Coating on Steel Discs with a Photocatalytic System CuO/SiO2 for the Degradation of the Ubiquitous Contaminants Methylene Blue and Amoxicillin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CuO Powders
2.2. Synthesis of the SiO2 Sol
2.3. Deposition and Characterization of the CuO/SiO2 Films via Dip Coating
2.4. Characterization
2.5. Evaluation of the Photocatalytic Efficiency of CuO Powders at Different Loadings
2.6. Photocatalytic Evaluation of the CuO/SiO2 Films
2.7. The CuO/SiO2 System in an Aerated and Stirred Tank
3. Results and Discussion
3.1. Characterization of the CuO Powders
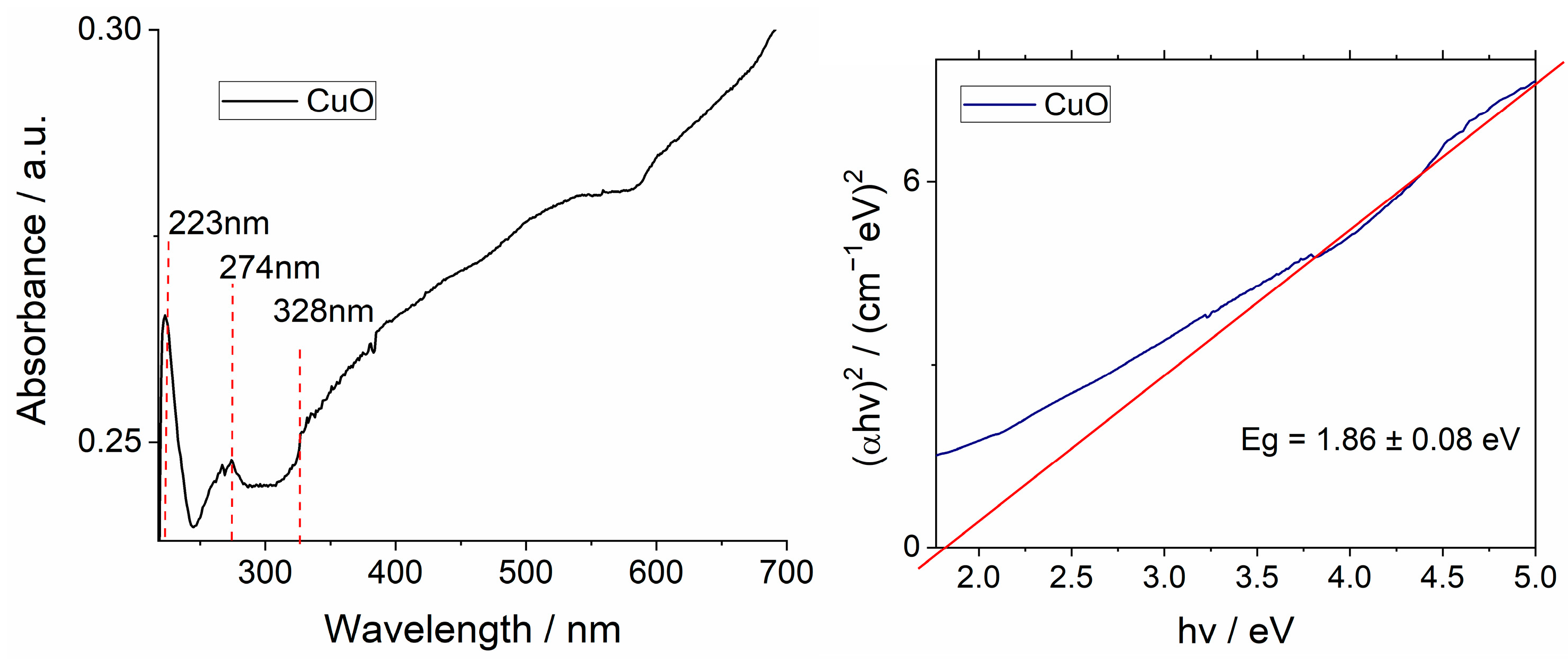
3.1.1. Ultraviolet–Visible (UV–Vis) Spectroscopy
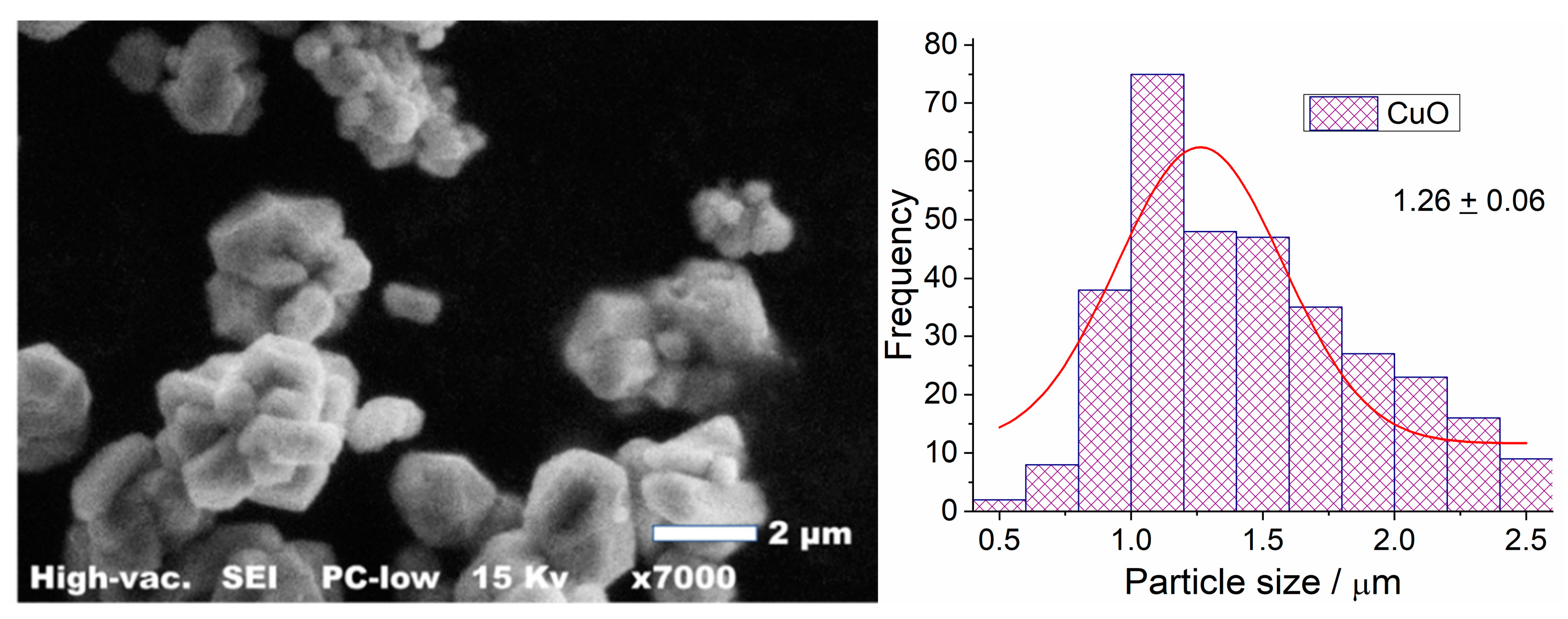
3.1.2. Scanning Electron Microscopy (SEM)
3.2. Characterization of the CuO/SiO2 Films
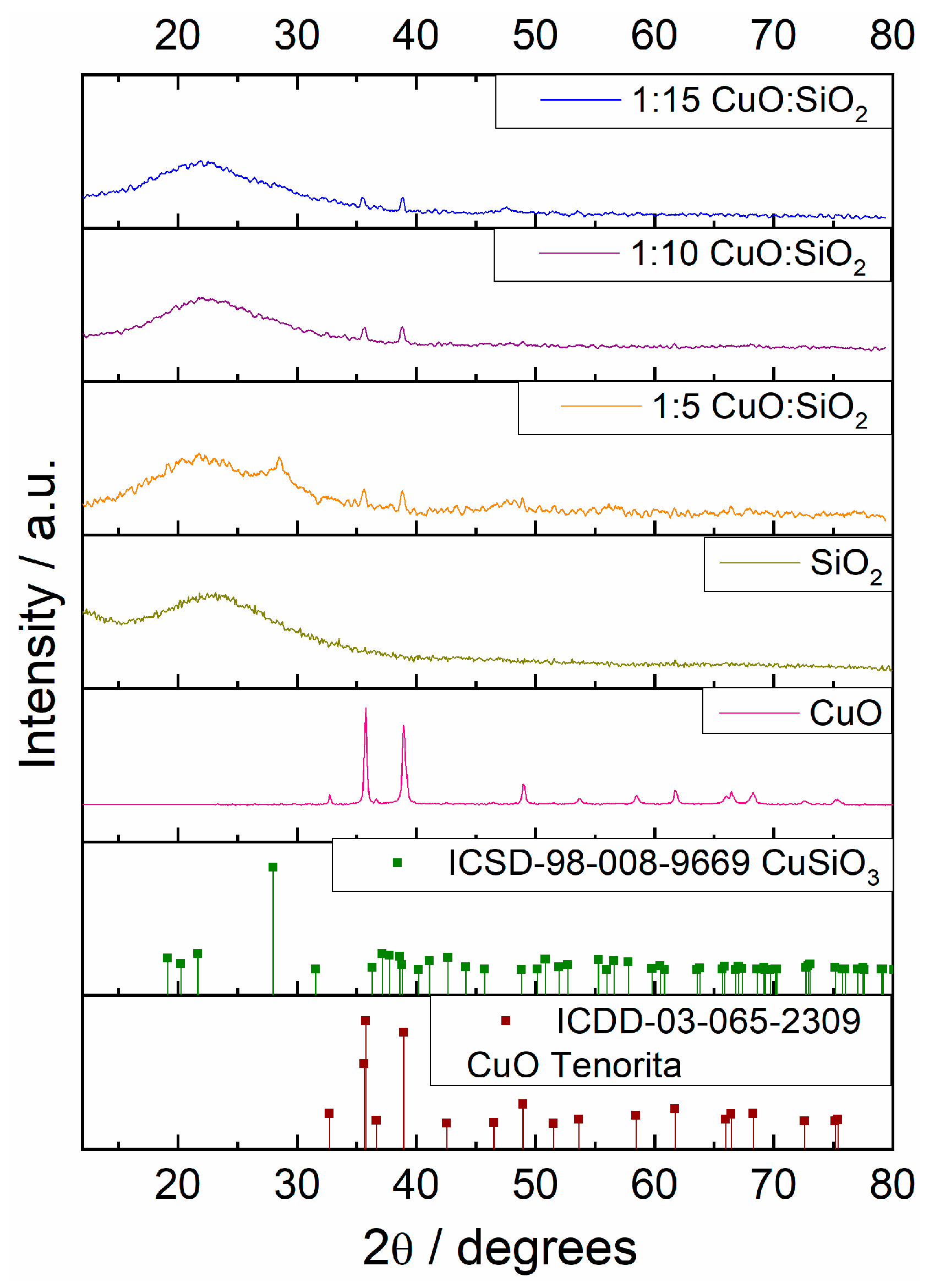
3.2.1. X-ray Diffraction
3.2.2. Fourier Transform Infrared (FTIR) Spectroscopy
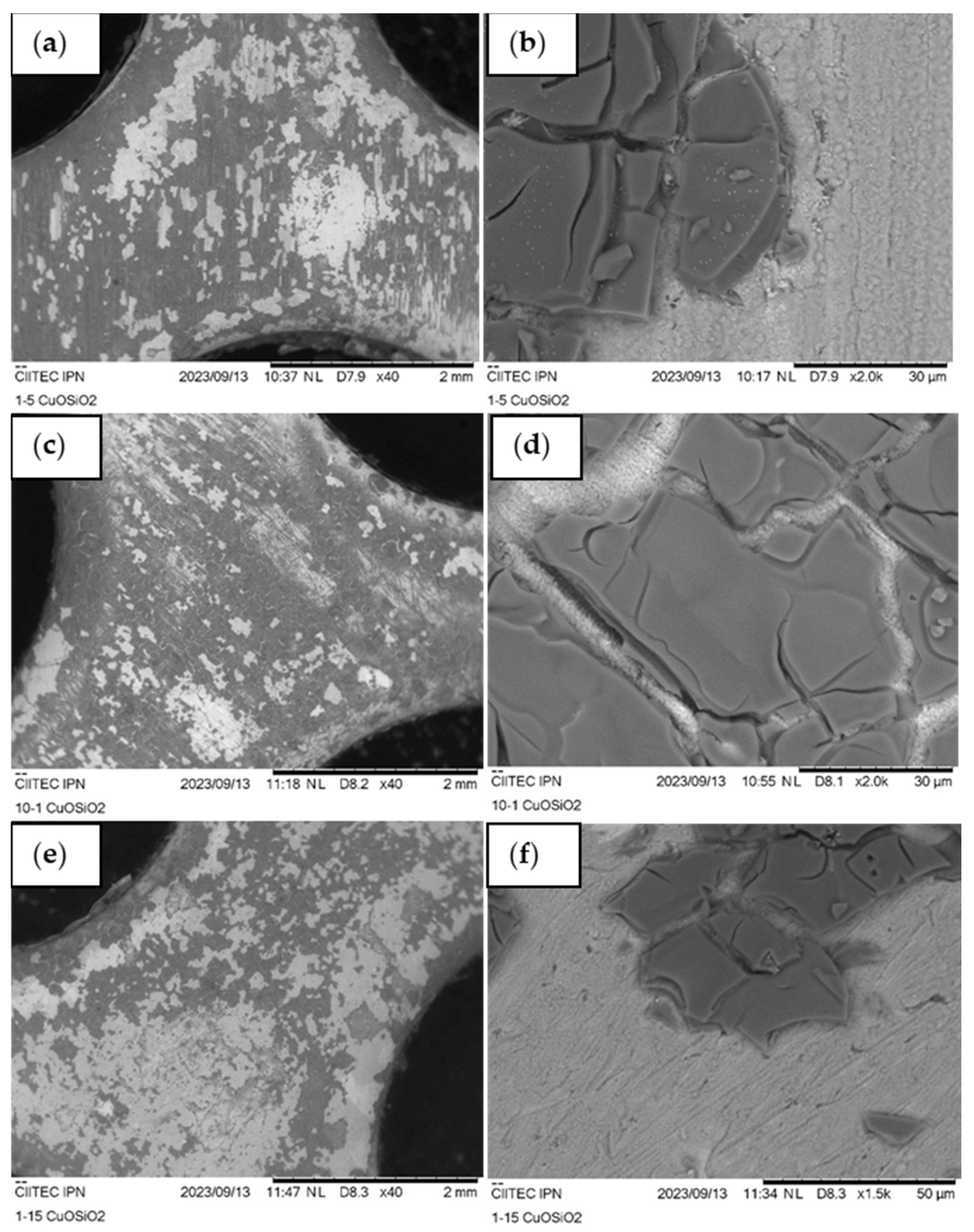
3.2.3. Scanning Electron Microscopy (SEM)
3.3. Photocatalytic Evaluation of CuO and CuO/SiO2
3.3.1. Methylene Blue (MB)
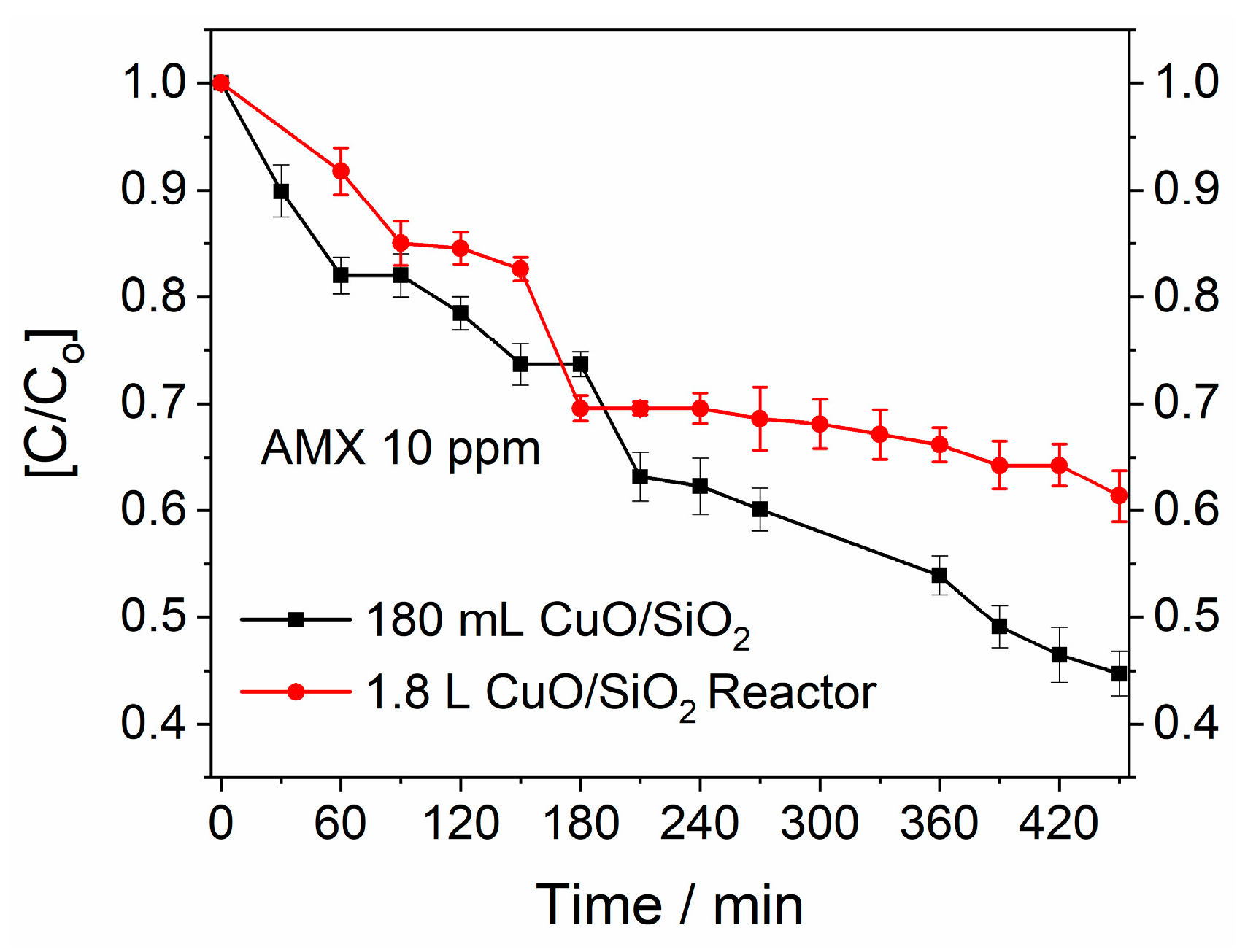
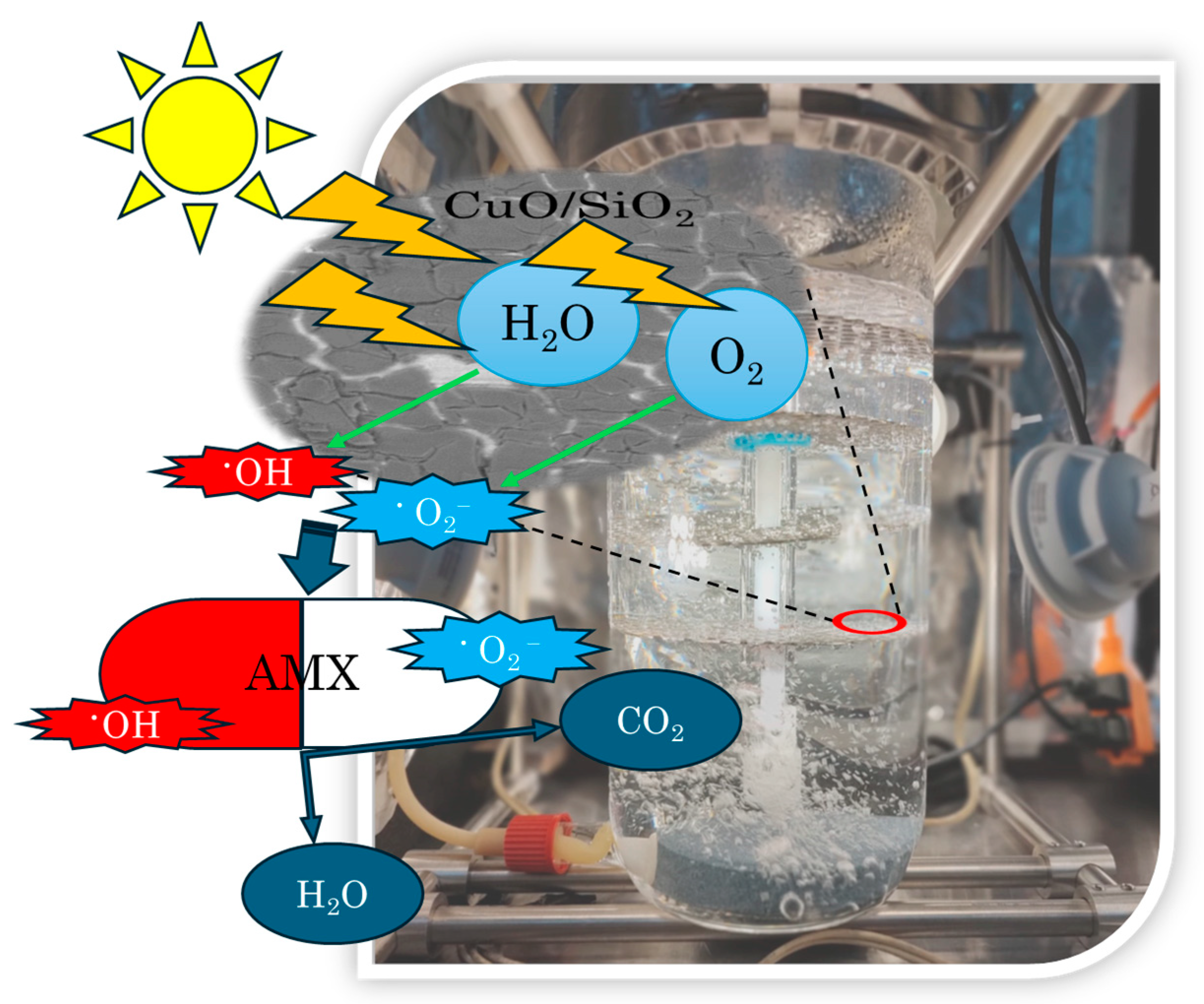
3.3.2. Amoxicillin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López-Pacheco, I.Y.; Silva-Núñez, A.; Salinas-Salazar, C.; Arévalo-Gallegos, A.; Lizarazo-Holguin, L.A.; Barceló, D.; Iqbal, H.M.N.; Parra-Saldívar, R. Anthropogenic contaminants of high concern: Existence in water resources and their adverse effects. Sci. Total Environ. 2019, 690, 1068–1088. [Google Scholar] [CrossRef] [PubMed]
- Shehu, Z.; Nyakairu, G.W.A.; Tebandeke, E.; Odume, O.N. Overview of African water resources contamination by contaminants of emerging concern. Sci. Total Environ. 2022, 852, 158303. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, H.; Li, J. Sources, distribution and potential risks of pharmaceuticals and personal care products in Qingshan Lake basin, Eastern China. Ecotoxicol. Environ. Saf. 2013, 96, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Pico, Y.; Belenguer, V.; Corcellas, C.; Diaz-Cruz, M.S.; Eljarrat, E.; Farré, M.; Gago-Ferrero, P.; Huerta, B.; Navarro-Ortega, A.; Petrovic, M.; et al. Contaminants of emerging concern in freshwater fish from four Spanish Rivers. Sci. Total Environ. 