Design and Preparation of Bending-Resistant Flexible All-Solid Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Dual-Porous TiO2 Film
2.3. Preparation and Filling of the PVDF/PEO-Based Electrolyte
2.4. Assemble of Solar Cells and Characterization of the Bending-Resistant Ability
3. Results and Discussion
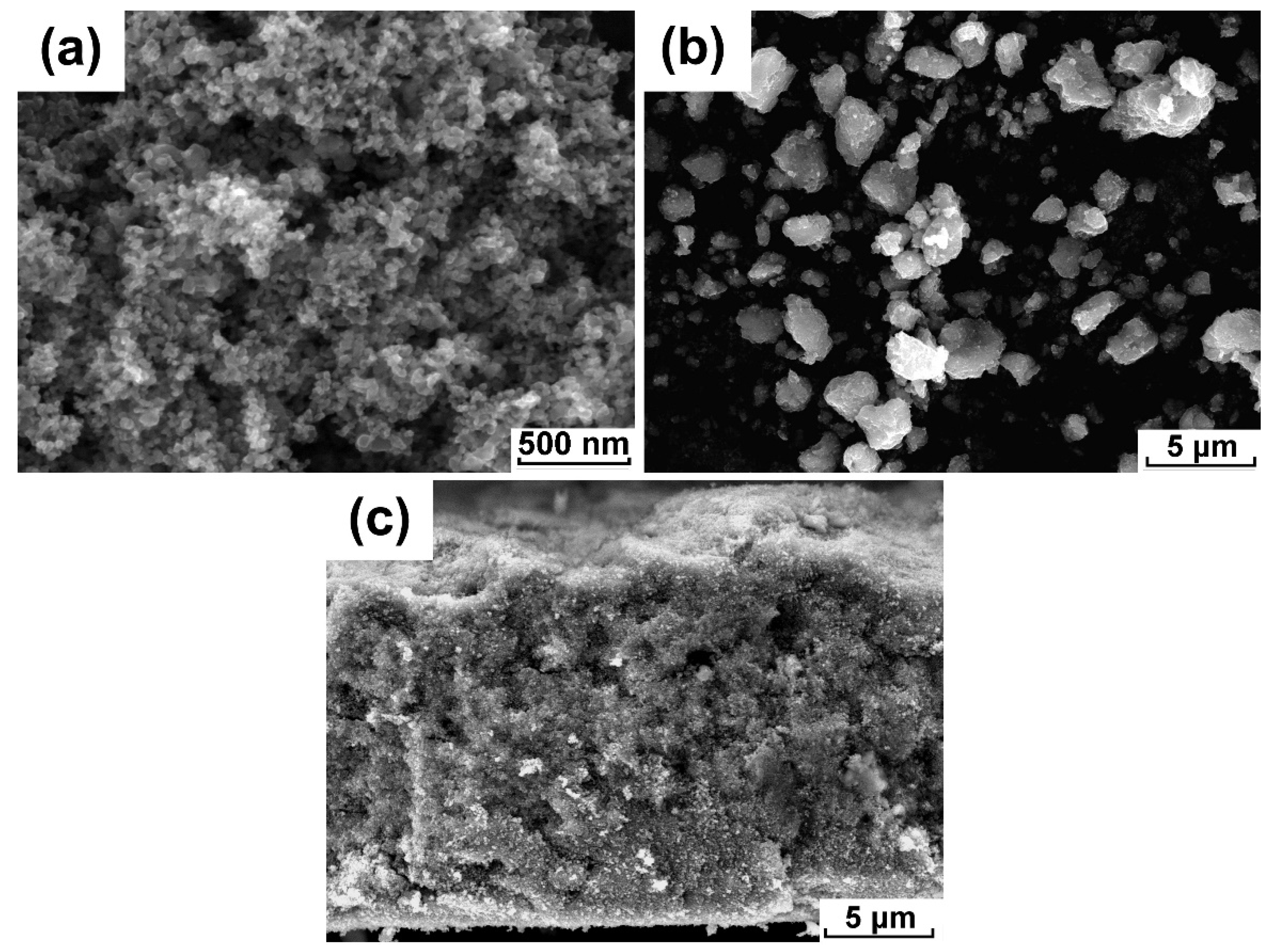
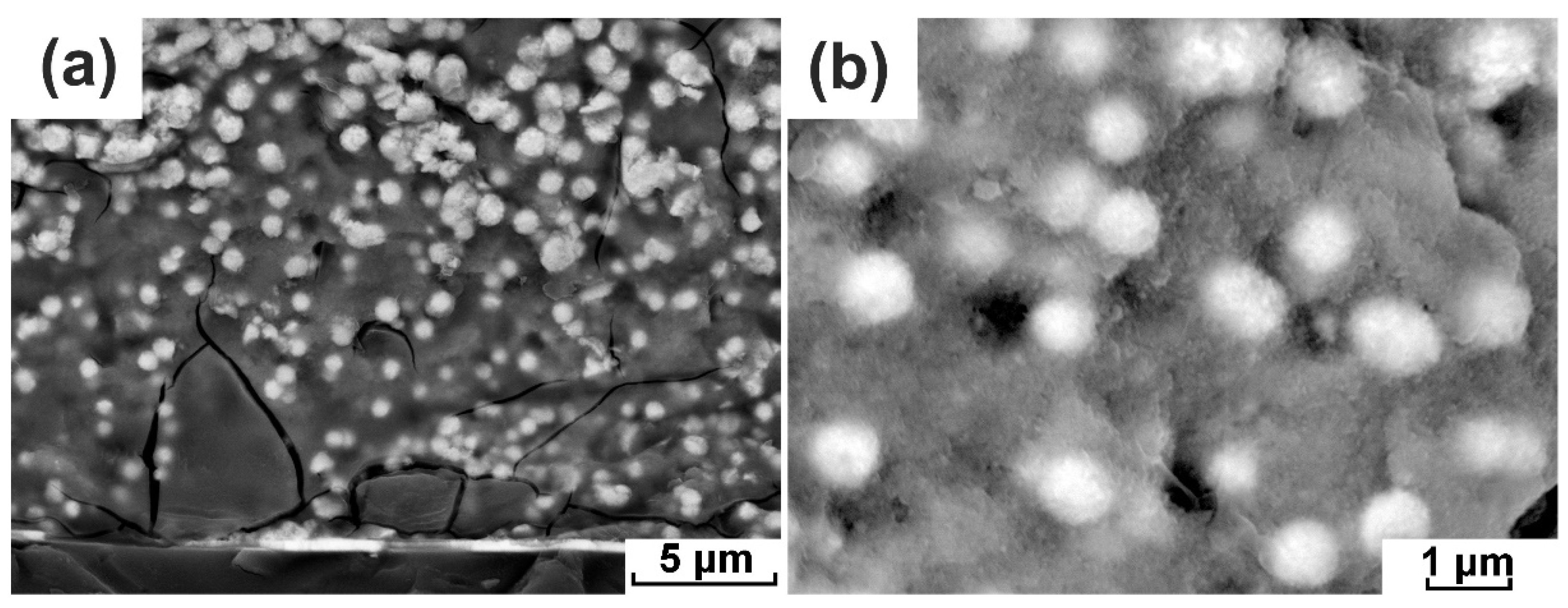
3.1. The Formation of the Submicron Pores in Dual-Porous Film
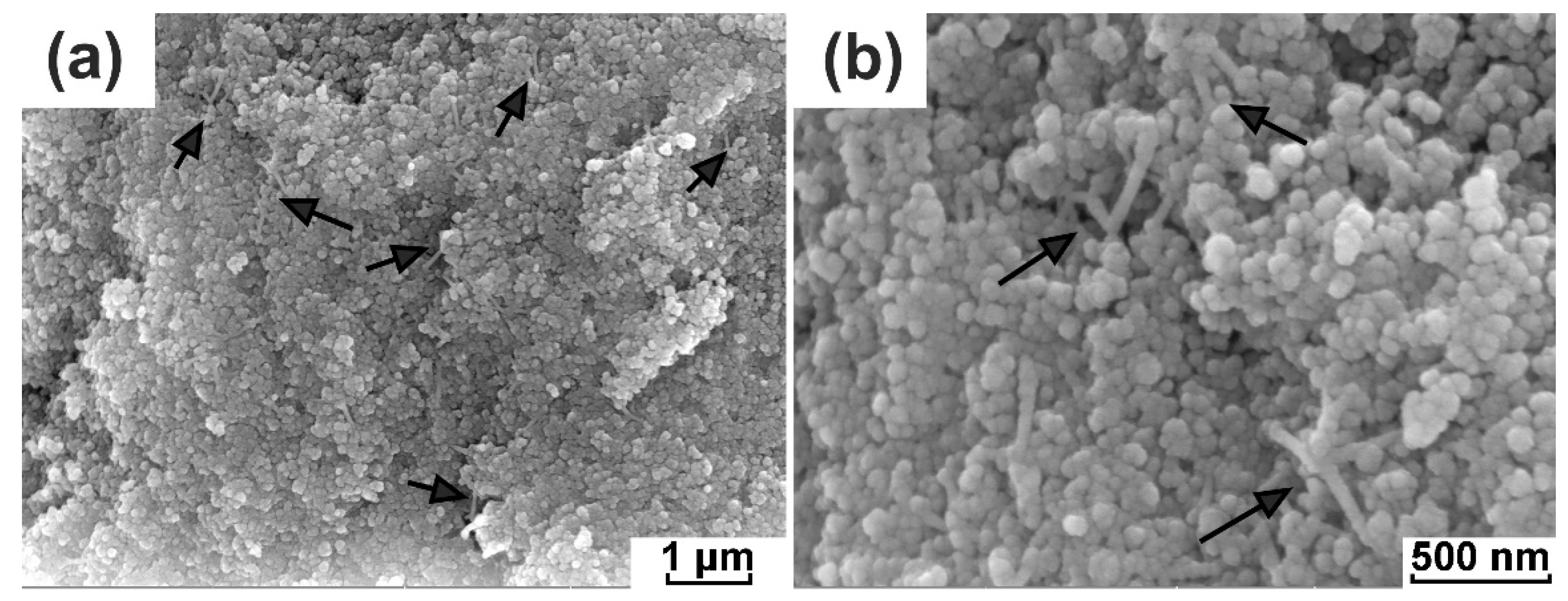
3.2. The Formation of PVDF/PEO Electrolyte Fibers in Submicron Pores
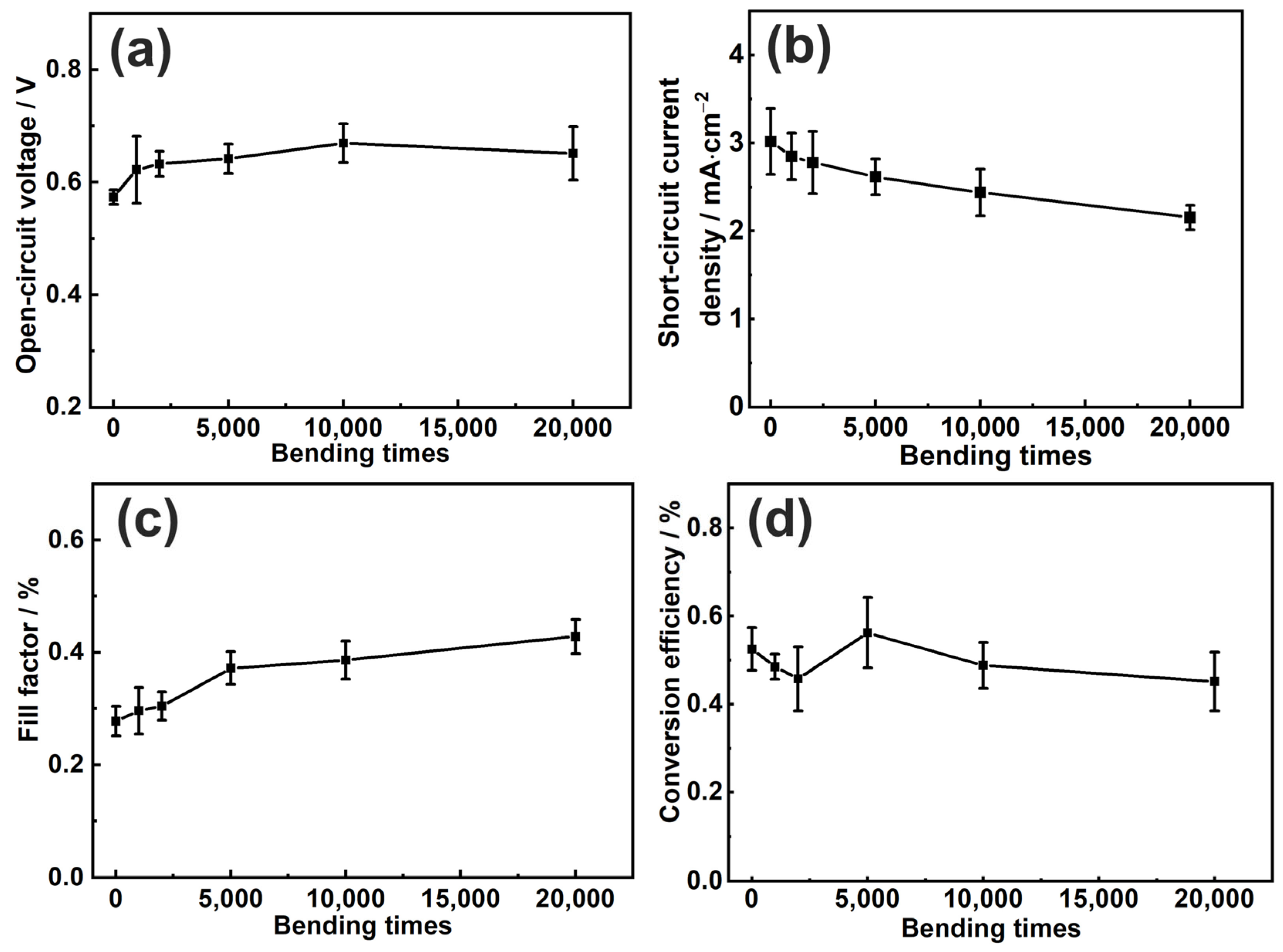
3.3. The Bending-Resistant Ability of the Flexible All-Solid-State Dye-Sensitized Solar Cell
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sekaran, P.D.; Marimuthu, R. An extensive analysis of dye-sensitized solar cell (DSSC). Brazilian J. Phys. 2024, 54, 28. [Google Scholar] [CrossRef]
- Wrede, S.; Cai, B.; Cheng, F.W.; Johansson, M.B.; Kubart, T.; Hägglund, C.; Tian, H.N. A solid-state p-n tandem dye-sensitized solar cell. Sustain. Energ. Fuel. 2024, 8, 1004–1011. [Google Scholar] [CrossRef]
- Wu, T.-C.; Huang, W.-M.; Tsai, J.-K.; Chang, C.-E.; Meen, T.-H. Effect of photoanode process sequence on efficiency of dye-sensitized solar cells. Coatings 2024, 14, 304. [Google Scholar] [CrossRef]
