Natural Light Rechargeable Night Peal-like Coatings for Expressway
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Phosphor
2.2. Preparation of Luminescent Coating
2.3. Characterization Techniques
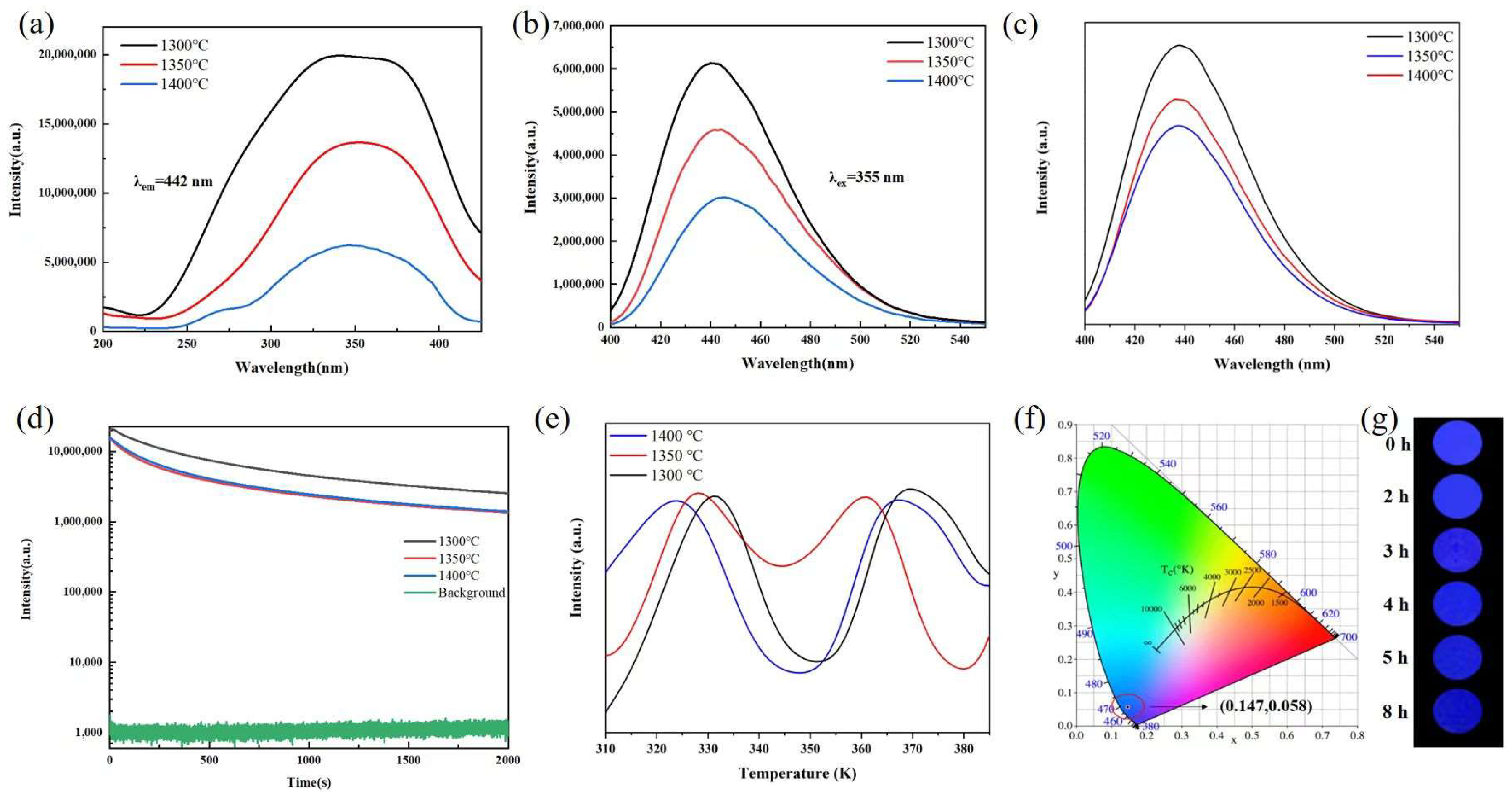
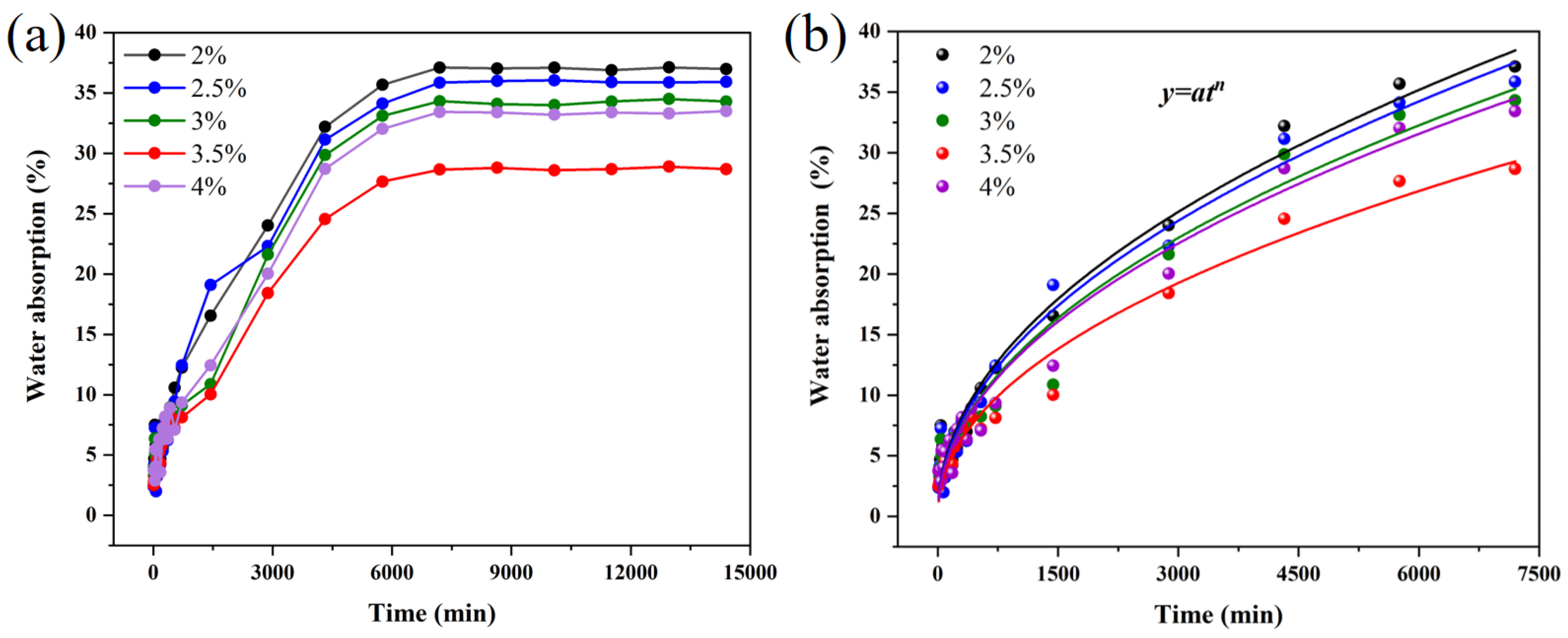
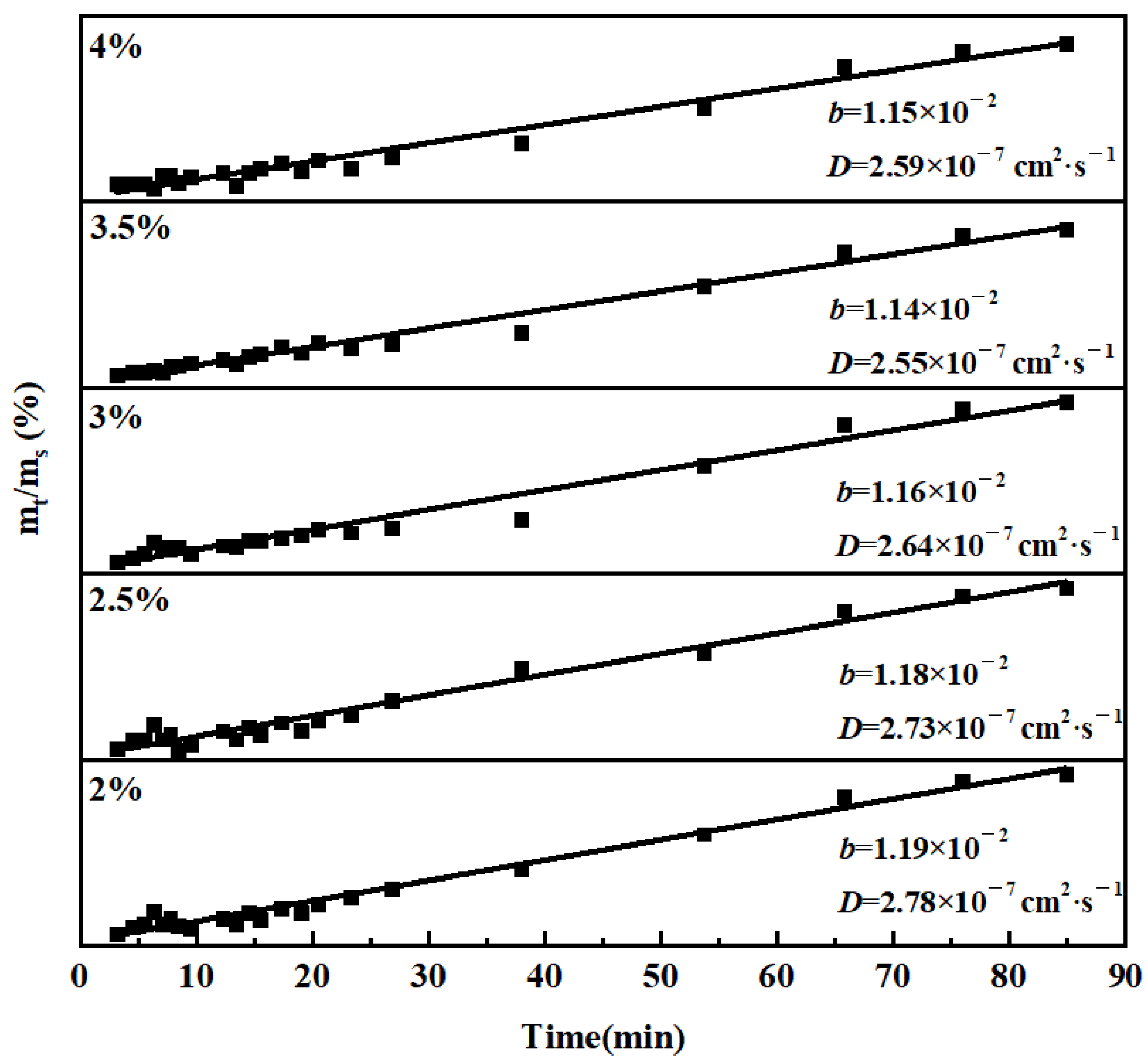
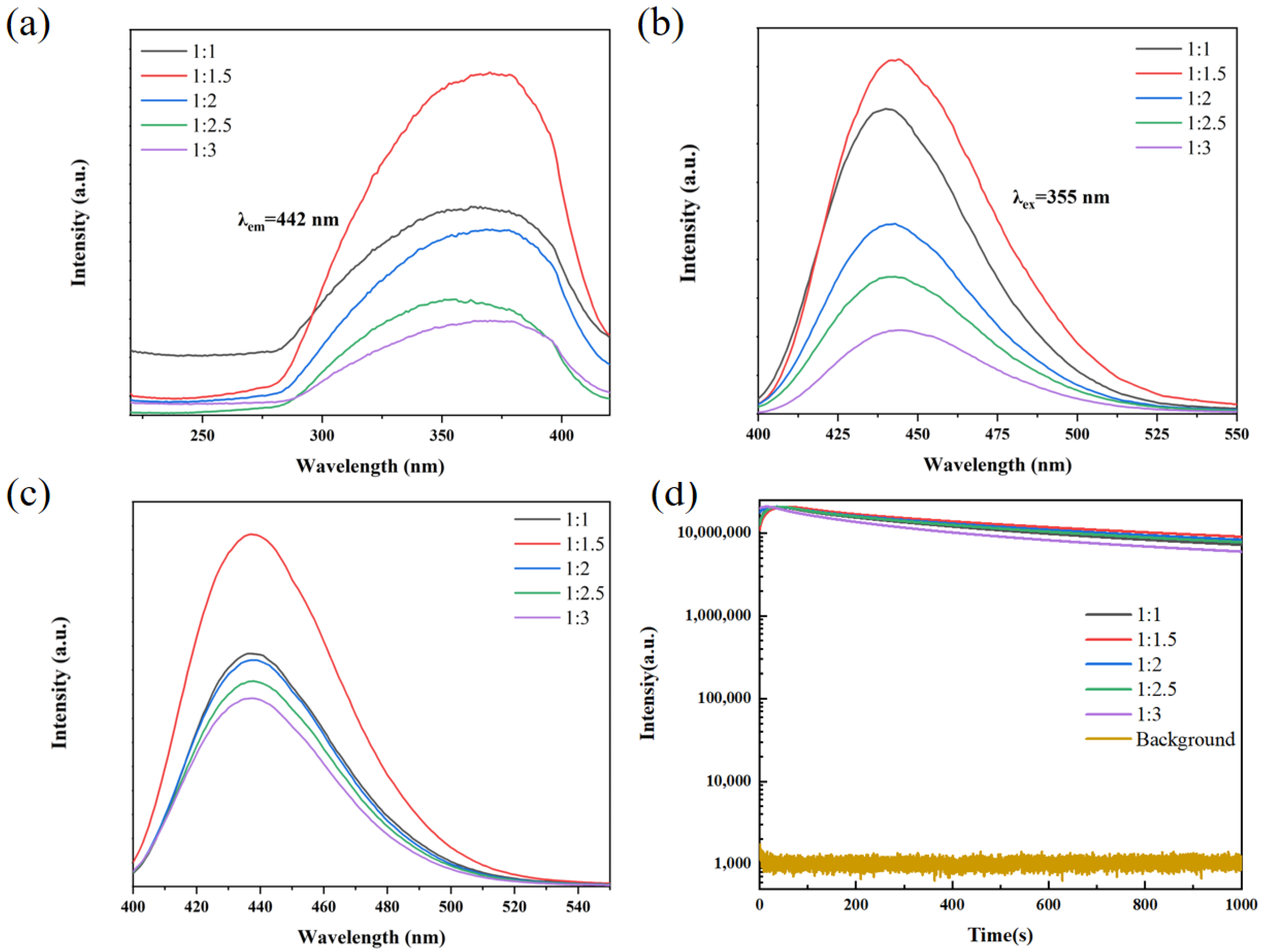
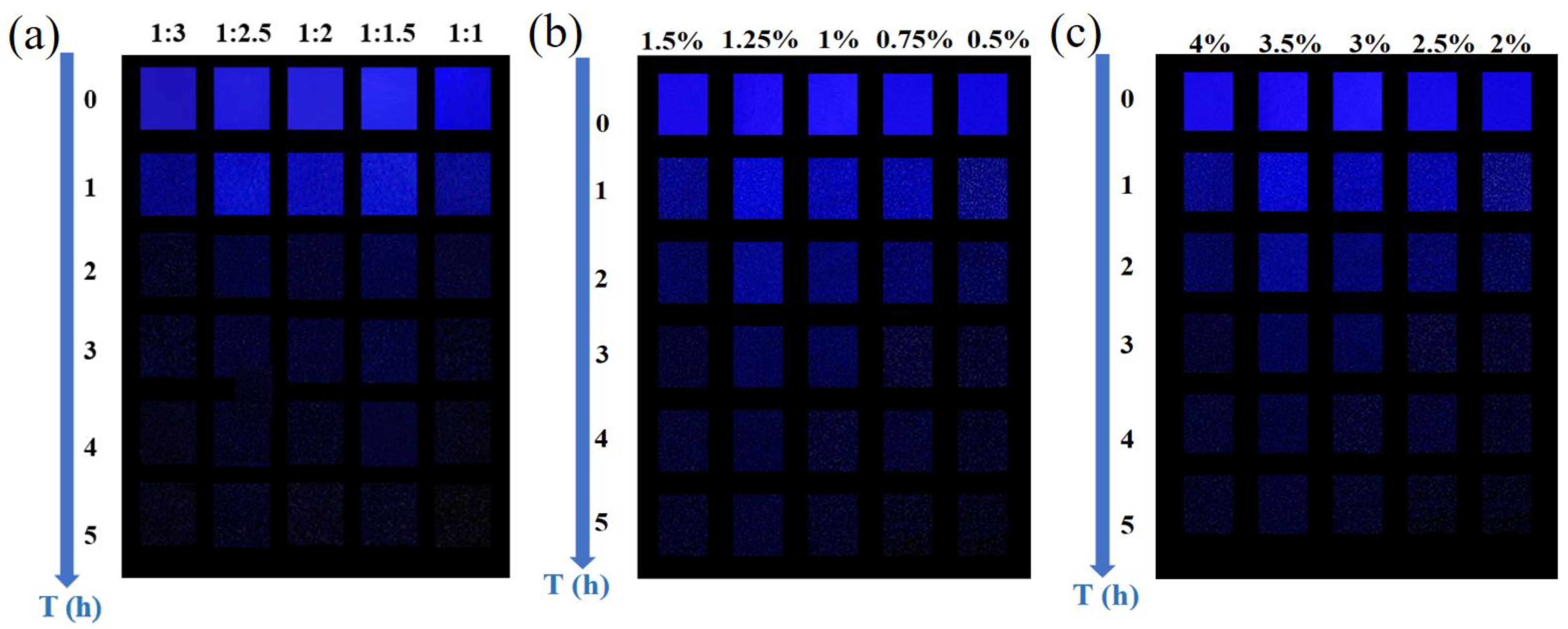
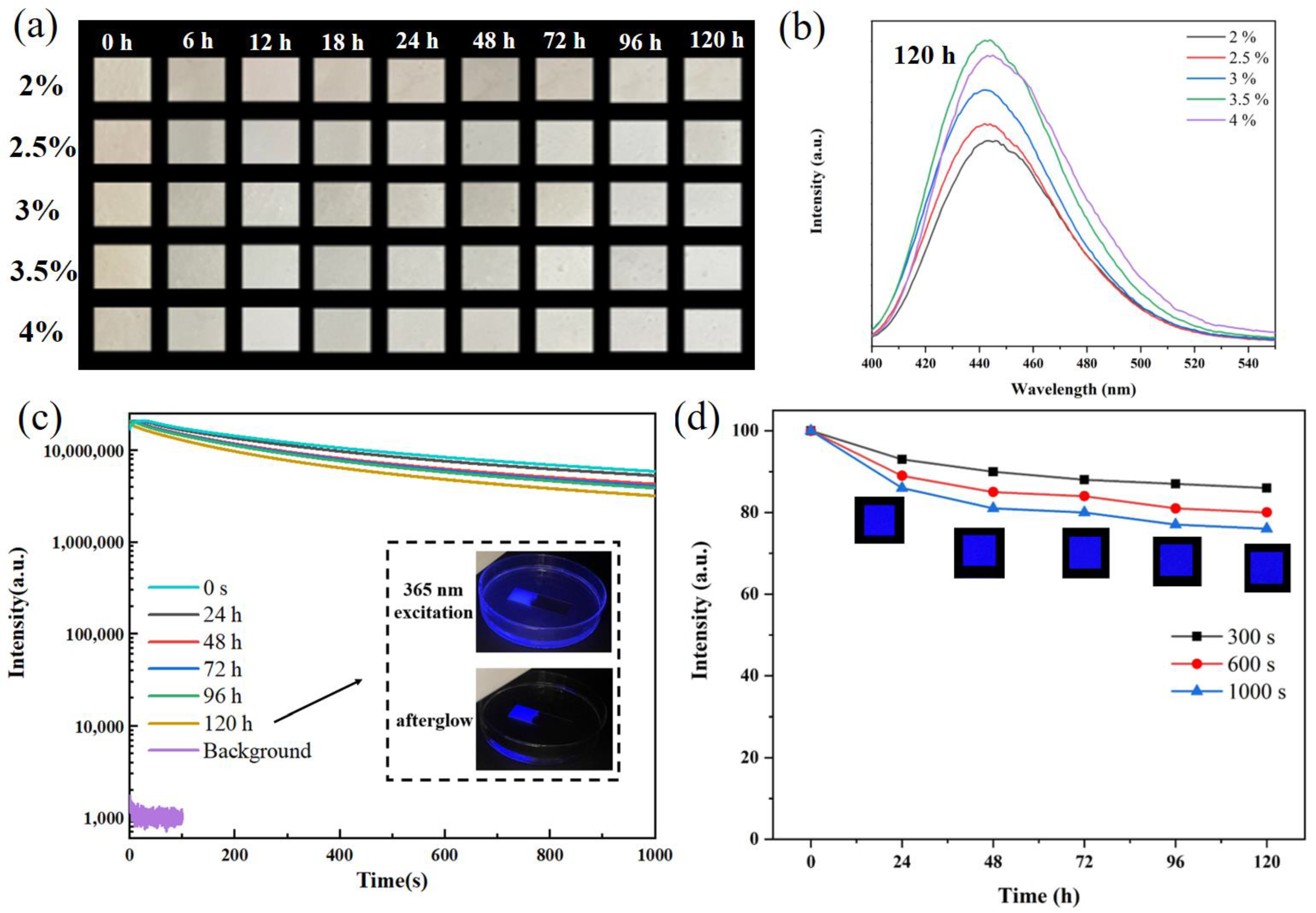
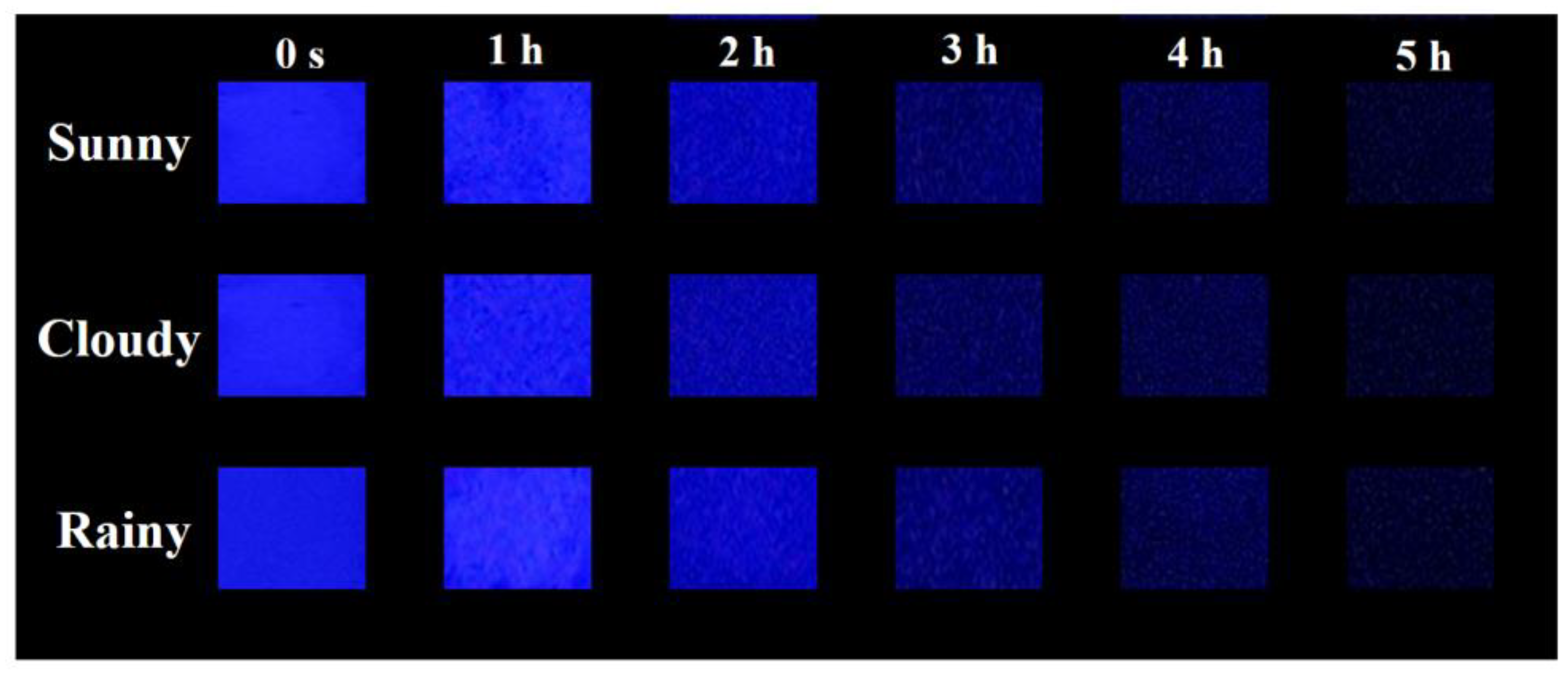
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Tanabe, S. Persistent luminescence instead of phosphorescence: History, mechanism, and perspective. J. Lumines. 2019, 205, 581–620. [Google Scholar] [CrossRef]
- Wang, P.; Xu, X.; Zhou, D.; Yu, X.; Qiu, J. Sunlight activated long-lasting luminescence from Ba5Si8O21:Eu2+,Dy3+ phosphor. Inorg. Chem. 2015, 54, 1690–1697. [Google Scholar] [CrossRef]
- Qu, B.; Zhang, B.; Wang, L.; Zhou, R.; Zeng, X. Mechanistic study of the persistent luminescence of CaAl2O4:Eu,Nd. Chem. Mat. 2015, 27, 2195–2202. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Aoki, Y.; Takeuchi, N.; Murayama, Y. A new long phosphorescent phosphor with high brightness, SrAl2O4:Eu2+,Dy3+. J. Electrochem. Soc. 1996, 143, 2670. [Google Scholar] [CrossRef]
- Wang, C.; Jin, Y.; Lv, Y.; Ju, G.; Liu, D.; Chen, L.; Li, Z.; Hu, Y. Trap distribution tailoring guided design of super-long-persistent phosphor Ba2SiO4:Eu2+,Ho3+ and photostimulable luminescence for optical information storage. J. Mater. Chem. C 2018, 6, 6058–6067. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, W.; Pang, R.; Ma, T.; Li, D.; Li, H.; Zhang, S.; Fu, J.; Jiang, L.; Li, C. Sunlight activated new long persistent luminescence phosphor BaSiO3:Eu2+,Nd3+,Tm3+: Optical properties and mechanism. Mater. Des. 2016, 90, 218–224. [Google Scholar] [CrossRef]
- Fu, X.; Zheng, S.; Meng, Y.; Sun, W.; Zhang, H. Long afterglow yellow luminescence from Pr3+ doped SrSc2O4. J. Rare Earths 2022, 40, 567–571. [Google Scholar] [CrossRef]
- Sun, W.; Jiao, M.; Fan, L.; Zhang, R.; Zhang, X.; Zhang, H. Design of a defect-induced orange persistent luminescence phosphor BaZnGeO4:Bi3+. J. Am. Ceram. Soc. 2022, 105, 2128–2139. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, W.; Song, E.; Hu, K.; Zhang, H.; Yun, R.; Huang, H.; Zhong, J.; Li, Y.; Li, X. Efficient visible light charging for rare earth-free persistent phosphor. Adv. Opt. Mater. 2022, 10, 2102496. [Google Scholar] [CrossRef]
- Dai, T.; Ju, G.; Lv, Y.; Jin, Y.; Wu, H.; Hu, Y. Luminescence properties of novel dual-emission (UV/red) long afterglow phosphor LiYGeO4:Eu3+. J. Lumines. 2021, 237, 118193. [Google Scholar] [CrossRef]
- Doke, G.; Antuzevics, A.; Krieke, G.; Kalnina, A.; Springis, M.; Sarakovskis, A. UV and X-ray excited red persistent luminescence in Mn2+ doped MgGeO3 material synthesized in air and reducing atmosphere. J. Lumines. 2021, 234, 117995. [Google Scholar] [CrossRef]
- Long, J.; Yuan, X.; Ma, C.; Du, M.; Ma, X.; Wen, Z.; Ma, R.; Wang, Y.; Cao, Y. Strongly enhanced luminescence of Sr4Al14O25:Mn4+ phosphor by co-doping B3+ and Na+ ions with red emission for plant growth LEDs. RSC Adv. 2018, 8, 1469–1476. [Google Scholar] [CrossRef]