2019, 659, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Sierra, E.M.; Gálvez, E.M.; Mendoza, I.A.G.; Mendoza, M.D.G.; Galvéz, M.B. Manejo Práctico de los Antibióticos: Farmacología, Indicaciones y Dosis en Pacientes Adultos y Pediátricos; Edición Kindle; 2016.
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene blue dye: Toxicity and potential elimination technology from wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Lorenzo, P.; Adriana, A.; Jessica, S.; Carles, B.; Marinella, F.; Marta, L.; Luis, B.J.; Pierre, S. Antibiotic resistance in urban and hospital wastewaters and their impact on a receiving freshwater ecosystem. Chemosphere 2018, 206, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, L.; Liu, Z.; Yang, J.; Ma, W.; Yang, X.; Li, X.; Wang, C.; Xin, Q.; Zhao, K. A “micro-explosion” strategy for preparing membranes with high porosity, permeability, and dye/salt separation efficiency. J. Ind. Eng. Chem. 2023, 119, 516–531. [Google Scholar] [CrossRef]
- Fonseca Couto, C.; Lange, L.C.; Santos Amaral, M.C. A critical review on membrane separation processes applied to remove pharmaceutically active compounds from water and wastewater. J. Water Process Eng. 2018, 26, 156–175. [Google Scholar] [CrossRef]
- Vieira, W.T.; de Farias, M.B.; Spaolonzi, M.P.; da Silva, M.G.C.; Vieira, M.G.A. Removal of endocrine disruptors in waters by adsorption, membrane filtration and biodegradation. A review. Environ. Chem. Lett. 2020, 18, 1113–1143. [Google Scholar] [CrossRef]
- Wang, X.; Sayed, M.; Ruzimuradov, O.; Zhang, J.; Fan, Y.; Li, X.; Bai, X.; Low, J. A review of step-scheme photocatalysts. Appl. Mater. Today 2022, 29, 101609. [Google Scholar] [CrossRef]
