Fabrication of Nanostructures Consisting of Composite Nanoparticles by Open-Air PLD
Abstract
:1. Introduction
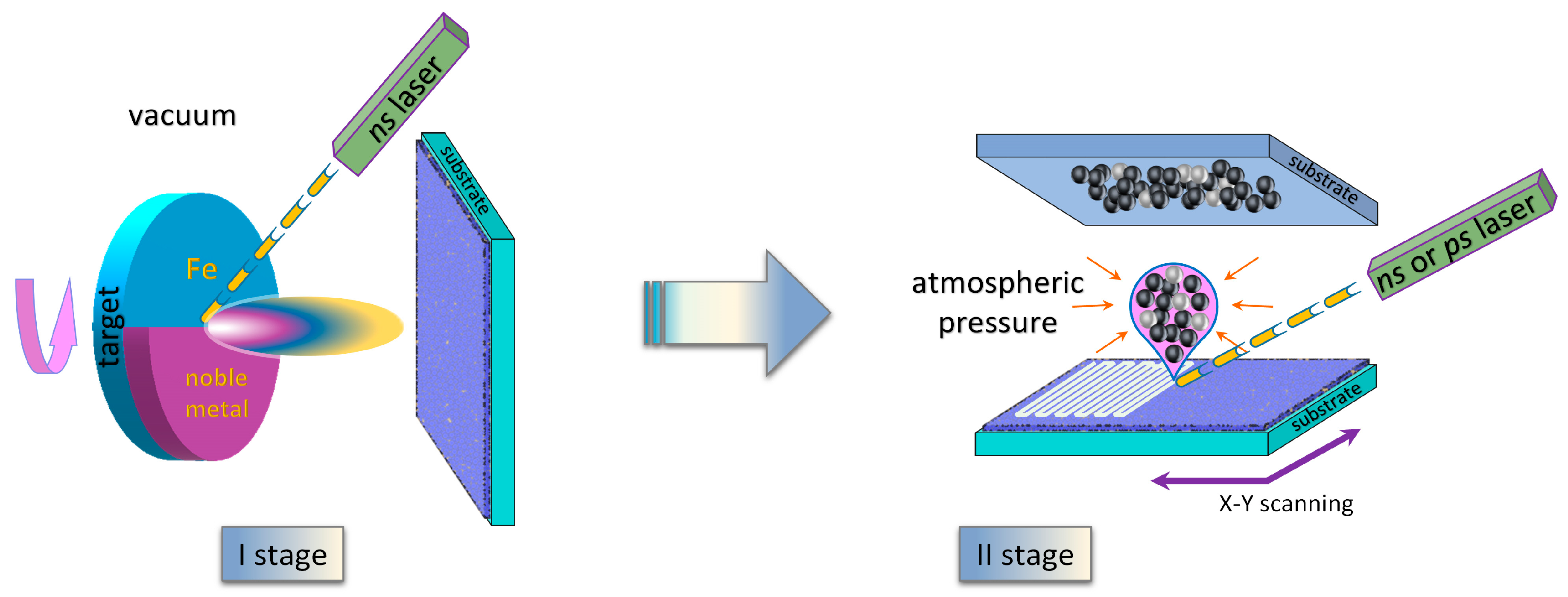
2. Materials and Methods
2.1. Experimental
2.2. Theoretical
2.3. Sample Characterization
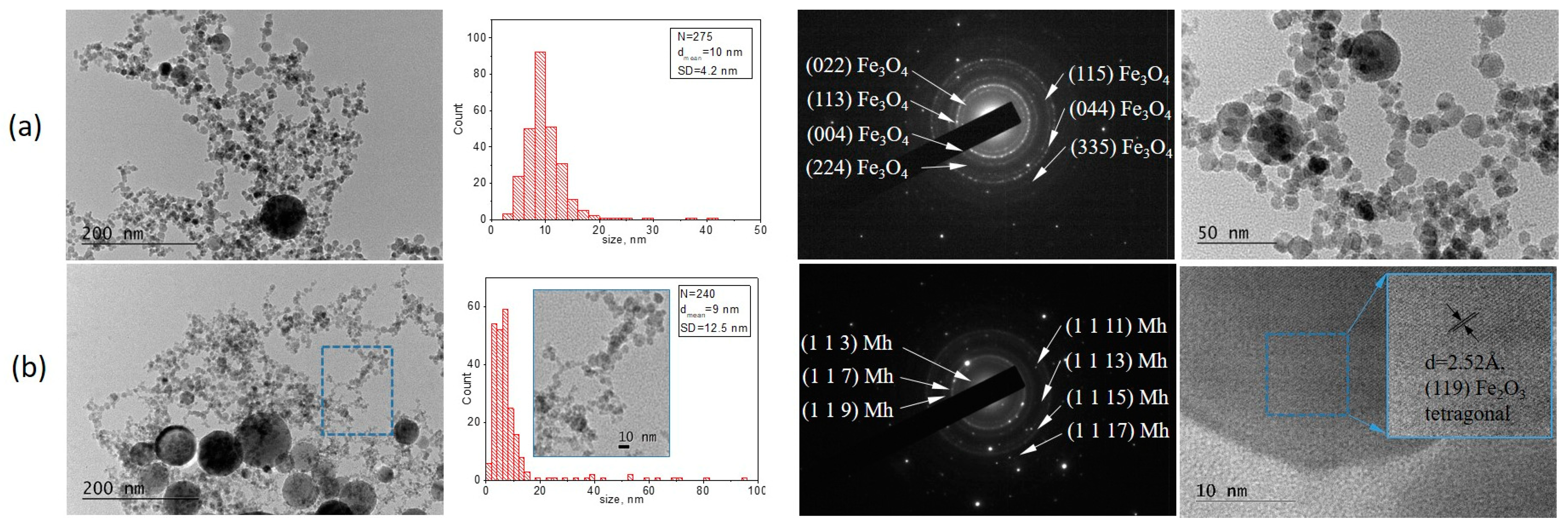
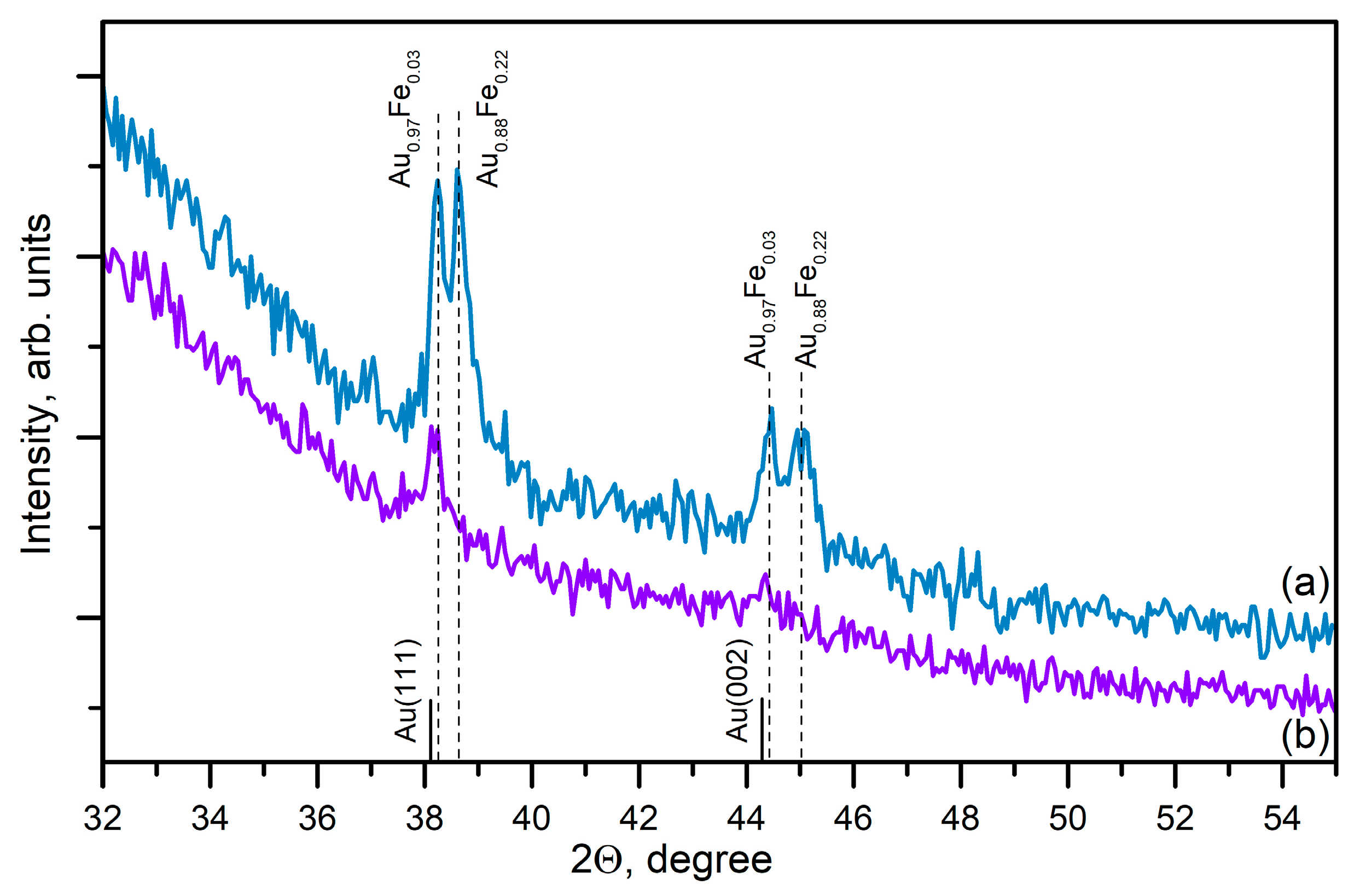
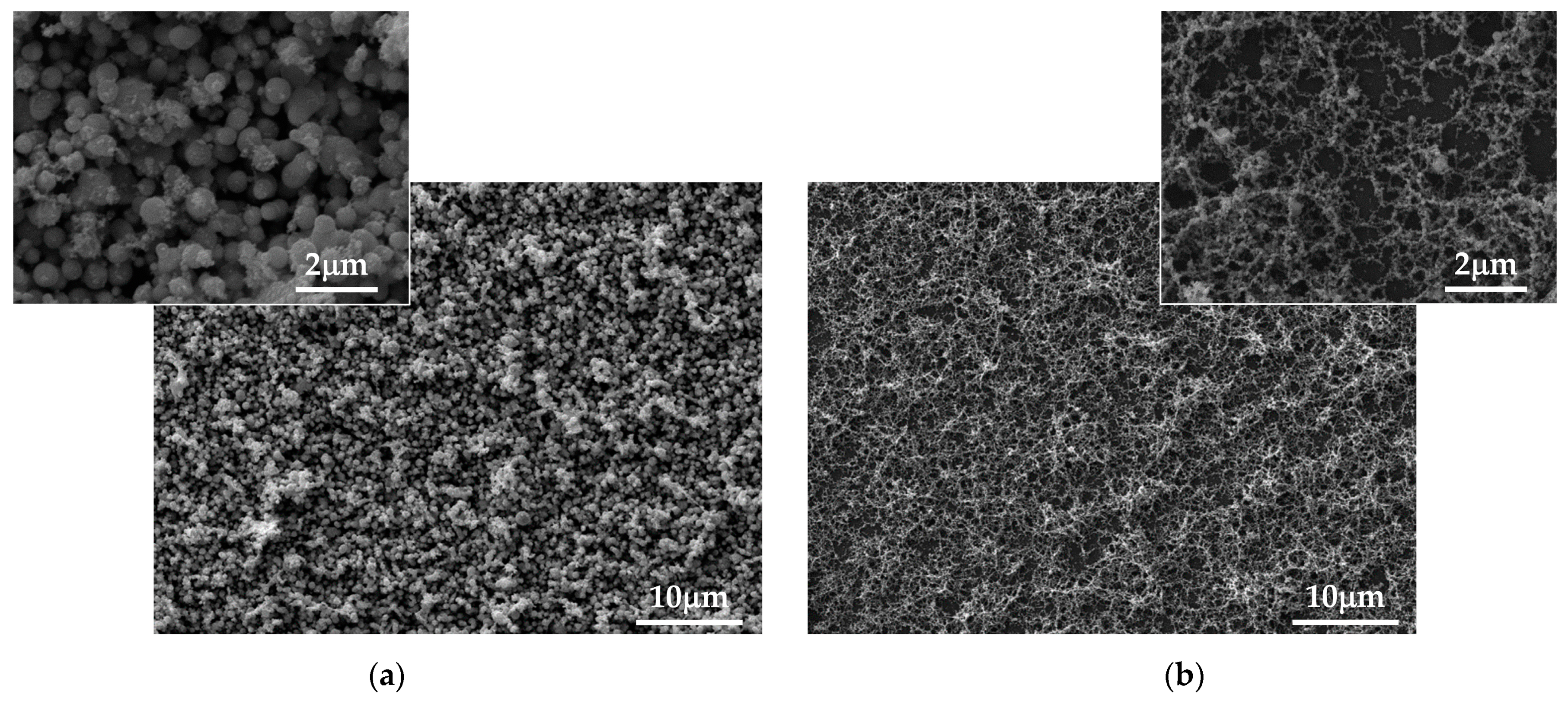
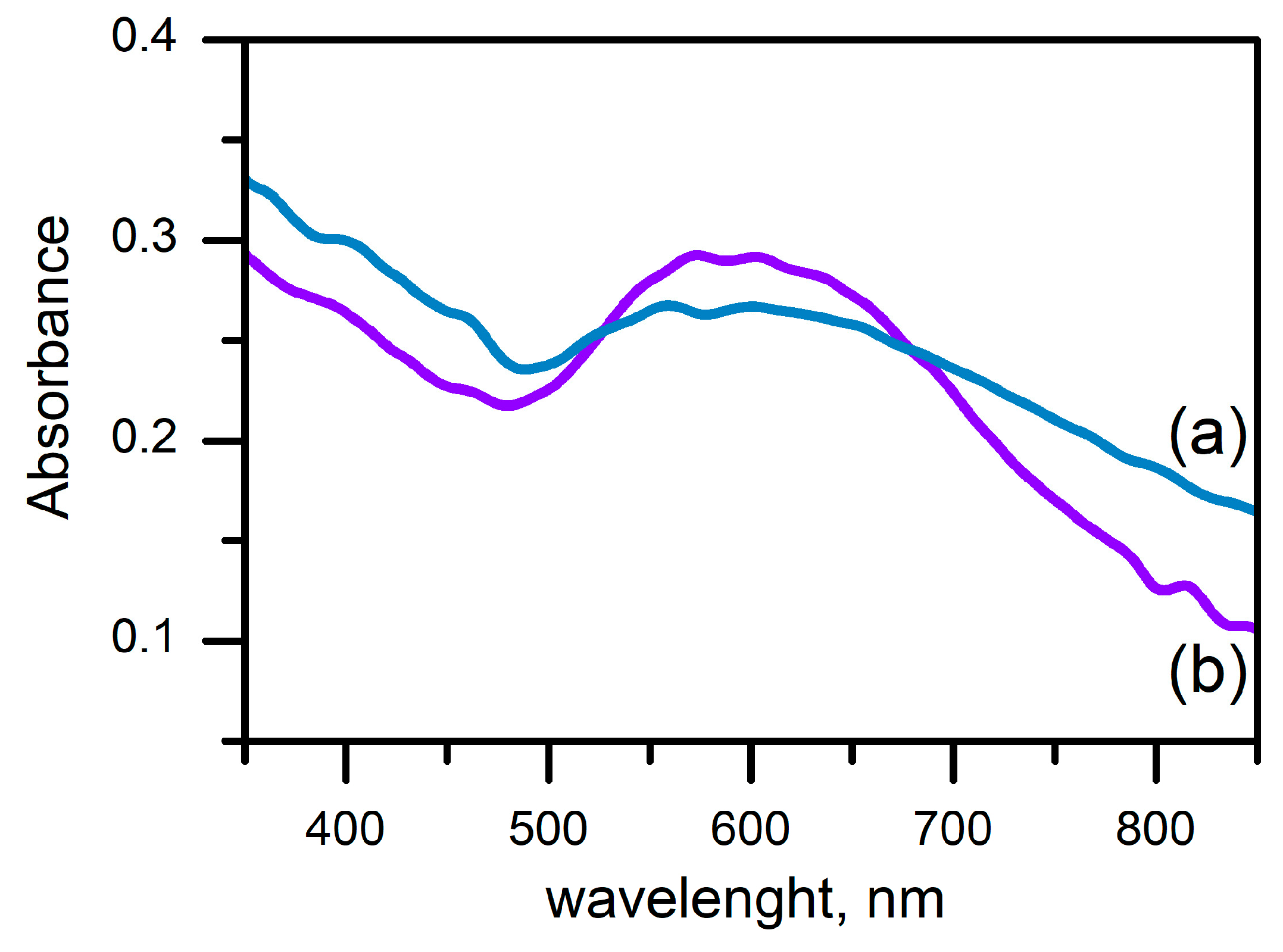
3. Results
3.1. Experimental
3.1.1. Laser Ablation of Bulk Fe
3.1.2. Laser Ablation of Pure Fe Film
3.1.3. Laser Ablation of Alloyed FeAu Metal Film
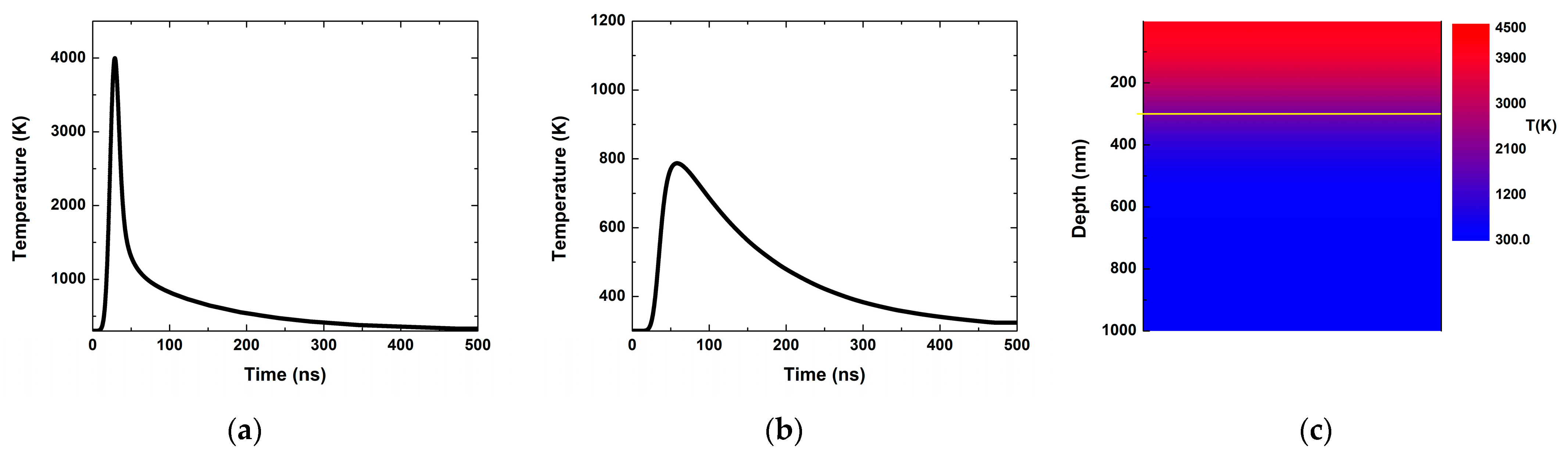
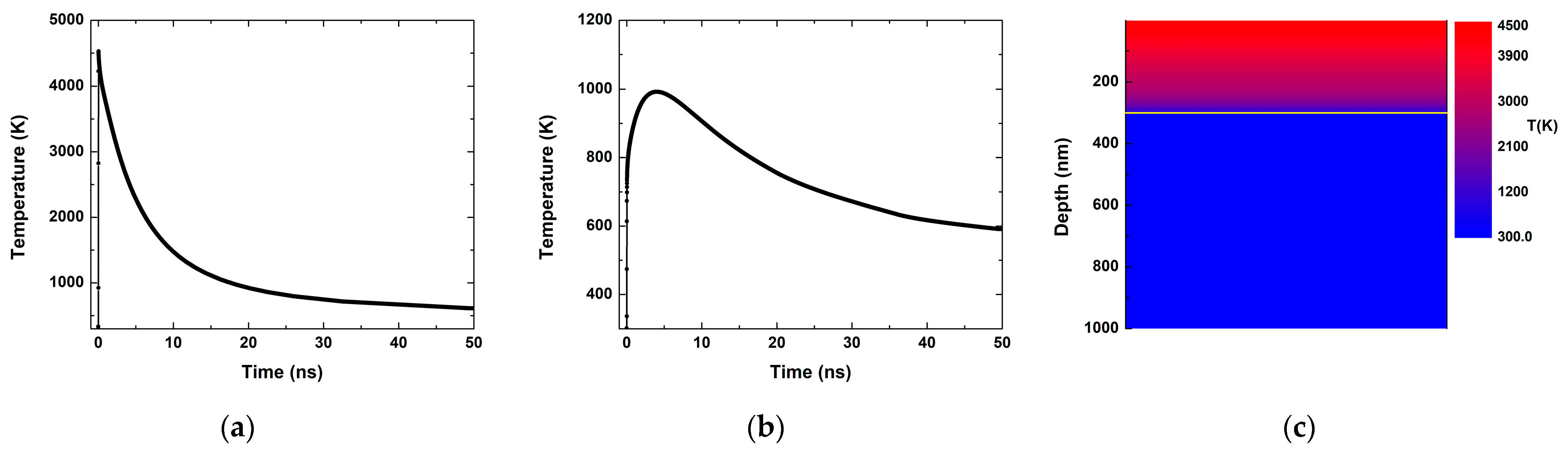
3.2. Theoretical
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jungwirth, T.; Marti, M.; Wadley, P.; Wunderlich, J. Antiferromagnetic spintronics. Nat. Nanotechnol. 2016, 11, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.Q.; Shi, Y.N.; Zhu, Y.P.; Liu, Y.Q.; Gu, L.W.; Liu, D.D.; Ma, A.; Xia, F.; Guo, Q.Y.; Xu, C.C.; et al. Recent trends in preparation and biomedical applications of iron oxide nanoparticles. J. Nanobiotechnol. 2024, 22, 24. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef]
