; Randolph, M.A. Bone grafts: A radiologic, histologic, and biomechanical model comparing autografts, allografts, and free vascularized bone grafts. Plast. Reconstr. Surg. 1984, 74, 368–379. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, M.; Jiang, F.; Yin, S.; Lin, S.; Yang, G.; Lu, Y.; Zhang, W.; Jiang, X. Marginal sealing around integral bilayer scaffolds for repairing osteochondral defects based on photocurable silk hydrogels. Bioact. Mater. 2021, 6, 3976–3986. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, Z.; Xiao, L.; Ai, M.; Cao, Y.; Mao, J.; Song, K. The Role of Gli1(+) Mesenchymal Stem Cells in Osteogenesis of Craniofacial Bone. Biomolecules 2023, 13, 1351. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, W.; Tang, Z.; Wu, H.; Liu, Y.; Dong, H.; Wang, N.; Huang, H.; Bao, S.; Shi, L.; et al. Magnesium surface-activated 3D printed porous PEEK scaffolds for in vivo osseointegration by promoting angiogenesis and osteogenesis. Bioact. Mater. 2023, 20, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Noden, D.M. Embryonic origins and assembly of blood vessels. Am. Rev. Respir. Dis. 1989, 140, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Díaz Del Moral, S.; Barrena, S.; Muñoz-Chápuli, R.; Carmona, R. Embryonic circulating endothelial progenitor cells. Angiogenesis 2020, 23, 531–541. [Google Scholar] [CrossRef]
- Moccia, F.; Negri, S.; Shekha, M.; Faris, P.; Guerra, G. Endothelial Ca2+ Signaling, Angiogenesis and Vasculogenesis: Just What It Takes to Make a Blood Vessel. Int. J. Mol. Sci. 2019, 20, 3962. [Google Scholar] [CrossRef]
- Duan, X.; Bradbury, S.R.; Olsen, B.R.; Berendsen, A.D. VEGF stimulates intramembranous bone formation during craniofacial skeletal development. Matrix Biol. 2016, 52–54, 127–140. [Google Scholar] [CrossRef]
- Hara, E.S.; Nagaoka, N.; Okada, M.; Nakano, T.; Matsumoto, T. Distinct Morphologies of Bone Apatite Clusters in Endochondral and Intramembranous Ossification. Adv. Biol. 2022, 6, e2200076. [Google Scholar] [CrossRef]
- Percival, C.J.; Richtsmeier, J.T. Angiogenesis and intramembranous osteogenesis. Dev. Dyn. 2013, 242, 909–922. [Google Scholar] [CrossRef]
- Ye, X.; He, J.; Wang, S.; Han, Q.; You, D.; Feng, B.; Zhao, F.; Yin, J.; Yu, M.; Wang, H.; et al. A hierarchical vascularized engineered bone inspired by intramembranous ossification for mandibular regeneration. Int. J. Oral Sci. 2022, 14, 31. [Google Scholar] [CrossRef]
- Rouwkema, J.; Koopman, B.; Blitterswijk, C.; Dhert, W.; Malda, J. Supply of nutrients to cells in engineered tissues. Biotechnol. Genet. Eng. Rev. 2010, 26, 163–178. [Google Scholar] [CrossRef]
- Place, T.L.; Domann, F.E.; Case, A.J. Limitations of oxygen delivery to cells in culture: An underappreciated problem in basic and translational research. Free Radic. Biol. Med. 2017, 113, 311–322. [Google Scholar] [CrossRef]
- Owen-Woods, C.; Kusumbe, A. Fundamentals of bone vasculature: Specialization, interactions and functions. Semin. Cell Dev. Biol. 2022, 123, 36–47. [Google Scholar] [CrossRef]
- Ramasamy, S.K.; Kusumbe, A.P.; Wang, L.; Adams, R.H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 2014, 507, 376–380. [Google Scholar] [CrossRef]
- Xu, Z.; Kusumbe, A.P.; Cai, H.; Wan, Q.; Chen, J. Type H blood vessels in coupling angiogenesis-osteogenesis and its application in bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 1434–1446. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Xu, C.; Zhang, Y.; Chen, H. Advances in the understanding of the role of type-H vessels in the pathogenesis of osteoporosis. Arch. Osteoporos. 2020, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, X.; Lin, H. The hypoxic microenvironment: A driving force for heterotopic ossification progression. Cell Commun. Signal. 2020, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Dibble, M.; Di Cio, S.; Luo, P.; Balkwill, F.; Gautrot, J.E. The impact of pericytes on the stability of microvascular networks in response to nanoparticles. Sci. Rep. 2023, 13, 5729. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Xiao, W.; Liu, R.; Kumar, P.; Li, Y.; Zhou, P.; Guo, F.; Farmer, D.L.; Lam, K.S.; Wang, F.; et al. Discovery and Characterization of a Potent and Specific Peptide Ligand Targeting Endothelial Progenitor Cells and Endothelial Cells for Tissue Regeneration. ACS Chem. Biol. 2017, 12, 1075–1086. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, Z.; Jiang, W.; Zhu, P.; Zhou, N.; Huang, X. A Review Into the Insights of the Role of Endothelial Progenitor Cells on Bone Biology. Front. Cell Dev. Biol. 2022, 10, 878697. [Google Scholar] [CrossRef]