- Pourret, O.; Bollinger, J.-C.; Hursthouse, A.; van Hullebusch, E.D. Sorption vs adsorption: The words they are a-changin’, not the phenomena. Sci. Total Environ. 2022, 838, 156545. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J.; Hameed, B.H. Removal of emerging pharmaceutical contaminants by adsorption in a fixed-bed column: A review. Ecotoxicol. Environ. Saf. 2018, 149, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yan, X.; Dai, W.; Lv, B.; Wang, W. Adsorption Properties and Mechanism of Sepiolite to Graphene Oxide in Aqueous Solution. Arab. J. Chem. 2023, 16, 104595. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, J.; Hu, S.; Zhang, G.; Lan, H.; Peng, J.; Liu, H. Evaluation of degradation performance toward antiviral drug ribavirin using advanced oxidation process and its relations to ecotoxicity evolution. Sci. Total Environ. 2022, 850, 157851. [Google Scholar] [CrossRef] [PubMed]
- Meropoulis, S.; Giannoulia, S.; Skandalis, S.; Rassias, G.; Aggelopoulos, C.A. Key-study on plasma-induced degradation of cephalosporins in water: Process optimization, assessment of degradation mechanisms and residual toxicity. Sep. Purif. Technol. 2022, 298, 121639. [Google Scholar] [CrossRef]
- Jin, D.; Lv, Y.; He, D.; Zhang, D.; Liu, Y.; Zhang, T.; Cheng, F.; Zhang, Y.; Sun, J.; Qu, J. Photocatalytic degradation of COVID-19 related drug arbidol hydrochloride by Ti3C2 MXene/supramolecular g-C3N4 Schottky junction photocatalyst. Chemosphere 2022, 308, 136461. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.L.; da Costa, C.S.D.; Silva, M.G.C.d.; Vieira, M.G.A. Overview of non-steroidal anti-inflammatory drugs degradation by advanced oxidation processes. J. Clean. Prod. 2022, 346, 131226. [Google Scholar] [CrossRef]
- Thanh Tung, M.H.; Thu Phuong, T.T.; Phuong Le Chi, N.T.; The, D.M.; Quoc, N.T.; Khan, D.T.; Pham, T.-D.; Khoa, N.V.; Thu Hien, T.T.; Dieu Cam, N.T. Novel amoxicillin degradation via photocatalysis of WO3/AgI heterojunction decorated on rGO. Ceram. Int. 2023, 49, 10881–10888. [Google Scholar] [CrossRef]
- Yahia, B.; Faouzi, S.; Ahmed, C.; Lounis, S.; Mohamed, T. A new hybrid process for Amoxicillin elimination by combination of adsorption and photocatalysis on (CuO/AC) under solar irradiation. J. Mol. Struct. 2022, 1261, 132769. [Google Scholar] [CrossRef]