- Revathy, R.; Sajini, T.; Augustine, C.; Joseph, N. Iron-based magnetic nanomaterials: Sustainable approaches of synthesis and applications. Results Eng. 2023, 18, 101114. [Google Scholar] [CrossRef]
- Chen, L.; Xie, J.; Wang, Z.; Zhao, Y.; Gou, J.; Wu, J. Amorphous Pt-decorated α-Fe2O3 sensor with superior triethylamine sensing performance prepared by a one-step impregnation method. J. Alloys Compd. 2024, 976, 173330. [Google Scholar] [CrossRef]
- Mikaeili Ghezeljeh, S.; Salehzadeh, A.; Ataei-e Jaliseh, S. Iron oxide nanoparticles coated with Glucose and conjugated with Safranal (Fe3O4@Glu-Safranal NPs) inducing apoptosis in liver cancer cell line (HepG2). BMC Chem. 2024, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.; Hyeon, T. Chemical Design of Biocompatible Iron Oxide Nanoparticles for Medical Applications. Small 2012, 9, 1450–1466. [Google Scholar] [CrossRef]
- Nana, A.B.A.; Marimuthu, T.; Kondiah, P.P.D.; Choonara, Y.E.; Du Toit, L.C.; Pillay, V. Multifunctional magnetic nanowires: Design, fabrication, and future prospects as cancer therapeutics. Cancers 2019, 11, 1956. [Google Scholar] [CrossRef]