- Zhuang, Y.; Chen, D.; Chen, W.; Zhang, W.; Su, X.; Deng, R.; An, Z.; Chen, H.; Xie, R. X-ray-charged bright persistent luminescence in NaYF4:Ln3+@NaYF4 nanoparticles for multidimensional optical information storage. Light Sci. Appl. 2021, 10, 132. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, L.; Lv, Y.; Zhou, T.; Xie, R. Optical data storage and multicolor emission readout on flexible films using deep-trap persistent luminescence materials. Adv. Funct. Mater. 2018, 28, 1705769. [Google Scholar] [CrossRef]
- Tian, S.; Wen, J.; Fan, H.; Chen, Y.; Yan, J. A thermochromic luminous polyurethane based on long persistent luminescent phosphors and thermochromic pigment. New J. Chem. 2018, 42, 5066–5070. [Google Scholar] [CrossRef]
- Rojas-Hernandez, R.; Rubio-Marcos, F.; Rodriguez, M.; Fernandez, J. Long lasting phosphors: SrAl2O4:Eu,Dy as the most studied material. Renew. Sust. Energ. Rev. 2018, 81, 2759–2770. [Google Scholar] [CrossRef]
- Kalyani, N.T.; Jain, A.; Dhoble, S. Persistent phosphors for luminous paints: A review. Luminescence 2022, 37, 524–542. [Google Scholar] [CrossRef]