- Mohamad, A.A. Physical properties of quasi-solid-state polymer electrolytes for dye-sensitised solar cells: A characterisation review. Sol. Energ. 2019, 190, 434–452. [Google Scholar] [CrossRef]
- Grätzel, M. Dye-sensitized solar cells. J. Photoch. Photobio. C 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Kamat, P.V. Quantum dot solar cells. The next big thing in photovoltaics. J. Phys. Chem. Lett. 2013, 4, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yoshida, T.; Minoura, H. Low-temperature fabrication of efficient porous titania photoelectrodes by hydrothermal crystallization at the solid/gas interface. Adv. Mater. 2003, 15, 814–817. [Google Scholar] [CrossRef]
- Lindström, H.; Magnusson, E.; Holmberg, A.; Södergren, S.; Lindquist, S.-E.; Hagfeldt, A. A new method for manufacturing nanostructured electrodes on glass substrates. Sol. Energy Mater. Sol. Cells 2002, 73, 91–101. [Google Scholar] [CrossRef]
- Miyasaka, T.; Kijitori, Y.; Murakami, T.N.; Kimura, M.; Uegusa, S. Efficient nonsintering type dye-sensitized photocells based on electrophoretically deposited TiO2 layers. Chem. Lett. 2002, 31, 1250–1251. [Google Scholar] [CrossRef]