- Zhan, K.; Bai, L.; Xu, J. Role of vascular endothelial progenitor cells in construction of new vascular loop. Microvasc. Res. 2013, 90, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Coelho, F.; Silva, F.; Gouveia-Fernandes, S.; Martins, C.; Lopes, N.; Domingues, G.; Brito, C.; Almeida, A.M.; Pereira, S.A.; Serpa, J. Monocytes as Endothelial Progenitor Cells (EPCs), Another Brick in the Wall to Disentangle Tumor Angiogenesis. Cells 2020, 9, 107. [Google Scholar] [CrossRef]
- Figueiredo, A.M.; Barbacena, P.; Russo, A.; Vaccaro, S.; Ramalho, D.; Pena, A.; Lima, A.P.; Ferreira, R.R.; Fidalgo, M.A.; El-Marjou, F.; et al. Endothelial cell invasion is controlled by dactylopodia. Proc. Natl. Acad. Sci. USA 2021, 118, e2023829118. [Google Scholar] [CrossRef]
- Potente, M.; Carmeliet, P. The Link Between Angiogenesis and Endothelial Metabolism. Annu. Rev. Physiol. 2017, 79, 43–66. [Google Scholar] [CrossRef]
- Eldridge, L.; Wagner, E.M. Angiogenesis in the lung. J. Physiol. 2019, 597, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Blanco, R.; Gerhardt, H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb. Perspect. Med. 2013, 3, a006569. [Google Scholar] [CrossRef]
- Ben-Shaul, S.; Landau, S.; Merdler, U.; Levenberg, S. Mature vessel networks in engineered tissue promote graft–host anastomosis and prevent graft thrombosis. Proc. Natl. Acad. Sci. USA 2019, 116, 2955–2960. [Google Scholar] [CrossRef]
- Gao, X.; Yourick, J.J.; Sprando, R.L. Comparative transcriptomic analysis of endothelial progenitor cells derived from umbilical cord blood and adult peripheral blood: Implications for the generation of induced pluripotent stem cells. Stem Cell Res. 2017, 25, 202–212. [Google Scholar] [CrossRef]
- Gao, X.; Yourick, J.J.; Sprando, R.L. Generation of Human Induced Pluripotent Stem Cells Using Endothelial Progenitor Cells Derived from Umbilical Cord Blood and Adult Peripheral Blood. Methods Mol. Biol. 2022, 2454, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Liu, X.; Ding, H.; Zhang, W. Paracrine mechanisms of endothelial progenitor cells in vascular repair. Acta Histochem. 2022, 124, 151833. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, J.; Zhang, W. Transplantation of Endothelial Progenitor Cells: Summary and prospect. Acta Histochem. 2023, 125, 151990. [Google Scholar] [CrossRef]
- Seebach, C.; Henrich, D.; Wilhelm, K.; Barker, J.H.; Marzi, I. Endothelial progenitor cells improve directly and indirectly early vascularization of mesenchymal stem cell-driven bone regeneration in a critical bone defect in rats. Cell Transplant. 2012, 21, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Xin, Q.; Yuan, R.; Yuan, Y.; Cong, W.; Chen, K. Neovascularization: The Main Mechanism of MSCs in Ischemic Heart Disease Therapy. Front. Cardiovasc. Med. 2021, 8, 633300. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/Pericyte Interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Payne, L.B.; Hoque, M.; Houk, C.; Darden, J.; Chappell, J.C. Pericytes in Vascular Development. Curr. Tissue Microenviron. Rep. 2020, 1, 143–154. [Google Scholar] [CrossRef]
- Selvam, S.; Kumar, T.; Fruttiger, M. Retinal vasculature development in health and disease. Prog. Retin. Eye Res. 2018, 63, 1–19. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Abdulkadir, S.; Li, C.; Jiang, W.; Zhao, X.; Sang, P.; Wei, L.; Hu, Y.; Li, Q.; Cai, J. Modulating Angiogenesis by Proteomimetics of Vascular Endothelial Growth Factor. J. Am. Chem. Soc. 2022, 144, 270–281. [Google Scholar] [CrossRef]
- Kivelä, R.; Hemanthakumar, K.A.; Vaparanta, K.; Robciuc, M.; Izumiya, Y.; Kidoya, H.; Takakura, N.; Peng, X.; Sawyer, D.B.; Elenius, K.; et al. Endothelial Cells Regulate Physiological Cardiomyocyte Growth via VEGFR2-Mediated Paracrine Signaling. Circulation 2019, 139, 2570–2584. [Google Scholar] [CrossRef]
- Sawada, N.; Arany, Z. Metabolic Regulation of Angiogenesis in Diabetes and Aging. Physiology 2017, 32, 290–307. [Google Scholar] [CrossRef]
- Tiemeijer, L.A.; Frimat, J.P.; Stassen, O.; Bouten, C.V.C.; Sahlgren, C.M. Spatial patterning of the Notch ligand Dll4 controls endothelial sprouting in vitro. Sci. Rep. 2018, 8, 6392. [Google Scholar] [CrossRef]
