Coating Red Phosphor on Green Luminescent Material for Multi-Mode Luminescence and Advanced Anti-Counterfeit Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of SrAl2O4:1%Eu,2%Dy
2.2. Preparation of Nitrate Solutions
2.3. Formation of Core-Shell Structures via a Sol-Gel Method
2.4. Preparation of Anti-Counterfeiting Logo
2.5. Characterization Techniques
3. Results and Discussion
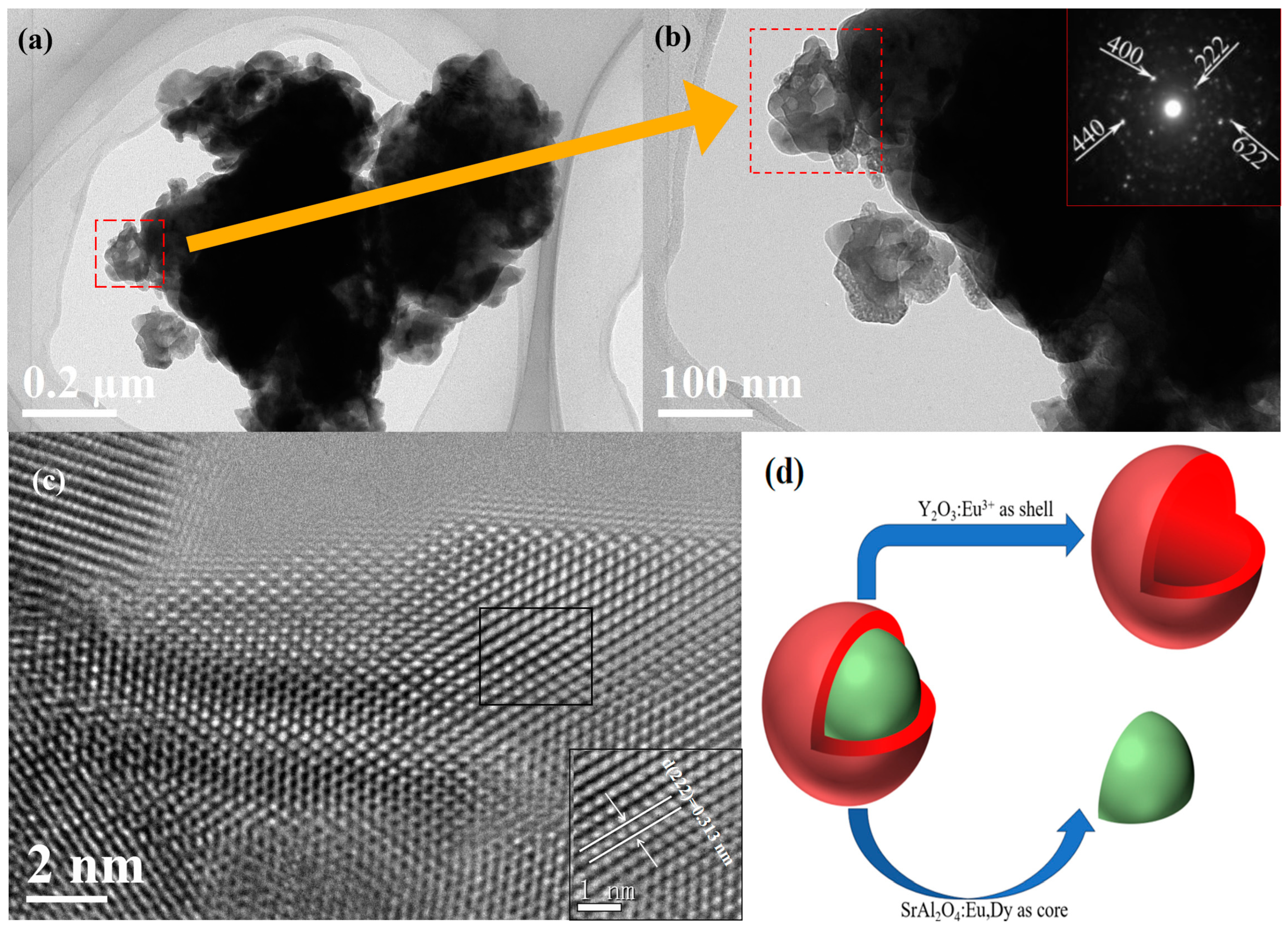