- Han, H.-G.; Weerasinghe, H.C.; Min Kim, K.; Soo Kim, J.; Cheng, Y.-B.; Jones, D.J.; Holmes, A.B.; Kwon, T.-H. Ultrafast fabrication of flexible dye-sensitized solar cells by ultrasonic spray-coating technology. Sci. Rep. 2015, 5, 14645. [Google Scholar] [CrossRef] [PubMed]
- Kijitori, Y.; Ikegami, M.; Miyasaka, T. Highly efficient plastic dye-sensitized photoelectrodes prepared by low-temperature binder-free coating of mesoscopic titania pastes. Chem. Lett. 2007, 36, 190–191. [Google Scholar] [CrossRef]
- Chen, H.-W.; Hsu, C.-Y.; Chen, J.-G.; Lee, K.-M.; Wang, C.-C.; Huang, K.-C.; Ho, K.-C. Plastic dye-sensitized photo-supercapacitor using electrophoretic deposition and compression methods. J. Power Sources 2010, 195, 6225–6231. [Google Scholar] [CrossRef]
- Shao, J.; Liu, F.; Dong, W.; Tao, R.; Deng, Z.; Fang, X.; Dai, S. Low temperature preparation of TiO2 films by cold isostatic pressing for flexible dye-sensitized solar cells. Mater. Lett. 2012, 68, 493–496. [Google Scholar] [CrossRef]
- He, X.-L.; Yang, G.-J.; Li, C.-J.; Li, C.-X.; Fan, S.-Q. Room temperature cold sprayed TiO2 scattering layer for high performance and bending resistant plastic-based dye-sensitized solar cells. J. Power Sources 2014, 251, 122–129. [Google Scholar] [CrossRef]