- Su, W.; Liu, G.; Liu, X.; Zhou, Y.; Sun, Q.; Zhen, G.; Wang, X.; Hu, Y.; Gao, P.; Demehri, S.; et al. Angiogenesis stimulated by elevated PDGF-BB in subchondral bone contributes to osteoarthritis development. JCI Insight 2020, 5, e135446. [Google Scholar] [CrossRef]
- Hosaka, K.; Yang, Y.; Nakamura, M.; Andersson, P.; Yang, X.; Zhang, Y.; Seki, T.; Scherzer, M.; Dubey, O.; Wang, X.; et al. Dual roles of endothelial FGF-2–FGFR1–PDGF-BB and perivascular FGF-2–FGFR2–PDGFRβ signaling pathways in tumor vascular remodeling. Cell Discov. 2018, 4, 3. [Google Scholar] [CrossRef]
- Hellström, M.; Phng, L.-K.; Hofmann, J.J.; Wallgard, E.; Coultas, L.; Lindblom, P.; Alva, J.; Nilsson, A.-K.; Karlsson, L.; Gaiano, N.; et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 2007, 445, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Noguera-Troise, I.; Daly, C.; Papadopoulos, N.J.; Coetzee, S.; Boland, P.; Gale, N.W.; Lin, H.C.; Yancopoulos, G.D.; Thurston, G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 2006, 444, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.M.; Presnell, J.S. Hypoxia Inducible Factor (HIF) transcription factor family expansion, diversification, divergence and selection in eukaryotes. PLoS ONE 2017, 12, e0179545. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wu, S.; Li, Y.; Crane, J.L. Type H blood vessels in bone modeling and remodeling. Theranostics 2020, 10, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Westra, J.; Molema, G.; Kallenberg, C.G.M. Hypoxia-inducible factor-1 as regulator of angiogenesis in rheumatoid arthritis—Therapeutic implications. Curr. Med. Chem. 2010, 17, 254–263. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Brown, N.J.; Jones, R.; Lewis, C.E.; Mujamammi, A.H.; Muthana, M.; Seed, M.P.; Barker, M.D. A peptide derived from TIMP-3 inhibits multiple angiogenic growth factor receptors and tumour growth and inflammatory arthritis in mice. Angiogenesis 2014, 17, 207–219. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, X.; Hu, Y.; Yang, B.; Tsui, C.-K.; Yu, S.; Lu, L.; Liang, X. Melatonin attenuated retinal neovascularization and neuroglial dysfunction by inhibition of HIF-1α-VEGF pathway in oxygen-induced retinopathy mice. J. Pineal Res. 2018, 64, e12473. [Google Scholar] [CrossRef]
- Chen, W.; Wu, P.; Yu, F.; Luo, G.; Qing, L.; Tang, J. HIF-1α Regulates Bone Homeostasis and Angiogenesis, Participating in the Occurrence of Bone Metabolic Diseases. Cells 2022, 11, 3552. [Google Scholar] [CrossRef] [PubMed]
- Garbo, C.; Locs, J.; D’Este, M.; Demazeau, G.; Mocanu, A.; Roman, C.; Horovitz, O.; Tomoaia-Cotisel, M. Advanced Mg, Zn, Sr, Si Multi-Substituted Hydroxyapatites for Bone Regeneration. Int. J. Nanomed. 2020, 15, 1037–1058. [Google Scholar] [CrossRef]
- Fiorati, A.; Linciano, C.; Galante, C.; Raucci, M.G.; Altomare, L. Bioactive Hydrogels: Design and Characterization of Cellulose-Derived Injectable Composites. Materials 2021, 14, 4511. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef]
- Hu, D.; Ren, Q.; Li, Z.; Zhang, L. Chitosan-Based Biomimetically Mineralized Composite Materials in Human Hard Tissue Repair. Molecules 2020, 25, 4785. [Google Scholar] [CrossRef]
- Yuan, Z.; Wan, Z.; Gao, C.; Wang, Y.; Huang, J.; Cai, Q. Controlled magnesium ion delivery system for in situ bone tissue engineering. J. Control Release 2022, 350, 360–376. [Google Scholar] [CrossRef]
- Hong, B.Z.; Piao, H.N.; Li, S.F.; Piao, H.; Jin, L.; Cao, P.A. Evidence for a major role of Mg2+ in VEGF165-mediated angiogenesis. Zhonghua Xin Xue Guan Bing Za Zhi 2007, 35, 260–264. [Google Scholar] [PubMed]
- Negri, S.; Faris, P.; Berra-Romani, R.; Guerra, G.; Moccia, F. Endothelial Transient Receptor Potential Channels and Vascular Remodeling: Extracellular Ca2+ Entry for Angiogenesis, Arteriogenesis and Vasculogenesis. Front. Physiol. 2019, 10, 1618. [Google Scholar] [CrossRef]
- Fan, L.; Körte, F.; Rudt, A.; Jung, O.; Burkhardt, C.; Barbeck, M.; Xiong, X. Encapsulated vaterite-calcite CaCO3 particles loaded with Mg2+ and Cu2+ ions with sustained release promoting osteogenesis and angiogenesis. Front. Bioeng. Biotechnol. 2022, 10, 983988. [Google Scholar] [CrossRef]
