Anticorrosion Method Combining Impressed Current Cathodic Protection and Coatings in Marine Atmospheric Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Specimens
2.2. Marine Atmospheric Exposure Site
2.3. Electrochemical Characterization
3. Results and Discussion
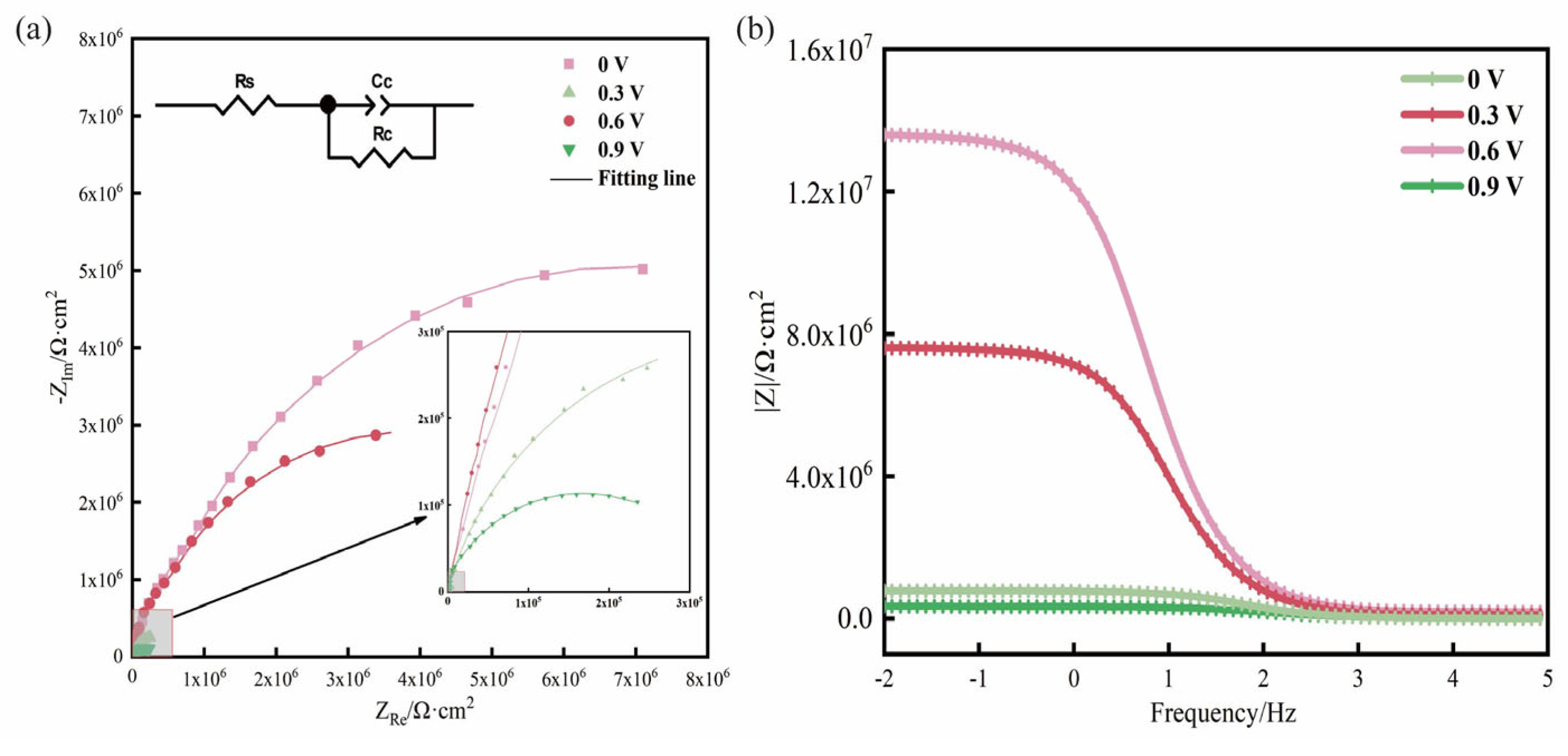