- He, X.-L.; Liu, M.; Yang, G.-J.; Yao, H.-L.; Fan, S.-Q.; Li, C.-J. Photovoltaic performance degradation and recovery of the flexible dye-sensitized solar cells by bending and relaxing. J. Power Sources 2013, 226, 173–178. [Google Scholar] [CrossRef]
- Chung, I.; Lee, B.; He, J.; Chang, R.P.H.; Kanatzidis, M.G. All-solid-state dye-sensitized solar cells with high efficiency. Nature 2012, 485, 486–489. [Google Scholar] [CrossRef]
- Zhang, L.; Boschloo, G.; Hammarström, L.; Tian, H. Solid state p-type dye-sensitized solar cells: Concept, experiment and mechanism. Phys. Chem. Chem. Phys. 2016, 18, 5080–5085. [Google Scholar] [CrossRef]
- Manceriu, L.; Bharwal, A.K.; Daem, N.; Dewalque, J.; Colson, P.; Boschini, F.; Cloots, R. Printability of (quasi-) solid polysiloxane electrolytes for online dye-sensitized solar cell fabrication. Coatings 2023, 13, 1164. [Google Scholar] [CrossRef]
- Li, Y.; He, X.-L.; Lian, C.-X.; Yao, H.-L.; Yang, G.-J.; Li, C.-X.; Li, C.-J.; Fang, B. In situ formation of continuous charge transfer pathways for highly efficient, solvent-free, polymer electrolyte-based dye-sensitized solar cells. ACS Sustain. Chem. Eng. 2016, 4, 4013–4020. [Google Scholar] [CrossRef]
- Li, C.; Luo, Y.; Guo, X.; Li, D.; Mi, J.; Sø, L.; Hald, P.; Meng, Q.; Iversen, B.B. Mesoporous TiO2 aggregate photoanode with high specific surface area and strong light scattering for dye-sensitized solar cells. J. Solid State Chem. 2012, 196, 504–510. [Google Scholar] [CrossRef]