- Andrade, R.G.D.; Veloso, S.R.S.; Castanheira, E.M.S. Shape Anisotropic Iron Oxide-Based Magnetic Nanoparticles: Synthesis and Biomedical Applications. Int. J. Mol. Sci. 2020, 21, 2455. [Google Scholar] [CrossRef]
- Mondal, P.; Anweshan, A.; Purkait, M.K. Green synthesis and environmental application of iron-based nanomaterials and nanocomposite: A review. Chemosphere 2020, 259, 127509. [Google Scholar] [CrossRef] [PubMed]
- Eason, R. Pulsed Laser Deposition of Thin Films: Applications-Led Growth of Functional Materials; John Wiley & Sons, Inc.: New York, NY, USA, 2007; ISBN 9780470052129. [Google Scholar]
- Boutinguiza, M.; Comesaña, R.; Lusquiños, F.; Riveiro, A.; del Val, J.; Pou, J. Production of silver nanoparticles by laser ablation in open air. Appl. Surf. Sci. 2015, 336, 108–111. [Google Scholar] [CrossRef]
- Białous, A.; Gazda, M.; Grochowska, K.; Atanasov, P.; Dikovska, A.; Nedyalkov, N.; Reszczyńska, J.; Zaleska-Medowska, A.; Śliwiński, G. Nanoporous TiO2 electrode grown by laser ablation of titanium in air at atmospheric pressure and room temperature. Thin Solid Films 2016, 601, 41–44. [Google Scholar] [CrossRef]
- Nikov, R.G.; Dikovska, A.O.; Atanasova, G.B.; Avdeev, G.V.; Nedyalkov, N.N. Magnetic field-assisted formation of oriented nanowires produced by PLD in open air. Appl. Surf. Sci. 2018, 458, 273–280. [Google Scholar] [CrossRef]