- Jeyarani, W.J.; Tenkyong, T.; Bachan, N.; Kumar, D.A.; Shyla, J.M. An investigation on the tuning effect of glucose-capping on the size and bandgap of CuO nanoparticles. Adv. Powder Technol. 2016, 27, 338–346. [Google Scholar] [CrossRef]
- Ahmad, I.; Shukrullah, S.; Naz, M.Y.; Ahmed, E.; Ahmad, M.; Akhtar, M.S.; Ullah, S.; Farooq, M.U.; Iqbal, S.; Assiri, M.A.; et al. Microwave-assisted one-pot hydrothermal synthesis of V and La co-doped ZnO/CNTs nanocomposite for boosted photocatalytic hydrogen production. Int. J. Hydrogen Energy 2022, 47, 15505–15515. [Google Scholar] [CrossRef]
- Bao, X.; Lv, X.; Wang, Z.; Wang, M.; Liu, M.; Dai, D.; Zheng, L.; Zheng, Z.; Cheng, H.; Wang, P.; et al. Nitrogen vacancy enhanced photocatalytic selective oxidation of benzyl alcohol in g-C3N4. Int. J. Hydrogen Energy 2021, 46, 37782–37791. [Google Scholar] [CrossRef]
- Bahadoran, A.; Masudy-Panah, S.; De Lile, J.R.; Li, J.; Gu, J.; Sadeghi, B.; Ramakrishna, S.; Liu, Q. Novel 0D/1D ZnBi2O4/ZnO S-scheme photocatalyst for hydrogen production and BPA removal. Int. J. Hydrogen Energy 2021, 46, 24094–24106. [Google Scholar] [CrossRef]
- Yu, Q.; Ma, X.; Wang, M.; Yu, C.; Bai, T. Influence of embedded particles on microstructure, corrosion resistance and thermal conductivity of CuO/SiO2 and NiO/SiO2 nanocomposite coatings. Appl. Surf. Sci. 2008, 254, 5089–5094. [Google Scholar] [CrossRef]
- Venkadesh, A.; Mathiyarasu, J.; Radhakrishnan, S. A highly uniform CuO@SiO2 porous sphere with improved electrochemical sensing performance for the accurate determination of vanillin in food samples. Mater. Today Chem. 2021, 22, 100554. [Google Scholar] [CrossRef]
- Zarzuela, R.; Almoraima Gil, M.L.; Carretero, J.; Carbú, M.; Cantoral, J.M.; Mosquera, M.J. Development of a novel engineered stone containing a CuO/SiO2 nanocomposite matrix with biocidal properties. Constr. Build. Mater. 2021, 303, 124459. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S. Construction of an efficient and durable hierarchical porous CuO/SiO2 monolith for synergistically boosting the visible-light-driven degradation of organic pollutants. Sep. Purif. Technol. 2021, 279, 119759. [Google Scholar] [CrossRef]