- Zhu, F.; Wu, D.; Li, Q.; Dong, H.; Li, J.; Jiang, K.; Xu, D. Hierarchical TiO2 microspheres: Synthesis, structural control and their applications in dye-sensitized solar cells. RSC Adv. 2012, 2, 11629–11637. [Google Scholar] [CrossRef]
- Chen, D.; Huang, F.; Cheng, Y.-B.; Caruso, R.A. Mesoporous anatase TiO2 beads with high surface areas and controllable pore sizes: A superior candidate for high-performance dye-sensitized solar cells. Adv. Mater. 2009, 21, 2206–2210. [Google Scholar] [CrossRef]
- He, X.-L.; Yang, G.-J.; Li, C.-J.; Li, C.-X. Influence of accelerating gas flow rate on the particle cohesion in room temperature cold sprayed scattering layer for plastic-based dye-sensitized solar cells. Appl. Surf. Sci. 2014, 288, 416–422. [Google Scholar] [CrossRef]
- Yang, G.-J.; Liao, K.-X.; Li, C.-J.; Fan, S.-Q.; Li, C.-X.; Li, S. Formation of pore structure and its influence on the mass transport property of vacuum cold sprayed TiO2 coatings using strengthened nanostructured powder. J. Therm. Spray Technol. 2012, 21, 505–513. [Google Scholar] [CrossRef]