- Finley, E.; Cobb, A.; Duke, A.; Paterson, A.; Brgoch, J. Optimizing blue persistent luminescence in (Sr1−δBaδ)2MgSi2O7:Eu2+,Dy3+ via solid solution for use in point-of-care diagnostics. ACS Appl. Mater. Interfaces 2016, 8, 26956–26963. [Google Scholar] [CrossRef]
- Xiao, L.; Zhou, J.; Liu, G.; Wang, L. Luminescent properties of R+ doped Sr2MgSi2O7:Eu2+,Dy3+ (R+ = Li+, Ag+) phosphors. J. Alloy. Compd. 2017, 712, 24–29. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Li, G.; Feng, P.; Liu, D.; Ye, Q. Activating persistent luminescence and thermal stability of K2Ba7Si16O40:Eu2+ via traps modulation. J. Alloy. Compd. 2019, 801, 295–301. [Google Scholar] [CrossRef]
- Zhang, M.; Li, F.; Lin, Y.; Li, Y.; Shen, Y. Enhanced afterglow performance of CaAl2O4:Eu2+,Nd3+ phosphors by co-doping Gd3+. J. Rare Earths 2021, 39, 930–937. [Google Scholar] [CrossRef]
- Chiatti, C.; Fabiani, C.; Pisello, A. Long persistent luminescence: A road map toward promising future developments in energy and environmental science. Ann. Rev. Mater. Res. 2021, 51, 409–433. [Google Scholar] [CrossRef]