3.1. Determination of the Optimum Protection Potential
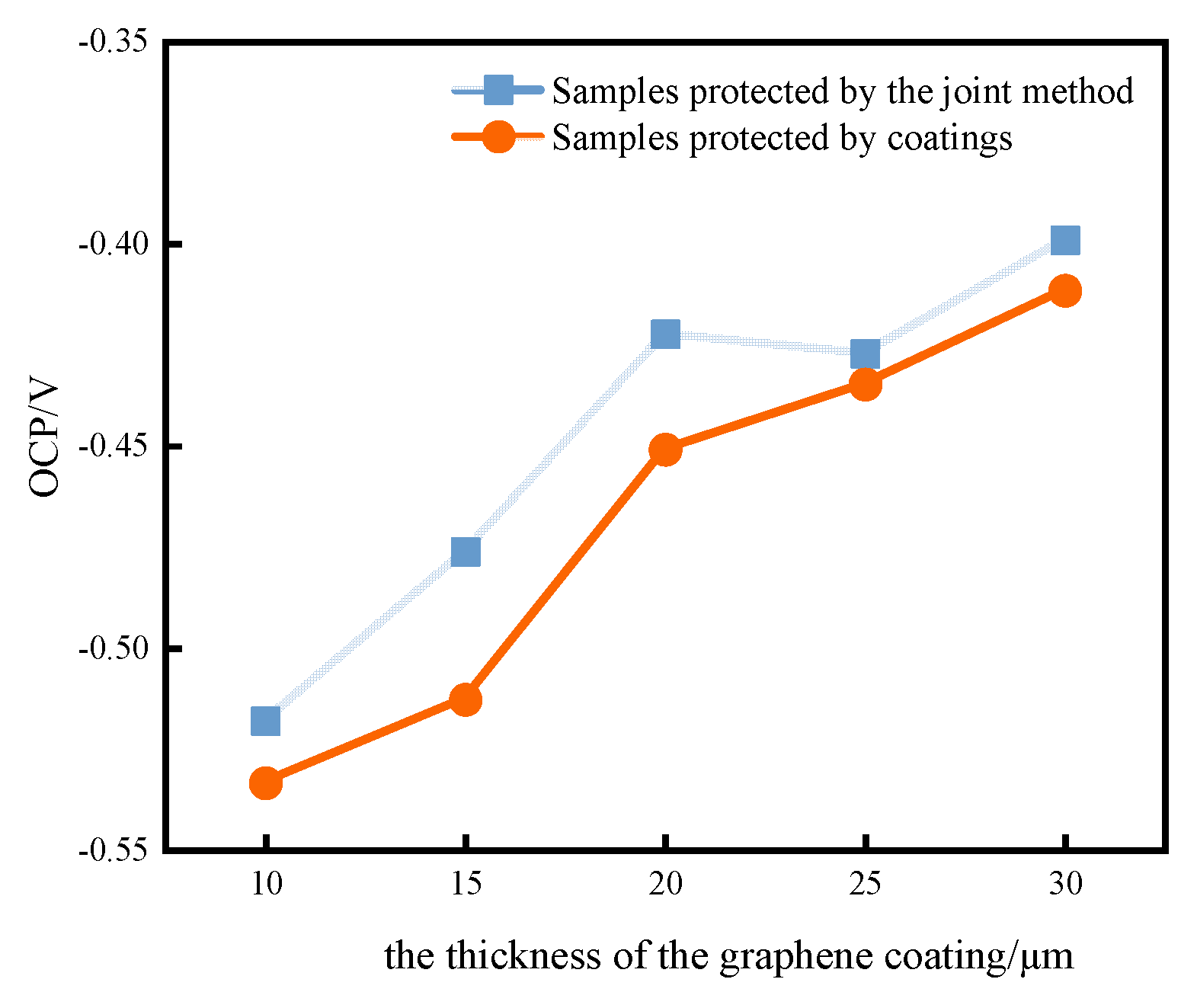
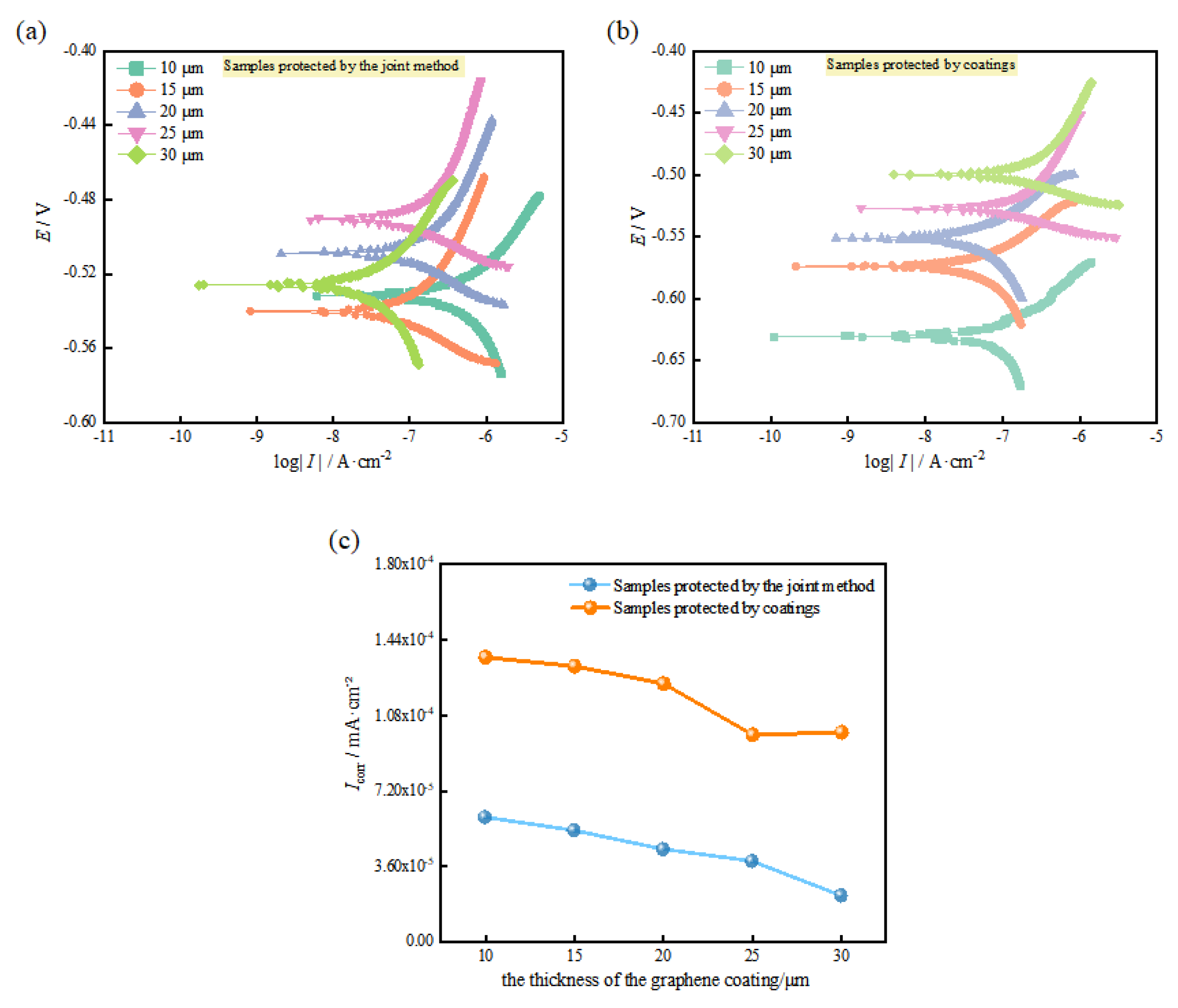
3.2. Determination of the Optimal Graphene Coating Thickness
4. Conclusions
- (1)
- In this paper, a novel corrosion prevention method that effectively combines the advantages of coatings and ICCP (impressed current cathodic protection) technology is proposed. The method constructs a sandwich composite coating system. The microscopic electrolyte membrane layer provides a pathway between the metal substrate and the conductive graphene coating, allowing ICCP to improve the corrosion protection of equipment in the marine atmospheric environment.