- Nikov, R.G.; Dikovska, A.O.; Avdeev, G.V.; Amoruso, S.; Ausanio, G.; Nedyalkov, N.N. PLD fabrication of oriented nanowires in magnetic field. Appl. Surf. Sci. 2019, 471, 368–374. [Google Scholar] [CrossRef]
- Nikov, R.G.; Dikovska, A.O.; Avdeev, G.V.; Atanasova, G.B.; Nedyalkov, N.N. Composite magnetic and non-magnetic oxide nanostructures fabricated by a laser-based technique. Appl. Surf. Sci. 2021, 549, 49204. [Google Scholar] [CrossRef]
- Nikov, R.G.; Dikovska, A.O.; Avdeev, G.V.; Atanasova, G.B.; Karashanova, D.B.; Amoruso, S.; Ausanio, G.; Nedyalkov, N.N. Single-step fabrication of oriented composite nanowires by pulsed laser deposition in magnetic field. Mater. Today Commun. 2021, 26, 101717. [Google Scholar] [CrossRef]
- Nedyalkov, N.; Nakajima, Y.; Terakawa, M. Magnetic nanoparticle composed nanowires fabricated by ultrashort laser ablation in air. Appl. Phys. Lett. 2016, 108, 043107. [Google Scholar] [CrossRef]
- Dikovska, A.; Atanasova, G.; Nikov, R.; Avdeev, G.; Cherkezova-Zheleva, Z.; Paneva, D.; Nedyalkov, N. Formation of Oriented Nanowires from Mixed Metal Oxides. Materials 2023, 16, 6446. [Google Scholar] [CrossRef]