- Li, Y.; Gecevicius, M.; Qiu, J. Long persistent phosphors—From fundamentals to applications. Chem. Soc. Rev. 2016, 45, 2090–2136. [Google Scholar] [CrossRef]
- Hai, O.; Ren, Q.; Wu, X.; Zhang, Q.; Zhang, Z.; Zhang, Z. Insights into the element gradient in the grain and luminescence mechanism of the long afterglow material Sr2MgSi2O7:Eu2+,Dy3+. J. Alloy. Compd. 2019, 779, 892–899. [Google Scholar] [CrossRef]
- Guo, C.; Tang, Q.; Zhang, C.; Huang, D.; Su, Q. Thermoluminescent properties of Eu2+ and RE3+ co-doped phosphors CaGa2S4:Eu2+,RE3+ (RE = Ln, excluding Pm, Eu and Lu). J. Lumines. 2007, 126, 333–338. [Google Scholar] [CrossRef]
- Cheng, S.; Xu, X.; Han, J.; Qiu, J.; Zhang, B. Design, synthesis and characterization of a novel orange-yellow long-lasting phosphor: Li2SrSiO4:Eu2+,Dy3+. Powder Technol. 2015, 276, 129–133. [Google Scholar] [CrossRef]
- Xu, X.; Yu, X.; Zhou, D.; Qiu, J. Effect of retrapping on the persistent luminescence in strontium silicate orange-yellow phosphor. J. Solid State Chem. 2013, 206, 66–68. [Google Scholar] [CrossRef]