- (2)
- Wherever the coatings are stripped before and after, the scratched sample with an impressed voltage of 0.6 V has the least corrosion, while the samples with impressed voltages of 0 V and 0.9 V have the most severe corrosion. The graphene conductive coating serving as the auxiliary anode suffers to an extent with increasing impressed voltage. However, the resistance of the scratch-free sample with an impressed voltage of 0.6 V is similar to the sample without an impressed voltage, and the protection effect is the greatest. The results show that 0.6 V can effectively protect the carbon steel substrate for a long time.
- (3)
- As the thickness of the graphene coating increases, it becomes increasingly difficult for the corrosion products to reach the metal substrate; thus, the open-circuit potentials shift in the positive direction. Moreover, the scratched samples with impressed voltages have higher open-circuit potentials and lower corrosion current densities than those without impressed voltages. This further confirms that the method provides excellent corrosion protection. Considering the efficiency of corrosion prevention methods and the cost of application, the protective characteristics of the combined anticorrosion system are ensured by the optimal thickness of the graphene coating of 20 μm.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Zhang, D.; Liu, Z.; Li, Z.; Du, C.; Dong, C. Materials science: Share corrosion data. Nature 2015, 527, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.; Kolekar, A.; Bhopale, A.; Kalendova, A.; Kohl, M. ICCP of mild steel in association with zinc based paint coating. Mater. Today Proc. 2022, 50, 1660–1665. [Google Scholar] [CrossRef]
- Wu, W.; Zeng, Z.; Cheng, X.; Li, X.; Liu, B. Atmospheric corrosion behavior and mechanism of a Ni-Advanced weathering steel in simulated tropical marine environment. J. Mater. Eng. Perform. 2017, 26, 6075–6086. [Google Scholar] [CrossRef]