- Dikovska, A.; Atanasova, G.; Dilova, T.; Baeva, A.; Avdeev, G.; Atanasov, P.; Nedyalkov, N. Picosecond Pulsed Laser Deposition of Metals and Metal Oxides. Materials 2023, 16, 6364. [Google Scholar] [CrossRef]
- Kim, B.; Nam, H.K.; Watanabe, S.; Park, S.; Kim, Y.; Kim, Y.-J.; Fushinobu, K.; Kim, S.-W. Selective Laser Ablation of Metal Thin Films Using Ultrashort Pulses. Int. J. Precis. Eng. Manuf.-Green. Technol. 2021, 8, 771–782. [Google Scholar] [CrossRef]
- Hallum, G.E.; Kurschner, D.; Redka, D.; Niethammer, D.; Schulz, W.; Huber, H.P. Time-resolved ultrafast laser ablation dynamics of thin film indium tin oxide. Opt. Express 2021, 29, 30062. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Nam, H.-K.; Kim, Y.-J.; Kim, S.-W. Lift-Off Ablation of Metal Thin Films for Micropatterning Using Ultrashort Laser Pulses. Metals 2021, 11, 1586. [Google Scholar] [CrossRef]
- Domke, M.; Nobile, L.; Rapp, S.; Eiselen, S.; Sotrop, J.; Huber, H.P.; Schmidt, M. Understanding thin film laser ablation: The role of the effective penetration depth and the film thickness. Phys. Procedia 2014, 56, 1007–1014. [Google Scholar] [CrossRef]