- Nogueira, A.C.; Gomes, L.E.; Ferencz, J.A.P.; Rodrigues, J.E.F.S.; Gonçalves, R.V.; Wender, H. Improved Visible Light Photoactivity of CuBi2O4/CuO Heterojunctions for Photodegradation of Methylene Blue and Metronidazole. J. Phys. Chem. C 2019, 123, 25680–25690. [Google Scholar] [CrossRef]
- Latif, A.; Memon, A.M.; Gadhi, T.A.; Bhurt, I.A.; Channa, N.; Mahar, R.B.; Ali, I.; Chiadò, A.; Bonelli, B. Bi2O3 immobilized 3D structured clay filters for solar photocatalytic treatment of wastewater from batch to scaleup reactors. Mater. Chem. Phys. 2022, 276, 125297. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Li, Y.; Fan, T.; Wu, Z. A facile construction strategy of SiO2-modified anodic film on 304 stainless steel by introducing TEOS into fluoride-based ethylene glycol electrolyte. Mater. Today Commun. 2023, 36, 106711. [Google Scholar] [CrossRef]
- Subasri, R.; Malathi, R.; Jyothirmayi, A.; Hebalkar, N.Y. Synthesis and characterization of CuO-hybrid silica nanocomposite coatings on SS 304. Ceram. Int. 2012, 38, 5731–5740. [Google Scholar] [CrossRef]
- Arige, S.; Mishra, V.; Miryala, M.; Rao, M.S.R.; Dixit, T. Plasmon-coupled sub-bandgap photoluminescence enhancement in ultra-wide bandgap CuO through hot-hole transfer. Opt. Mater. 2022, 134, 113149. [Google Scholar] [CrossRef]
- Hanif, M.A.; Lee, I.; Akter, J.; Islam, M.A.; Zahid, A.A.S.M.; Sapkota, K.P.; Hahn, J.R. Enhanced Photocatalytic and Antibacterial Performance of ZnO Nanoparticles Prepared by an Efficient Thermolysis Method. Catalysts 2019, 9, 608. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Chen, Y.-S.; Lin, S.-F.; Chen, Y.-Y.; Hung, W.-Y.; Lin, H.-P.; Tang, C.-Y.; Lin, C.-Y.; Hsu, C.-H. Transformation of Cu(OH)2 to mesostructural copper-silicate in alkaline silicate solution. Mater. Today Sustain. 2023, 24, 100535. [Google Scholar] [CrossRef]
- Cao, G.; Guo, M.; Yang, F.; Xu, H.; Shao, G.; Hu, J. The effect of nanometre-scale kinetic competition on the phase selection in Zr/Si superstructure. Mater. Charact. 2024, 208, 113666. [Google Scholar] [CrossRef]