- Price, J.S.; Figueira, B.R.; Soucek, D.M. Corrosion protection systems and fatigue corrosion in offshore wind structures: Current status and future perspectives. Coatings 2017, 7, 25. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, W.; Wang, L.; Wang, J.; Wang, S.; Liu, G. A mechanically and chemically stable superhydrophobic coating for preventing marine atmospheric corrosion. Surf. Interfaces 2021, 27, 101537. [Google Scholar] [CrossRef]
- Mitolo, M.; Pettinger, A. Interactions between cathodically protected pipelines and grounding systems. IEEE Trans. Ind. Appl. 2016, 52, 3694–3698. [Google Scholar] [CrossRef]
- Guo, B.; Qiao, G.; Li, D.; Ou, J. Multi-species reactive transport modeling of electrochemical corrosion control in saturated concrete structures including electrode reactions and thermodynamic equilibrium. Constr. Build. Mater. 2021, 278, 122228. [Google Scholar] [CrossRef]
- Feng, R.; Wang, J.; Zhu, J.; Dong, Z. Fatigue behavior of corroded reinforced concrete continuous beams with multi-intervention system. Eng. Struct. 2021, 231, 111748. [Google Scholar] [CrossRef]
- Anwar, S.M.; Sujitha, B.; Vedalakshmi, R. Light-weight cementitious conductive anode for ICCP of steel reinforced concrete application. Constr. Build. Mater. 2014, 71, 167–180. [Google Scholar] [CrossRef]
- Refait, P.; Jeannin, M.; Sabot, R.; Antony, H.; Pineau, S. Corrosion and cathodic protection of carbon steel in the tidal zone: Products, mechanisms and kinetics. Corros. Sci. 2015, 90, 375–382. [Google Scholar] [CrossRef]
- Ge, D.; Zhang, Z.; Zhang, C.; Xu, N. Novel technique of impressed current cathodic protection in atmospheric environments. Br. Corros. J. 2013, 24, 265–268. [Google Scholar]