- Amendola, V.; Amans, D.; Ishikawa, Y.; Koshizaki, N.; Scirè, S.; Compagnini, G.; Reichenberger, S.; Barcikowski, S. Room-Temperature Laser Synthesis in Liquid of Oxide, Metal-Oxide Core-Shells, and Doped Oxide Nanoparticles. Chem. Eur. J. 2020, 26, 9206. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V. Laser-Assisted Synthesis of Non-Equilibrium Nanoalloys. Chem. Phys. Chem. 2021, 22, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Coviello, V.; Forrer, D.; Amendola, V. Recent Developments in Plasmonic Alloy Nanoparticles: Synthesis, Modelling, Properties and Applications. Chem. Phys. Chem. 2022, 23, e202200136. [Google Scholar] [CrossRef]
- Press, W.H.; Teukolsky, S.A.; Vetterling, W.T.; Flannery, B.P. Numerical Recipes in Fortran, 1st ed.; University Press: Cambridge, UK, 1992; ISBN 10: 0521383307. [Google Scholar]
- Lasemia, N.; Pachera, U.; Zhigilei, L.V.; Bomatí-Miguela, O.; Lahozd, R.; Kautek, W. Pulsed laser ablation and incubation of nickel, iron and tungsten in liquids and air. Appl. Surf. Sci. 2018, 433, 772–779. [Google Scholar] [CrossRef]