- Zhang, M.; Li, F.; Jiang, S.; Lin, Y.; Chen, F.; Zhao, X.; Shen, Y. CaAl2O4:Eu2+,Nd3+ anti-corrosive coating and its afterglow-Catalytic process. Opt. Mater. 2021, 116, 111049. [Google Scholar] [CrossRef]
- Shi, X.; Dou, R.; Ma, T.; Liu, W.; Lu, X.; Shea, K.; Song, Y.; Jiang, L. Bioinspired lotus-like self-illuminous coating. ACS Appl. Mater. Interfaces 2015, 7, 18424–18428. [Google Scholar] [CrossRef]
- Wan, M.; Jiang, X.; Nie, J.; Cao, Q.; Zheng, W.; Dong, X.; Fan, Z.; Zhou, W. Phosphor powders-incorporated polylactic acid polymeric composite used as 3D printing filaments with green luminescence properties. J. Appl. Polym. Sci. 2020, 137, 48644. [Google Scholar] [CrossRef]
- Perez, C.; Collazo, A.; Izquierdo, M.; Merino, P.; Novoa, X. Characterisation of the barrier properties of different paint systems: Part II. non-ideal diffusion and water uptake kinetics. Prog. Org. Coat. 1999, 37, 169–177. [Google Scholar] [CrossRef]
- Perez, C.; Collazo, A.; Izquierdo, M.; Merino, P.; Novoa, X. Characterisation of the barrier properties of different paint systems: Part I. experimental set-up and ideal Fickian diffusion. Prog. Org. Coat. 1999, 36, 102–108. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Zhang, J.; Cao, C. Studies of impedance models and water transport behaviors of polypropylene coated metals in NaCl solution. Prog. Org. Coat. 2004, 49, 293–301. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Zhang, J.; Cao, C. Studies of water transport behavior and impedance models of epoxy-coated metals in NaCl solution by EIS. Prog. Org. Coat. 2004, 51, 145–151. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Chen, R.; Xiao, R.; Li, W.; Si, T.; Li, P.; Zhu, Q. Natural Light Rechargeable Night Peal-like Coatings for Expressway. Coatings 2024, 14, 566. https://doi.org/10.3390/coatings14050566
Li X, Chen R, Xiao R, Li W, Si T, Li P, Zhu Q. Natural Light Rechargeable Night Peal-like Coatings for Expressway. Coatings. 2024; 14(5):566. https://doi.org/10.3390/coatings14050566
Chicago/Turabian StyleLi, Xin, Rong Chen, Rui Xiao, Wenjie Li, Te Si, Peiyang Li, and Qi Zhu. 2024. "Natural Light Rechargeable Night Peal-like Coatings for Expressway" Coatings 14, no. 5: 566. https://doi.org/10.3390/coatings14050566