- Cao, G.; Chen, C.; Xu, H.; Ban, J.; Liu, F.; Yuan, G.; Lei, H.; Su, Y.; Hu, J. Chemical regulation in the bonding reconstruction stage of amorphous Zr-Si oxidation and the resultant phase selection. Mater. Des. 2023, 233, 112291. [Google Scholar] [CrossRef]
- Shi, B.; Xie, L.; Ma, B.; Zhou, Z.; Xu, B.; Qu, L. Preparation and Properties of Highly Transparent SiO2 Aerogels for Thermal Insulation. Gels 2022, 8, 744. [Google Scholar] [CrossRef] [PubMed]
- Zawada, A.; Lubas, M.; Przerada, I. Application of Mössbauer spectroscopy and FT-IR to describe coordination of amphoteric ions in structure of glasses from the SiO2–Na2O–MgO–CaO–Al2O3–Fe2O3 system. J. Mol. Struct. 2023, 1285, 135368. [Google Scholar] [CrossRef]
- Akter, J.; Sapkota, K.P.; Hanif, M.A.; Islam, M.A.; Abbas, H.G.; Hahn, J.R. Kinetically controlled selective synthesis of Cu2O and CuO nanoparticles toward enhanced degradation of methylene blue using ultraviolet and sun light. Mater. Sci. Semicond. Process. 2021, 123, 105570. [Google Scholar] [CrossRef]
- Raul, P.K.; Senapati, S.; Sahoo, A.K.; Umlong, I.M.; Devi, R.R.; Thakur, A.J.; Veer, V. CuO nanorods: A potential and efficient adsorbent in water purification. RSC Adv. 2014, 4, 40580–40587. [Google Scholar] [CrossRef]
- Mohan, M.; Thangavel, K.; Balaprakash, V. Characterization of CZO thin films prepared by Sol-Gel dip coating technique with varied concentrations and annealed at different temperatures. Chem. Phys. 2023, 573, 111981. [Google Scholar] [CrossRef]
- Mohd Yusoff, M.F.; Abdul Kadir, M.R.; Iqbal, N.; Hassan, M.A.; Hussain, R. Dipcoating of poly (ε-caprolactone)/hydroxyapatite composite coating on Ti6Al4V for enhanced corrosion protection. Surf. Coat. Technol. 2014, 245, 102–107. [Google Scholar] [CrossRef]
- Patil, D.; Manjanna, J.; Chikkamath, S.; Uppar, V.; Chougala, M. Facile synthesis of stable Cu and CuO particles for 4-nitrophenol reduction, methylene blue photodegradation and antibacterial activity. J. Hazard. Mater. Adv. 2021, 4, 100032. [Google Scholar] [CrossRef]