- Wang, L.; Pang, Y.; Tang, Y.; Wang, X.; Zhang, D.; Zhang, X.; Yu, Y.; Yang, X.; Cai, Q. A biomimetic piezoelectric scaffold with sustained Mg2+ release promotes neurogenic and angiogenic differentiation for enhanced bone regeneration. Bioact. Mater. 2023, 25, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Guo, S.; Tang, Z.; Wei, X.; Gao, P.; Wang, N.; Li, X.; Guo, Z. Magnesium promotes bone formation and angiogenesis by enhancing MC3T3-E1 secretion of PDGF-BB. Biochem. Biophys. Res. Commun. 2020, 528, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Yuan, Z.; Li, Y.; Zhang, Y.; Wang, Y.; Yu, Y.; Mao, J.; Cai, Q.; Yang, X. Hierarchical Therapeutic Ion-Based Microspheres with Precise Ratio-Controlled Delivery as Microscaffolds for In Situ Vascularized Bone Regeneration. Adv. Funct. Mater. 2022, 32, 2113280. [Google Scholar] [CrossRef]
- Bosch-Rué, E.; Díez-Tercero, L.; Rodríguez-González, R.; Bosch-Canals, B.M.; Perez, R.A. Assessing the potential role of copper and cobalt in stimulating angiogenesis for tissue regeneration. PLoS ONE 2021, 16, e0259125. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Cao, Y.; Zou, J.; Zhu, F.; Gao, Y.; Zheng, X.; Wang, H.; Zhang, T.; Wu, T. Improved osteogenesis and angiogenesis of a novel copper ions doped calcium phosphate cement. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111032. [Google Scholar] [CrossRef] [PubMed]
- Tai, Z.; Li, L.; Zhao, G.; Liu, J.X. Copper stress impairs angiogenesis and lymphangiogenesis during zebrafish embryogenesis by down-regulating pERK1/2-foxm1-MMP2/9 axis and epigenetically regulating ccbe1 expression. Angiogenesis 2022, 25, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yao, A.; Ai, F.; Lin, J.; Fu, Q.; Wang, D. Cobalt-containing borate bioactive glass fibers for treatment of diabetic wound. J. Mater. Sci. Mater. Med. 2023, 34, 42. [Google Scholar] [CrossRef]
- Poliseno, L.; Tuccoli, A.; Mariani, L.; Evangelista, M.; Citti, L.; Woods, K.; Mercatanti, A.; Hammond, S.; Rainaldi, G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood 2006, 108, 3068–3071. [Google Scholar] [CrossRef]
- Zhuang, Y.; Cheng, M.; Li, M.; Cui, J.; Huang, J.; Zhang, C.; Si, J.; Lin, K.; Yu, H. Small extracellular vesicles derived from hypoxic mesenchymal stem cells promote vascularized bone regeneration through the miR-210-3p/EFNA3/PI3K pathway. Acta Biomater. 2022, 150, 413–426. [Google Scholar] [CrossRef]
- Castaño, I.M.; Raftery, R.M.; Chen, G.; Cavanagh, B.; Quinn, B.; Duffy, G.P.; Curtin, C.M.; O’Brien, F.J. Dual scaffold delivery of miR-210 mimic and miR-16 inhibitor enhances angiogenesis and osteogenesis to accelerate bone healing. Acta Biomater. 2023, 172, 480–493. [Google Scholar] [CrossRef]
- Besnier, M.; Gasparino, S.; Vono, R.; Sangalli, E.; Facoetti, A.; Bollati, V.; Cantone, L.; Zaccagnini, G.; Maimone, B.; Fuschi, P.; et al. miR-210 Enhances the Therapeutic Potential of Bone-Marrow-Derived Circulating Proangiogenic Cells in the Setting of Limb Ischemia. Mol. Ther. 2018, 26, 1694–1705. [Google Scholar] [CrossRef] [PubMed]
- Nan, K.; Zhang, Y.; Zhang, X.; Li, D.; Zhao, Y.; Jing, Z.; Liu, K.; Shang, D.; Geng, Z.; Fan, L. Exosomes from miRNA-378-modified adipose-derived stem cells prevent glucocorticoid-induced osteonecrosis of the femoral head by enhancing angiogenesis and osteogenesis via targeting miR-378 negatively regulated suppressor of fused (Sufu). Stem Cell Res. Ther. 2021, 12, 331. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Deng, Z.; Wang, C.H.; Yang, B.B. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc. Natl. Acad. Sci. USA 2007, 104, 20350–20355. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, L.; Rong, Y.; Qian, D.; Chen, J.; Zhou, Z.; Luo, Y.; Jiang, D.; Cheng, L.; Zhao, S.; et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020, 103, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhu, Y.; Qiu, S.; Xu, J.; Chai, Y. Exosomes secreted by endothelial progenitor cells accelerate bone regeneration during distraction osteogenesis by stimulating angiogenesis. Stem Cell Res. Ther. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Liu, L.; Cui, Y.; Liu, H.; Yi, J.; Bing, W.; Liu, C.; Jiang, D.; Bi, Y. miR-126-3p containing exosomes derived from human umbilical cord mesenchymal stem cells promote angiogenesis and attenuate ovarian granulosa cell apoptosis in a preclinical rat model of premature ovarian failure. Stem Cell Res. Ther. 2022, 13, 352. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, Y.; Guo, Q.; Zhang, L.; Liu, H.; Wang, S.; Wu, X.; Shen, X.; Tao, L. MiR-126-Loaded Immunoliposomes against Vascular Endothelial Inflammation In Vitro and Vivo Evaluation. Pharmaceutics 2023, 15, 1379. [Google Scholar] [CrossRef]