- Ashok, S.S.K.; Shahid, B.; Ramesh, K.; Ramesh, S. New perspectives on graphene/graphene oxide based polymer nanocomposites for corrosion applications: The relevance of the graphene/polymer barrier coatings. Prog. Org. Coat. 2021, 154, 106215. [Google Scholar]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; Van Der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable atomic membranes from graphene sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Berry, V. Impermeability of graphene and its applications. Carbon 2013, 62, 1–10. [Google Scholar] [CrossRef]
- Papageorgiou, G.D.; Kinloch, A.I.; Young, J.R. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Yao, W.; Wu, J.; Xiang, J.; Dai, X.; Wu, T.; Yuan, Y.; Wang, J.; Jiang, B.; et al. Development of metal-organic framework (MOF) decorated graphene oxide/MgAl-layered double hydroxide coating via microstructural optimization for anticorrosion micro-arc oxidation coatings of magnesium alloy. J. Mater. Sci. Technol. 2022, 130, 12–26. [Google Scholar] [CrossRef]
- Aidin, B.K.; Benyamin, Y.; Masoud, M. Effect of ZnO pore-sealing layer on anticorrosion and in-vitro bioactivity behavior of plasma electrolytic oxidized AZ91 magnesium alloy. Mater. Lett. 2020, 258, 126779. [Google Scholar]
- Yang, Z.; Che, J.; Zhang, Z.; Yu, L.; Hu, M.; Sun, W.; Gao, W.; Fan, J.; Wang, L.; Liu, G. High-efficiency graphene/epoxy composite coatings with outstanding thermal conductive and anticorrosion performance. Compos. Part A 2024, 181, 108152. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Zhang, F.; Ju, P.; Pan, M.; Zhang, D.; Huang, Y.; Li, G.; Li, X. Self-healing mechanisms in smart protective coatings: A review. Corros. Sci. 2018, 144, 74–88. [Google Scholar] [CrossRef]