- Riaz, R.; Bibi, I.; Majid, F.; Kamal, S.; Al Huwayz, M.; Jilani, K.; Ghafoor, A.; Raza, Q.; Alwadai, N.; Iqbal, M. NiFe2O4/CuO heterostructures optical, magnetic and photocatalytic properties: Methylene blue dye degradation under solar light irradiation. J. Mol. Struct. 2024, 1309, 138174. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, M.; Khan, S.; Luque, R.; Abualnaja, K.M.; Alduaij, O.K.; Yousef, T.A. Bio-Construction of CuO Nanoparticles Using Texas Sage Plant Extract for catalytical degradation of Methylene blue Via Photocatalysis. J. Mol. Struct. 2022, 1256, 132522. [Google Scholar] [CrossRef]
- Manassero, A.; Satuf, M.L.; Alfano, O.M. Photocatalytic reactors with suspended and immobilized TiO2: Comparative efficiency evaluation. Chem. Eng. J. 2017, 326, 29–36. [Google Scholar] [CrossRef]
- Althamthami, M.; EIhachmi, G.T.; Ben Temam, H.; Hasan, G.G.; Rahmane, S.; Gasmi, B. Effect of Different Cu:Co Film Concentrations on Photocatalytic Reactions of Ethanol, MB, AMX, and Cr(VI): A Study of Film Properties & Effects of Photoxidation. J. Environ. Chem. Eng. 2023, 11, 111247. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Li, X.-D.; Wang, N.; Liu, Y.-J.; Tian, F.; Wang, C.-X. Robust superhydrophobic SiO2/epoxy composite coating prepared by one-step spraying method for corrosion protection of aluminum alloy: Experimental and theoretical studies. Mater. Des. 2023, 228, 111833. [Google Scholar] [CrossRef]
- Xijing, L.; Yong, C. Effect of SiO2 nanoparticles on the hardness and corrosion resistance of NiW/SiO2 nano composite coating prepared by electrodeposition. Int. J. Electrochem. Sci. 2023, 18, 100138. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Chen, C.; Tian, Y.; Wang, Y.; Cao, S.; Ma, J. Corrosion-resistant SiO2-graphene oxide/epoxy coating reinforced by effective electron beam curing. Prog. Org. Coat. 2023, 184, 107855. [Google Scholar] [CrossRef]
- He, L.; Chen, X.; Ma, J.; He, H.; Wang, W. Characterization and catalytic performance of sol–gel derived Cu/SiO2 catalysts for hydrogenolysis of diethyl oxalate to ethylene glycol. J. Sol-Gel Sci. Technol. 2010, 55, 285–292. [Google Scholar] [CrossRef]
- Vezzoli, M.; Farrell, T.; Baker, A.; Psaltis, S.; Martens, W.N.; Bell, J.M. Optimal catalyst thickness in titanium dioxide fixed film reactors: Mathematical modelling and experimental validation. Chem. Eng. J. 2013, 234, 57–65. [Google Scholar] [CrossRef]