- Chamorro-Jorganes, A.; Lee, M.Y.; Araldi, E.; Landskroner-Eiger, S.; Fernández-Fuertes, M.; Sahraei, M.; Quiles Del Rey, M.; van Solingen, C.; Yu, J.; Fernández-Hernando, C.; et al. VEGF-Induced Expression of miR-17-92 Cluster in Endothelial Cells Is Mediated by ERK/ELK1 Activation and Regulates Angiogenesis. Circ. Res. 2016, 118, 38–47. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Yu, Z.; Li, L.; Chen, X.; Yang, A.; Lyu, F.; Dong, Y. Exosomal let-7f-5p derived from mineralized osteoblasts promotes the angiogenesis of endothelial cells via the DUSP1/Erk1/2 signaling pathway. J. Tissue Eng. Regen. Med. 2022, 16, 1184–1195. [Google Scholar] [CrossRef]
- Li, Q.; Yu, T.; Wang, F.; Liu, X.; Wang, Z. Endothelial progenitor cells with stem cells enhance osteogenic efficacy. Am. J. Transl. Res. 2020, 12, 2409–2424. [Google Scholar]
- Zigdon-Giladi, H.; Bick, T.; Lewinson, D.; Machtei, E.E. Co-transplantation of endothelial progenitor cells and mesenchymal stem cells promote neovascularization and bone regeneration. Clin. Implant Dent. Relat. Res. 2015, 17, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, H.; He, Y.; Li, Y.; He, X. Endothelial progenitor cells promote osteogenic differentiation in co-cultured with mesenchymal stem cells via the MAPK-dependent pathway. Stem Cell Res. Ther. 2020, 11, 537. [Google Scholar] [CrossRef]
- Goerke, S.M.; Obermeyer, J.; Plaha, J.; Stark, G.B.; Finkenzeller, G. Endothelial progenitor cells from peripheral blood support bone regeneration by provoking an angiogenic response. Microvasc. Res. 2015, 98, 40–47. [Google Scholar] [CrossRef]
- Gianni-Barrera, R.; Butschkau, A.; Uccelli, A.; Certelli, A.; Valente, P.; Bartolomeo, M.; Groppa, E.; Burger, M.G.; Hlushchuk, R.; Heberer, M.; et al. PDGF-BB regulates splitting angiogenesis in skeletal muscle by limiting VEGF-induced endothelial proliferation. Angiogenesis 2018, 21, 883–900. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Z.; Qiao, Q.; Li, S.; Yu, W.; Guan, X.; Schneider, A.; Weir, M.D.; Xu, H.H.K.; Zhang, K.; et al. Injectable periodontal ligament stem cell-metformin-calcium phosphate scaffold for bone regeneration and vascularization in rats. Dent. Mater. J. 2023, 39, 872–885. [Google Scholar] [CrossRef]
- Luo, R.; Huang, Y.; Yuan, X.; Yuan, Z.; Zhang, L.; Han, J.; Zhao, Y.; Cai, Q. Controlled co-delivery system of magnesium and lanthanum ions for vascularized bone regeneration. Biomed. Mater. 2021, 16, 065024. [Google Scholar] [CrossRef] [PubMed]
- Putranti, N.A.R.; Kunimatsu, R.; Rikitake, K.; Hiraki, T.; Nakajima, K.; Abe, T.; Tsuka, Y.; Sakata, S.; Nakatani, A.; Nikawa, H.; et al. Combination of Carbonate Hydroxyapatite and Stem Cells from Human Deciduous Teeth Promotes Bone Regeneration by Enhancing BMP-2, VEGF and CD31 Expression in Immunodeficient Mice. Cells 2022, 11, 1914. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Meng, C.X.; Lv, Z.Y.; Zhang, Y.J.; Li, J.; Li, K.Y.; Liu, F.Z.; Zhang, B.; Cui, F.Z. Enhancement of BMP-2 and VEGF carried by mineralized collagen for mandibular bone regeneration. Regen. Biomater. 2020, 7, 435–440. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; He, J.; Liu, L.; Hu, S.; Guo, M.; Liu, T.; Liu, J.; Wang, J.; Guo, B.; et al. ROS Scavenging Graphene-Based Hydrogel Enhances Type H Vessel Formation and Vascularized Bone Regeneration via ZEB1/Notch1 Mediation. Macromol. Biosci. 2023, 23, e2200502. [Google Scholar] [CrossRef]
- Omar, O.; Engstrand, T.; Kihlström Burenstam Linder, L.; Åberg, J.; Shah, F.A.; Palmquist, A.; Birgersson, U.; Elgali, I.; Pujari-Palmer, M.; Engqvist, H.; et al. In situ bone regeneration of large cranial defects using synthetic ceramic implants with a tailored composition and design. Proc. Natl. Acad. Sci. USA 2020, 117, 26660–26671. [Google Scholar] [CrossRef]
- Tang, Y.; Tong, X.; Conrad, B.; Yang, F. Injectable and in situ crosslinkable gelatin microribbon hydrogels for stem cell delivery and bone regeneration in vivo. Theranostics 2020, 10, 6035–6047. [Google Scholar] [CrossRef]
- He, Y.; Lin, S.; Ao, Q.; He, X. The co-culture of ASCs and EPCs promotes vascularized bone regeneration in critical-sized bone defects of cranial bone in rats. Stem Cell. Res. Ther. 2020, 11, 338. [Google Scholar] [CrossRef]