- Artyukov, I.A.; Zayarniy, D.A.; Ionin, A.A.; Kudryashov, S.I.; Makarov, S.V.; Saltuganov, P.N. Relaxation Phenomena in Electronic and Lattice Subsystems on Iron Surface during Its Ablation by Ultrashort Laser Pulses. JETP Lett. 2014, 99, 51–55. [Google Scholar] [CrossRef]
- Fernandez-Pañella, A.; Ogitsu, T.; Engelhorn, K.; Correa, A.A.; Barbrel, B.; Hamel, S.; Prendergast, D.G.; Pemmaraju, D.; Beckwith, M.A.; Bae, L.J.; et al. Reduction of electron-phonon coupling in warm dense iron. Phys. Rev. B 2020, 101, 184309. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Shokrollahi, H. A review of the magnetic properties, synthesis methods and applications of maghemite. J. Magn. Magn. Mater. 2017, 426, 74–81. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Pannu, C.; Bala, M.; Khan, S.A.; Srivastava, S.K.; Kabiraj, D.; Avasthi, D.K. Synthesis and characterization of Au–Fe alloy nanoparticles embedded in a silica matrix by atom beam sputtering. RSC Adv. 2015, 5, 92080–92088. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, W.; Wang, T.; Wang, D.; Liu, L.; Ye, J. High Performance Au-Cu Alloy for Enhanced Visible-light Water Splitting Driven by Coinage Metals. Chem. Commun. 2016, 52, 4694–4697. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Wu, J.H.; Min, J.H.; Kim, Y.K. Synthesis of monosized magnetic-optical AuFe alloy nanoparticles. J. Appl. Phys. 2008, 103, 07D529. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M.; Bakr, O.M.; Riello, P.; Polizzi, S.; Anjum, D.H.; Fiameni, S.; Arosio, P.; Orlando, T.; Fernandez, C.J.; et al. Coexistence of plasmonic and magnetic properties in Au89Fe11 nanoalloys. Nanoscale 2013, 5, 5611–5619. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dikovska, A.O.; Karashanova, D.; Atanasova, G.; Avdeev, G.; Atanasov, P.; Nedyalkov, N.N. Fabrication of Nanostructures Consisting of Composite Nanoparticles by Open-Air PLD. Coatings 2024, 14, 527. https://doi.org/10.3390/coatings14050527
Dikovska AO, Karashanova D, Atanasova G, Avdeev G, Atanasov P, Nedyalkov NN. Fabrication of Nanostructures Consisting of Composite Nanoparticles by Open-Air PLD. Coatings. 2024; 14(5):527. https://doi.org/10.3390/coatings14050527
Chicago/Turabian StyleDikovska, Anna Og, Daniela Karashanova, Genoveva Atanasova, Georgi Avdeev, Petar Atanasov, and Nikolay N. Nedyalkov. 2024. "Fabrication of Nanostructures Consisting of Composite Nanoparticles by Open-Air PLD" Coatings 14, no. 5: 527. https://doi.org/10.3390/coatings14050527