- Tapia-tlatelpa, T.; Buscio, V.; Trull, J.; Sala, V. Performance analysis and methodology for replacing conventional lamps by optimized LED arrays for photocatalytic processes. Chem. Eng. Res. Des. 2020, 156, 456–468. [Google Scholar] [CrossRef]
- Aksu Demirezen, D.; Yıldız, Y.Ş.; Demirezen Yılmaz, D. Amoxicillin degradation using green synthesized iron oxide nanoparticles: Kinetics and mechanism analysis. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100219. [Google Scholar] [CrossRef]
- Sun, X.; He, K.; Chen, Z.; Yuan, H.; Guo, F.; Shi, W. Construction of visible-light-response photocatalysis-self-Fenton system for the efficient degradation of amoxicillin based on industrial waste red mud/CdS S-scheme heterojunction. Sep. Purif. Technol. 2023, 324, 124600. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Reyes, A.; Lijanova, I.V.; Garrido-Hernández, A.; Morales-Ramirez, Á.d.J.; Hernández-Fuentes, C.; Calvillo-Muñoz, E.Y.; Likhanova, N.V.; Olivares-Xometl, O. Coating on Steel Discs with a Photocatalytic System CuO/SiO2 for the Degradation of the Ubiquitous Contaminants Methylene Blue and Amoxicillin. Coatings 2024, 14, 523. https://doi.org/10.3390/coatings14050523
Hernández-Reyes A, Lijanova IV, Garrido-Hernández A, Morales-Ramirez ÁdJ, Hernández-Fuentes C, Calvillo-Muñoz EY, Likhanova NV, Olivares-Xometl O. Coating on Steel Discs with a Photocatalytic System CuO/SiO2 for the Degradation of the Ubiquitous Contaminants Methylene Blue and Amoxicillin. Coatings. 2024; 14(5):523. https://doi.org/10.3390/coatings14050523
Chicago/Turabian StyleHernández-Reyes, Alberto, Irina V. Lijanova, Aristeo Garrido-Hernández, Ángel de J. Morales-Ramirez, Carlos Hernández-Fuentes, Evelyn Y. Calvillo-Muñoz, Natalya V. Likhanova, and Octavio Olivares-Xometl. 2024. "Coating on Steel Discs with a Photocatalytic System CuO/SiO2 for the Degradation of the Ubiquitous Contaminants Methylene Blue and Amoxicillin" Coatings 14, no. 5: 523. https://doi.org/10.3390/coatings14050523