- Wang, M.M.; Flores, R.L.; Witek, L.; Torroni, A.; Ibrahim, A.; Wang, Z.; Liss, H.A.; Cronstein, B.N.; Lopez, C.D.; Maliha, S.G.; et al. Dipyridamole-loaded 3D-printed bioceramic scaffolds stimulate pediatric bone regeneration in vivo without disruption of craniofacial growth through facial maturity. Sci. Rep. 2019, 9, 18439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Saxena, S.; Fakhrzadeh, A.; Rudolph, S.; Young, S.; Kohn, J.; Yelick, P.C. Use of Human Dental Pulp and Endothelial Cell Seeded Tyrosine-Derived Polycarbonate Scaffolds for Robust in vivo Alveolar Jaw Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 796. [Google Scholar]
- Dewey, M.J.; Milner, D.J.; Weisgerber, D.; Flanagan, C.L.; Rubessa, M.; Lotti, S.; Polkoff, K.M.; Crotts, S.; Hollister, S.J.; Wheeler, M.B.; et al. Repair of critical-size porcine craniofacial bone defects using a collagen-polycaprolactone composite biomaterial. Biofabrication 2021, 14, 014102. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.Q.; Jiao, K.; Ma, Y.X.; Gao, B.; Mu, Z.; Wang, Y.R.; Wang, Y.H.; Duan, L.; Xu, K.H.; Gu, J.T.; et al. Smart, Biomimetic Periosteum Created from the Cerium(III, IV) Oxide-Mineralized Eggshell Membrane. ACS Appl. Mater. Interfaces 2022, 14, 14103–14119. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Xu, J.; Meng, L.; Su, Y.; Fang, H.; Liu, J.; Cheng, Y.Y.; Jiang, D.; Nie, Y.; Song, K. 3D bioprinting of dECM/Gel/QCS/nHAp hybrid scaffolds laden with mesenchymal stem cell-derived exosomes to improve angiogenesis and osteogenesis. Biofabrication 2023, 15, 024103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Sun, Y.; Qiao, Q.; Weir, M.D.; Schneider, A.; Masri, R.; Lynch, C.D.; Zhang, N.; Zhang, K.; Bai, Y.; et al. Calvaria defect regeneration via human periodontal ligament stem cells and prevascularized scaffolds in athymic rats. J. Dent. 2023, 138, 104690. [Google Scholar] [CrossRef]
- Autefage, H.; Allen, F.; Tang, H.M.; Kallepitis, C.; Gentleman, E.; Reznikov, N.; Nitiputri, K.; Nommeots-Nomm, A.; O’Donnell, M.D.; Lange, C.; et al. Multiscale analyses reveal native-like lamellar bone repair and near perfect bone-contact with porous strontium-loaded bioactive glass. Biomaterials 2019, 209, 152–162. [Google Scholar] [CrossRef]
- Saskianti, T.; Nugraha, A.P.; Prahasanti, C.; Ernawati, D.S.; Tanimoto, K.; Riawan, W.; Kanawa, M.; Kawamoto, T.; Fujimoto, K. Study of Alveolar Bone Remodeling Using Deciduous Tooth Stem Cells and Hydroxyapatite by Vascular Endothelial Growth Factor Enhancement and Inhibition of Matrix Metalloproteinase-8 Expression in vivo. Clin. Cosmet. Investig. Dent. 2022, 14, 71–78. [Google Scholar] [CrossRef]
- Ma, W.; Lyu, H.; Pandya, M.; Gopinathan, G.; Luan, X.; Diekwisch, T.G.H. Successful Application of a Galanin-Coated Scaffold for Periodontal Regeneration. J. Dent. Res. 2021, 100, 1144–1152. [Google Scholar] [CrossRef]
- Kurobane, T.; Shiwaku, Y.; Anada, T.; Hamai, R.; Tsuchiya, K.; Baba, K.; Iikubo, M.; Takahashi, T.; Suzuki, O. Angiogenesis involvement by octacalcium phosphate-gelatin composite-driven bone regeneration in rat calvaria critical-sized defect. Acta Biomater. 2019, 88, 514–526. [Google Scholar] [CrossRef]
- Pandya, M.; Saxon, M.; Bozanich, J.; Tillberg, C.; Luan, X.; Diekwisch, T.G.H. The Glycoprotein/Cytokine Erythropoietin Promotes Rapid Alveolar Ridge Regeneration In Vivo by Promoting New Bone Extracellular Matrix Deposition in Conjunction with Coupled Angiogenesis/Osteogenesis. Int. J. Mol. Sci. 2021, 22, 2788. [Google Scholar] [CrossRef]
- Tenkumo, T.; Kruse, B.; Kostka, K.; Sokolova, V.; Ogawa, T.; Yoda, N.; Prymak, O.; Suzuki, O.; Sasaki, K.; Epple, M. Development of triple-functionalized calcium phosphate nanoparticles as an advanced drug delivery system for bone tissue repair. Regen. Ther. 2024, 25, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Collignon, A.M.; Lepry, W.C.; Ramirez-GarciaLuna, J.L.; Rosenzweig, D.H.; Chaussain, C.; Nazhat, S.N. Acellular dense collagen-S53P4 bioactive glass hybrid gel scaffolds form more bone than stem cell delivered constructs. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111743. [Google Scholar] [CrossRef] [PubMed]