- Quezada-Renteria, A.J.; Chazaro-Ruiz, F.L.; Rangel-Mendez, R.J. Poorly conductive electrochemically reduced graphene oxide films modified with alkyne chains to avoid the corrosion-promoting effect of graphene-based materials on carbon steel. Carbon 2020, 167, 512–522. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, X.; Yang, Q.; Cui, G.; Li, Z. The role of graphene in anticorrosion coatings: A review. Constr. Build. Mater. 2021, 294, 123613. [Google Scholar] [CrossRef]
- Zhou, S.; Yao, W.; Wang, Z.; Ma, L.; Lu, Z.; Hou, C. The first-principles calculations to explore the mechanism of oxygen diffusion on vacancy defective graphene in marine environment. Appl. Surf. Sci. 2020, 525, 136585. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Chen, Y.; Song, X.; Yang, Y. Effect of a DC Stray Current on the corrosion of X80 pipeline steel and the cathodic disbondment behavior of the protective 3PE coating in 3.5% NaCl solution. Coatings 2019, 9, 29. [Google Scholar] [CrossRef]

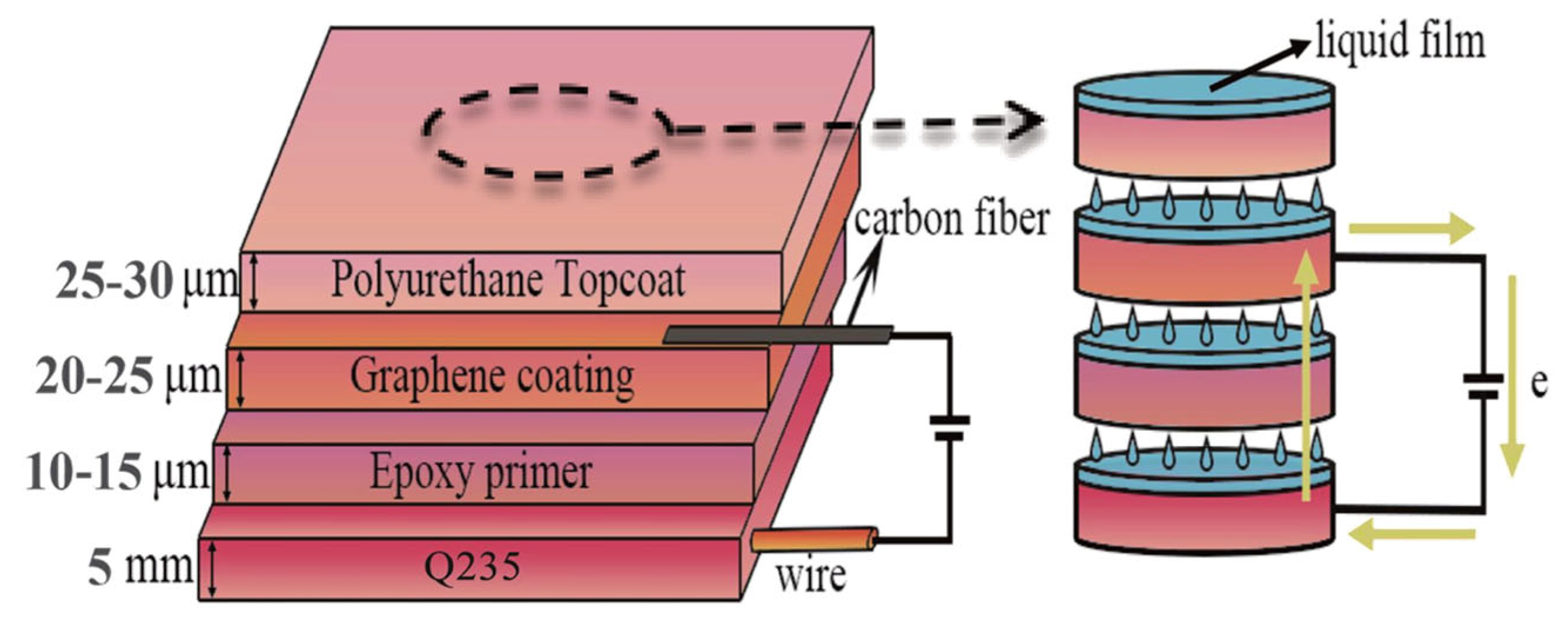


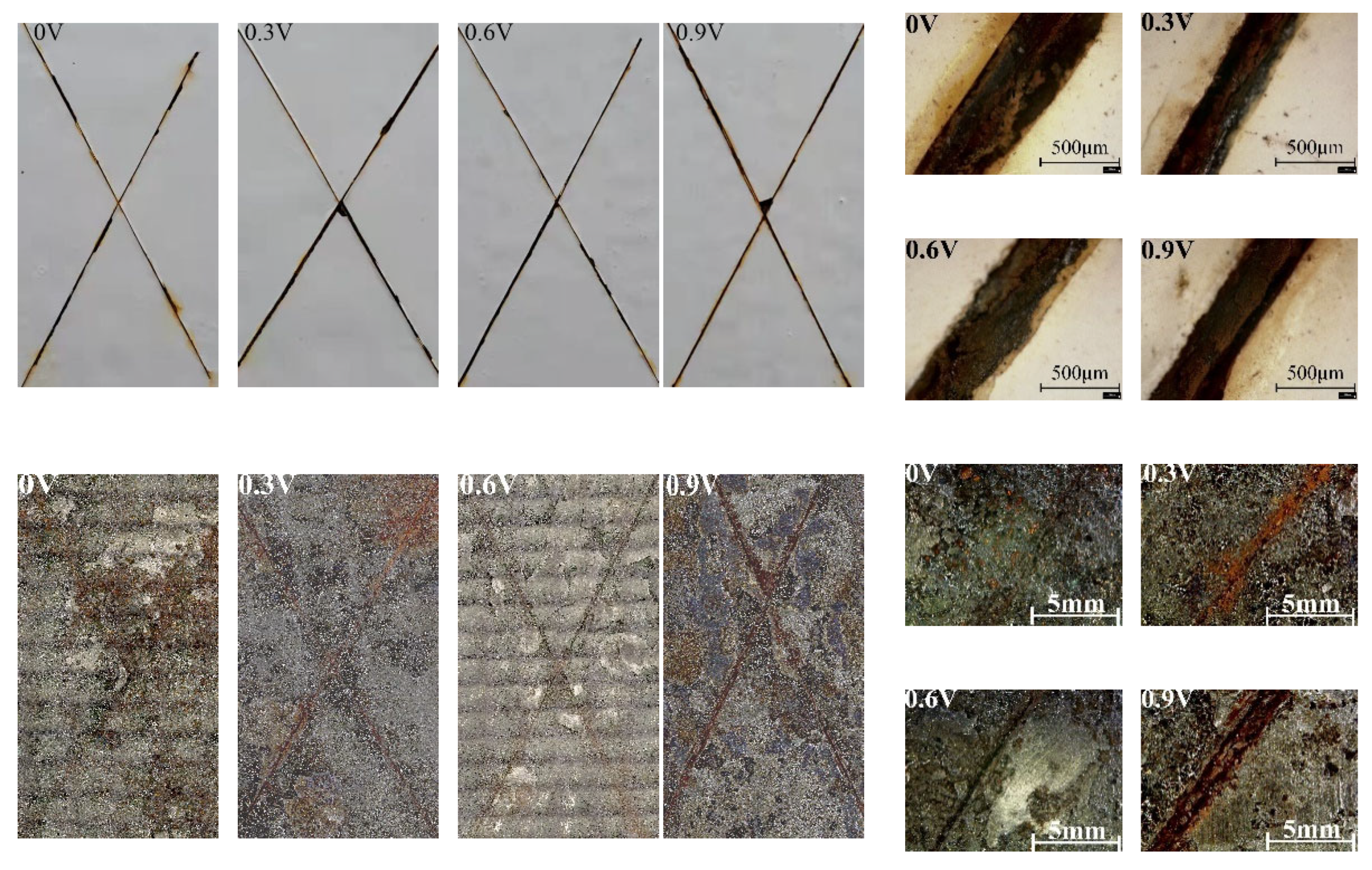
| Element wt (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Material | C | Si | Mn | P | S | Cr | Ni | Cu |
| Q235 | 0.15 | 0.19 | 0.5 | 0.023 | 0.015 | ≤0.3 | ≤0.3 | ≤0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, P.; Shangguan, J.; Hu, J.; Huang, H.; Zhou, L. Anticorrosion Method Combining Impressed Current Cathodic Protection and Coatings in Marine Atmospheric Environment. Coatings 2024, 14, 524. https://doi.org/10.3390/coatings14050524
Deng P, Shangguan J, Hu J, Huang H, Zhou L. Anticorrosion Method Combining Impressed Current Cathodic Protection and Coatings in Marine Atmospheric Environment. Coatings. 2024; 14(5):524. https://doi.org/10.3390/coatings14050524
Chicago/Turabian StyleDeng, Peichang, Juyu Shangguan, Jiezhen Hu, Huan Huang, and Lingbo Zhou. 2024. "Anticorrosion Method Combining Impressed Current Cathodic Protection and Coatings in Marine Atmospheric Environment" Coatings 14, no. 5: 524. https://doi.org/10.3390/coatings14050524


.jpg)