- Yang, G.-J.; Li, C.-J.; Fan, S.-Q.; Gao, J.-C. Influence of pore structure on ion diffusion property in porous TiO2 coating and photovoltaic performance of dye-sensitized solar cells. Surf. Coatings Technol. 2011, 205, 3205–3210. [Google Scholar] [CrossRef]
- Zaine, S.N.A.; Mohamed, N.M.; Khatani, M.; Shahid, M.U. Enhancement of charge transport of a dye-sensitized solar cell utilizing TiO2 quantum dot photoelectrode film. Coatings 2021, 11, 1442. [Google Scholar] [CrossRef]
- Al-Attafi, K.; Dwech, M.H.; Mezher, H.A.; Nattestad, A.; Kim, J.H. A Comparative study of organic dye-sensitized solar cells based on anatase TiO2 and amorphous free mixed phase’s anatase/rutile P25 TiO2 photoanodes. Coatings 2023, 13, 121. [Google Scholar] [CrossRef]
- Wu, T.C.; Huang, W.M.; Meen, T.H.; Tsai, J.K. Performance improvement of dye-sensitized solar cells with pressed TiO2 nanoparticles layer. Coatings 2023, 13, 907. [Google Scholar] [CrossRef]
- Bisquert, J. Theory of the impedance of electron diffusion and recombination in a thin layer. J. Phys. Chem. B 2002, 106, 325–333. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Bisquert, J.; Garcia-Belmonte, G.; Boschloo, G.; Hagfeldt, A. Influence of electrolyte in transport and recombination in dye-sensitized solar cells studied by impedance spectroscopy. Sol. Energy Mater. Sol. Cells 2005, 87, 117–131. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Bisquert, J.; Palomares, E.; Otero, L.; Kuang, D.; Zakeeruddin, S.M.; Gratzel, M. Correlation between photovoltaic performance and impedance spectroscopy of dye-sensitized solar cells based on ionic liquids. J. Phys. Chem. C 2007, 111, 6550–6560. [Google Scholar] [CrossRef]

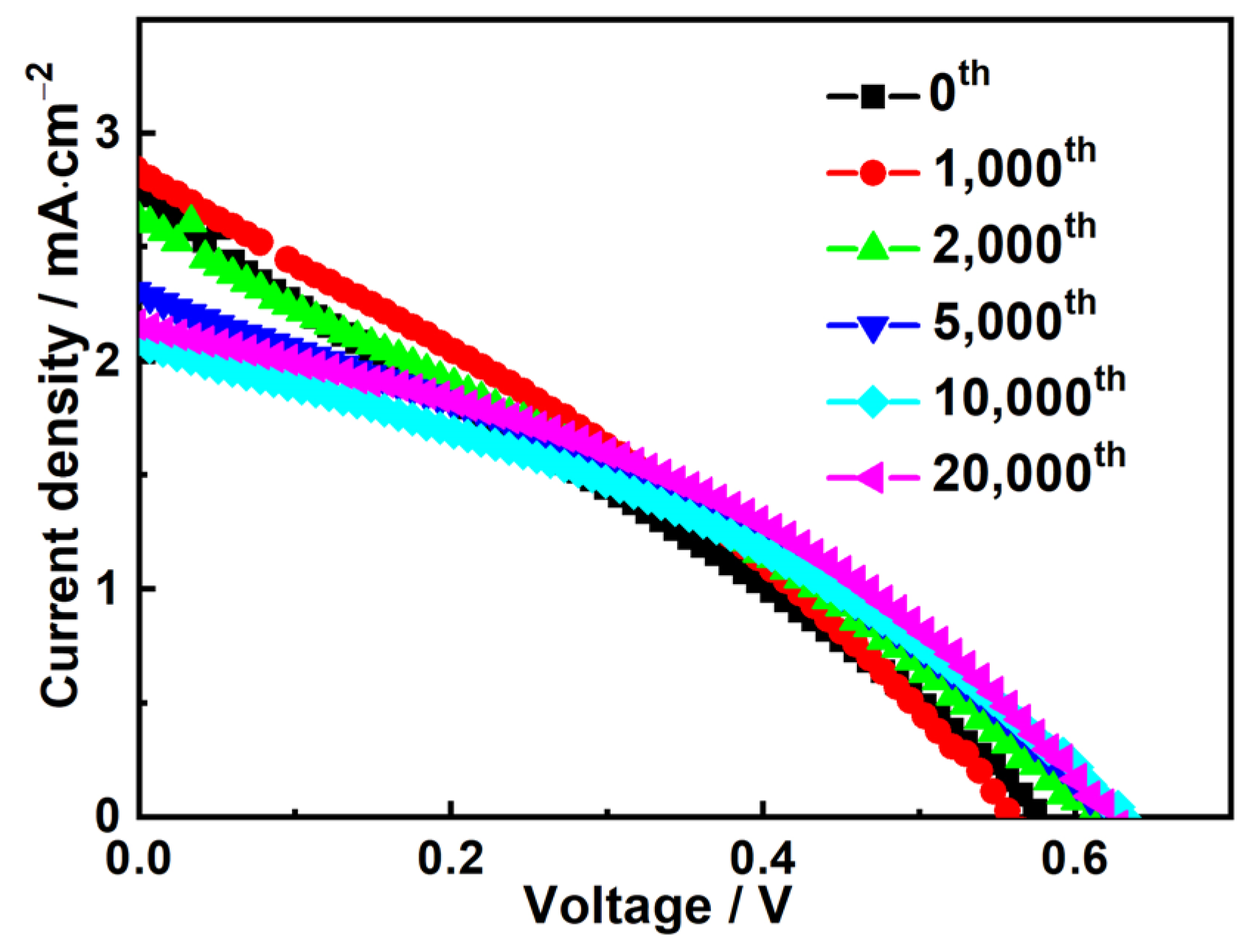

| Bending Times | Rt/Ω | Rct/×103 Ω | Rw/×103 Ω |
|---|---|---|---|
| 0 | 10.7 | 1.79 | 1.28 |
| 1000 | 11.9 | 1.80 | 1.35 |
| 20,000 | 11.0 | 1.79 | 1.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Hou, Y.-X.; Dang, W.-W.; Liu, L.; Chen, J.-H.; Gu, X. Design and Preparation of Bending-Resistant Flexible All-Solid Dye-Sensitized Solar Cells. Coatings 2024, 14, 504. https://doi.org/10.3390/coatings14040504
Li Y, Hou Y-X, Dang W-W, Liu L, Chen J-H, Gu X. Design and Preparation of Bending-Resistant Flexible All-Solid Dye-Sensitized Solar Cells. Coatings. 2024; 14(4):504. https://doi.org/10.3390/coatings14040504
Chicago/Turabian StyleLi, Yan, Yu-Xuan Hou, Wei-Wu Dang, Li Liu, Jian-Hua Chen, and Xian Gu. 2024. "Design and Preparation of Bending-Resistant Flexible All-Solid Dye-Sensitized Solar Cells" Coatings 14, no. 4: 504. https://doi.org/10.3390/coatings14040504