- Asri, R.I.M.; Harun, W.S.W.; Hassan, M.A.; Ghani, S.A.C.; Buyong, Z. A review of hydroxyapatite-based coating techniques: Sol–gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huse, R.O.; de Groot, K.; Buser, D.; Hunziker, E.B. Delivery mode and efficacy of BMP-2 in association with implants. J. Dent. Res. 2007, 86, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, L.; Jiang, X.; Rowe, D.; Wei, M. Biomimetic CaP coating incorporated with parathyroid hormone improves the osseointegration of titanium implant. J. Mater. Sci. Mater. Med. 2012, 23, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Hagi, T.T.; Wu, G.; Liu, Y.; Hunziker, E.B. Cell-mediated BMP-2 liberation promotes bone formation in a mechanically unstable implant environment. Bone 2010, 46, 1322–1327. [Google Scholar] [CrossRef]
- Jacobs, E.E.; Gronowicz, G.; Hurley, M.M.; Kuhn, L.T. Biomimetic calcium phosphate/polyelectrolyte multilayer coatings for sequential delivery of multiple biological factors. J. Biomed. Mater. Res. A 2017, 105, 1500–1509. [Google Scholar] [CrossRef]
- Alhamdi, J.R.; Peng, T.; Al-Naggar, I.M.; Hawley, K.L.; Spiller, K.L.; Kuhn, L.T. Controlled M1-to-M2 transition of aged macrophages by calcium phosphate coatings. Biomaterials 2019, 196, 90–99. [Google Scholar] [CrossRef]
- Siebers, M.C.; Walboomers, X.F.; Leeuwenburgh, S.C.; Wolke, J.G.; Jansen, J.A. Electrostatic spray deposition (ESD) of calcium phosphate coatings, an in vitro study with osteoblast-like cells. Biomaterials 2004, 25, 2019–2027. [Google Scholar] [CrossRef]
- Graziani, G.; Bianchi, M.; Sassoni, E.; Russo, A.; Marcacci, M. Ion-substituted calcium phosphate coatings deposited by plasma-assisted techniques: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 219–229. [Google Scholar] [CrossRef]
- Hadjizadeh, A.; Doillon, C.J. Directional migration of endothelial cells towards angiogenesis using polymer fibres in a 3D co-culture system. J. Tissue Eng. Regen. Med. 2010, 4, 524–531. [Google Scholar] [CrossRef]
- Yu, J.; Gu, Y.; Du, K.T.; Mihardja, S.; Sievers, R.E.; Lee, R.J. The effect of injected RGD modified alginate on angiogenesis and left ventricular function in a chronic rat infarct model. Biomaterials 2009, 30, 751–756. [Google Scholar] [CrossRef]
- Hao, D.; Fan, Y.; Xiao, W.; Liu, R.; Pivetti, C.; Walimbe, T.; Guo, F.; Zhang, X.; Farmer, D.L.; Wang, F.; et al. Rapid endothelialization of small diameter vascular grafts by a bioactive integrin-binding ligand specifically targeting endothelial progenitor cells and endothelial cells. Acta Biomater. 2020, 108, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Walimbe, T.; Dehghani, T.; Casella, A.; Lin, J.; Wang, A.; Panitch, A. Proangiogenic Collagen-Binding Glycan Therapeutic Promotes Endothelial Cell Angiogenesis. ACS Biomater. Sci. Eng. 2021, 7, 3281–3292. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Dreyer, C.H.; Kjaergaard, K.; Ding, M.; Qin, L. Vascular endothelial growth factor for in vivo bone formation: A systematic review. J. Orthop. Translat. 2020, 24, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Wernike, E.; Montjovent, M.O.; Liu, Y.; Wismeijer, D.; Hunziker, E.B.; Siebenrock, K.A.; Hofstetter, W.; Klenke, F.M. VEGF incorporated into calcium phosphate ceramics promotes vascularisation and bone formation in vivo. Eur. Cell Mater. 2010, 19, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.G.; Grosso, A.; Briquez, P.S.; Born, G.M.E.; Lunger, A.; Schrenk, F.; Todorov, A.; Sacchi, V.; Hubbell, J.A.; Schaefer, D.J.; et al. Robust coupling of angiogenesis and osteogenesis by VEGF-decorated matrices for bone regeneration. Acta Biomater. 2022, 149, 111–125. [Google Scholar] [CrossRef] [PubMed]
- De Pieri, A.; Rochev, Y.; Zeugolis, D.I. Scaffold-free cell-based tissue engineering therapies: Advances, shortfalls and forecast. NPJ Regen. Med. 2021, 6, 18. [Google Scholar] [CrossRef]
- Shimizu, T.; Sekine, H.; Yang, J.; Isoi, Y.; Yamato, M.; Kikuchi, A.; Kobayashi, E.; Okano, T. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 708–710. [Google Scholar] [CrossRef]
- Sasagawa, T.; Shimizu, T.; Sekiya, S.; Haraguchi, Y.; Yamato, M.; Sawa, Y.; Okano, T. Design of prevascularized three-dimensional cell-dense tissues using a cell sheet stacking manipulation technology. Biomaterials 2010, 31, 1646–1654. [Google Scholar] [CrossRef]



| Target Pathway | miRNA | Target Signaling Pathway |
|---|---|---|
| Activation of ECs | miR-210-3p | EFNA3/PI3K/AKT [81,83] |
| miR-378 | Sufu [84] Fus1 [85] | |
| miR-126 | SPRED1/Ras/Erk [86,87] PIK3R2 [88] VCAM-1 [89] | |
| Sprouting, migration, and tubulogenesis of ECs | miR-17-92 | ERK/ELK1 [90] |
| Let-7f-5p | DUSP1/Erk1/2 [91] |
| Author and Year | Biomaterial Scaffolds | Bioactive Agent | Implanted Cells | Animal Model | Observation Period | Osteogenesis | Angiogenesis |
|---|---|---|---|---|---|---|---|
| Yaxi Sun, Dent Mater, 2023 [97] | Calcium phosphate cement scaffold (CPC) | Metformin | hPDLSCs | Critical-sized defect of rat cranium | 12 weeks | 9 folds by control | 3 folds by control |
| Ruochen Luo, Biomed Mater, 2021 [98] | Poly(lactide-co-glycolide) microspheres | Mg2+ and La3+ | --- | Critical-sized defect of rat cranium | 8 weeks | Enhanced | Enhanced |
| Nurul Aisyah Rizky Putranti Cells, 2022 [99] | Carbonate hydroxyapatite (CAP) granules | BMP-2 | SHED | Critical-sized defect of immunodeficient mice cranium | 12 weeks | Enhanced | Enhanced |
| Kun Liu, Regen Biomater, 2020 [100] | Mineralized collagen | BMP-2 and VEGF | --- | Mandibular defects of rabbits | 12 weeks | Enhanced | Enhanced |
| Junpeng Zhou, Macromol Biosci, 2023 [101] | GM/Ac-CD/rGO hydrogel | --- | --- | Critical-sized defect of rat and mice cranium | 8 weeks | Enhanced | Promotes type H vessel formation |
| Omar Omar, Proc Natl Acad Sci U S A, 2020 [102] | Bioceramic (biocer) implants | --- | --- | Skull defect of ovine | 12 months | Enhanced | Enhanced |
| Yaohui Tang, Theranostics, 2020 [103] | Injectable gelatin-based μRB hydrogel | BMP-2 | ASC | Critical-sized defect of immunodeficient mice cranium | 8 weeks | Enhanced | --- |
| Yuanjia He, Stem Cell Res Ther, 2020 [104] | HA/Col scaffold | --- | EPCs and ASC | Critical-sized defect of rat cranium | 8 weeks | Enhanced | Enhanced |
| Maxime M Wang, Sci Rep, 2019 [105] | 3D-printed bioceramic scaffolds | Dipyridamole | --- | Unilateral alveolar defect of rabbits | 24 weeks | Enhanced | --- |
| Weibo Zhang, Front Bioeng Biotechnol, 2020 [106] | E1001(1K)/β-TCP scaffolds | Tyrosine-derived polycarbonate | hDPSCs and HUVECs | Mandible defect of rabbits | 3 months | Enhanced | Enhanced |
| Marley J Dewey, Biofabrication, 2021 [107] | Mineralized collagen/PCL composites | --- | --- | Critical-sized defect of porcine ramus | 10 months | Enhanced | Enhanced |
| Qian-Qian Wan, ACS Appl Mater Interfaces, 2022 [108] | Eggshell membranes | Cerium oxide | --- | Critical-sized defect of mice cranium | 8 weeks | Enhanced | Enhanced |
| Yue Kang, Biofabrication, 2023 [109] | Hybrid scaffolds | Exos isolated from hASC | --- | Critical-sized defect of immunodeficient mice cranium | 10 weeks | Enhanced | Enhanced |
| Zeqing Zhao, J Dent, 2023 [110] | Calcium phosphate cement (CPC) scaffolds | Human platelet lysate | hPDLSCs and hUVECs | Critical-sized defect of immunodeficient mice cranium | 12 weeks | 4 folds by control | 7.9 folds by control |
| H Autefage, Biomaterials, 2019 [111] | Bioactive glass-based scaffold | Strontium | --- | Femoral condyle defect of ovine | 12 weeks | Enhanced | --- |
| Tania Saskianti, Clin Cosmet Investig Dent, 2022 [112] | Hydroxyapatite | --- | SHED | Mandibular defect of rats | Downregulation of MMP-8 | Upregulation VEGF expressions | |
| W Ma, J Dent Res, 2021 [113] | Col scaffold | Galanin | --- | Periodontitis-treated mice | 6 weeks | Enhanced | --- |
| Tsuyoshi Kurobane, Acta Biomater, 2019 [114] | Octacalcium phosphate/gelatin composite (OCP/Gel) | --- | --- | Critical-sized defect of immunodeficient mice cranium | 4 weeks | --- | Enhanced |
| Mirali Pandya, Int J Mol Sci, 2021 [115] | Collagen/erythropoietin (EPO) scaffold | EPO | First maxillary molars extracted rats | 8 weeks | Enhanced | enhanced | |
| TaichiTenkumo, Regen Ther, 2023 [116] | A triple-functionalized paste of CAP | DNA and siRNA | --- | Femoral head defect of rats | 21 days | Enhanced | --- |
| Hyeree Park, Mater Sci Eng C Mater Biol Appl, 2021 [117] | DC-S53P4 bioactive glass hybrid gels | --- | DPSCs | Critical-sized defect of immunodeficient mice cranium | 8 weeks | Enhanced | Enhanced |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Da, Y.; Yang, J.; Zhu, G.; Qin, H. Vascularization Reconstruction Strategies in Craniofacial Bone Regeneration. Coatings 2024, 14, 357. https://doi.org/10.3390/coatings14030357
Chen J, Da Y, Yang J, Zhu G, Qin H. Vascularization Reconstruction Strategies in Craniofacial Bone Regeneration. Coatings. 2024; 14(3):357. https://doi.org/10.3390/coatings14030357
Chicago/Turabian StyleChen, Jiping, Yu Da, Jing Yang, Guirong Zhu, and Haiyan Qin. 2024. "Vascularization Reconstruction Strategies in Craniofacial Bone Regeneration" Coatings 14, no. 3: 357. https://doi.org/10.3390/coatings14030357