3.1. Phase Identification and Crystal structure Characterization
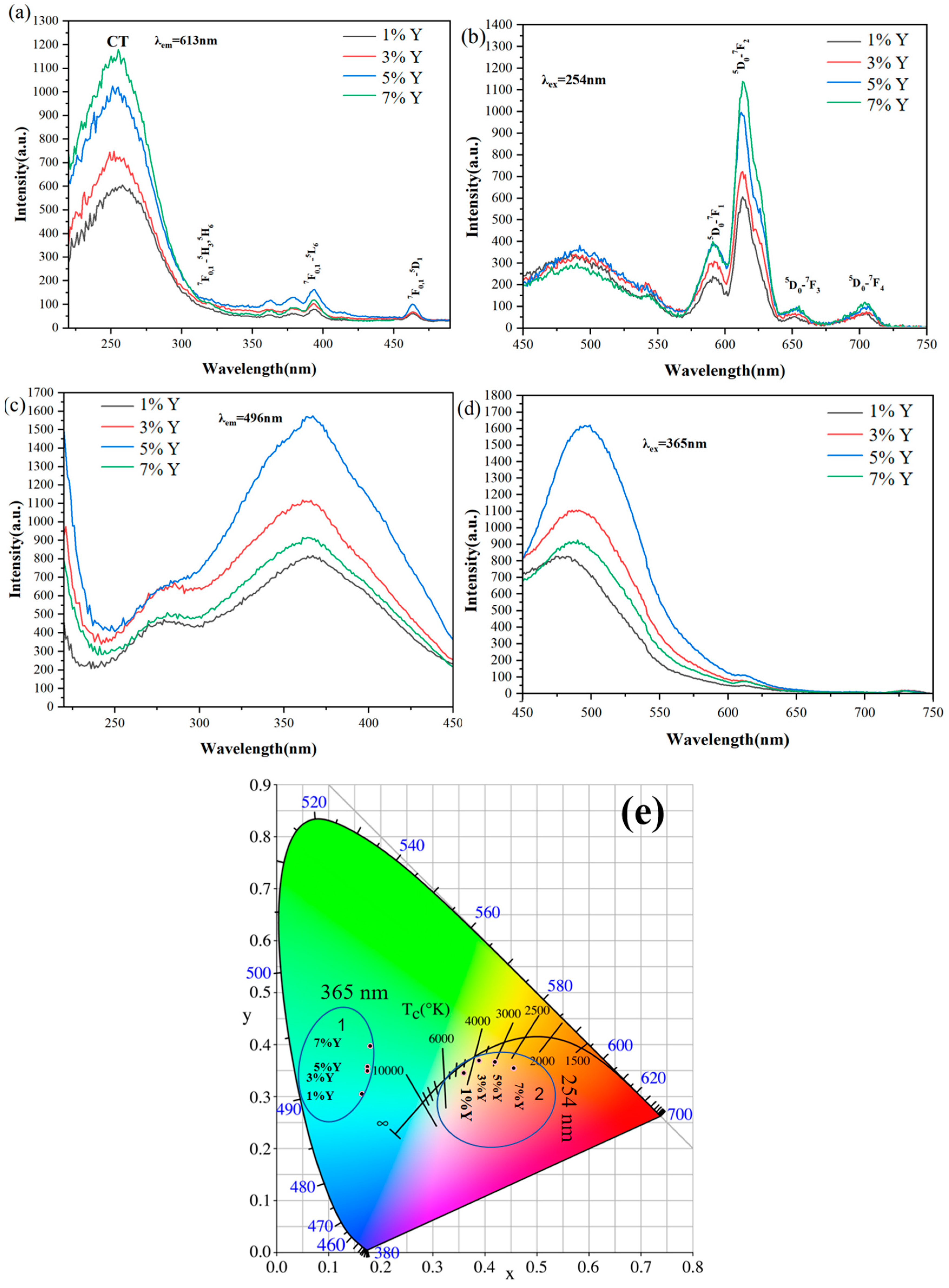
3.2. Photoluminescence of the Phosphors
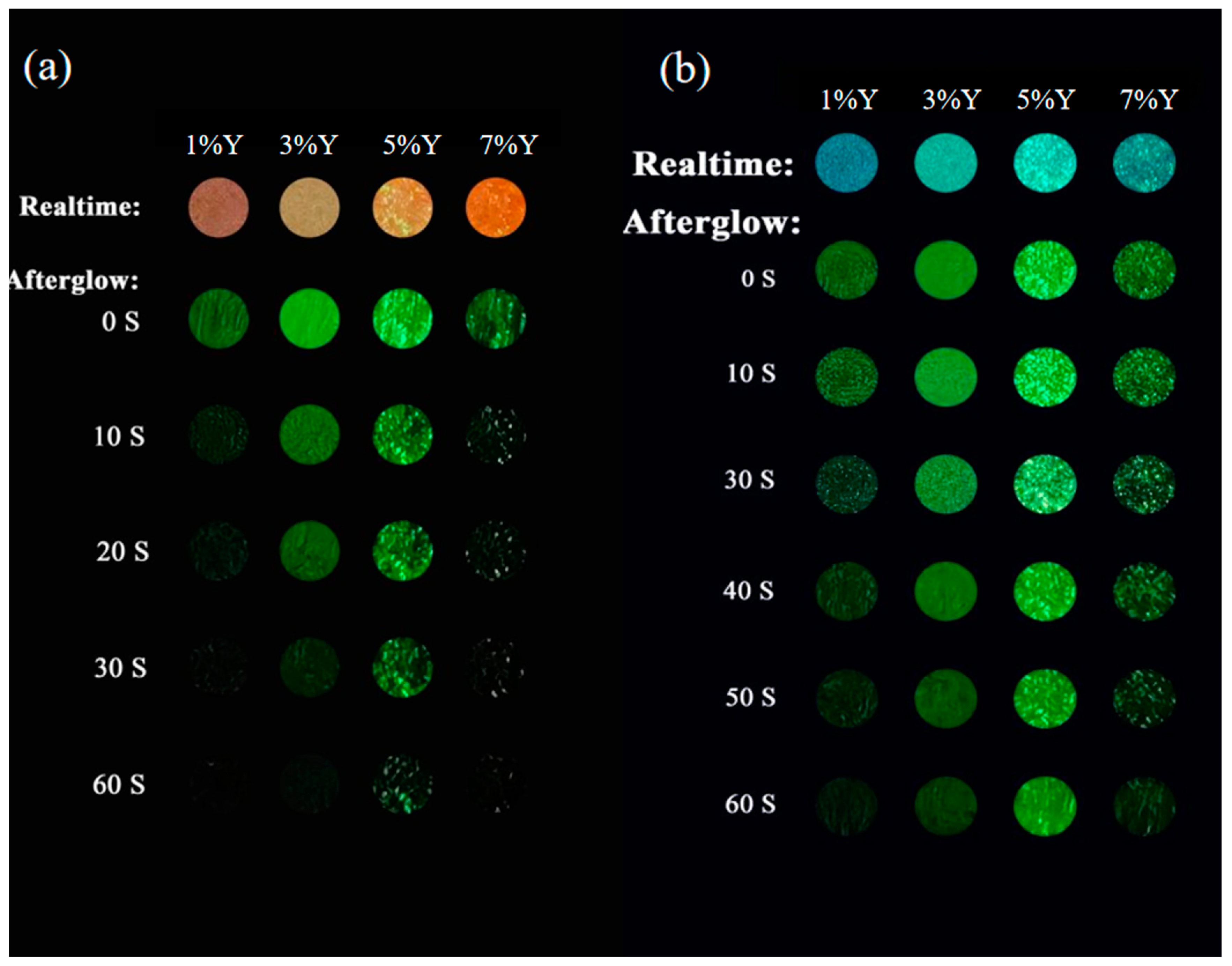
3.3. Persistent Luminescence and Energy Transfer of the Phosphors
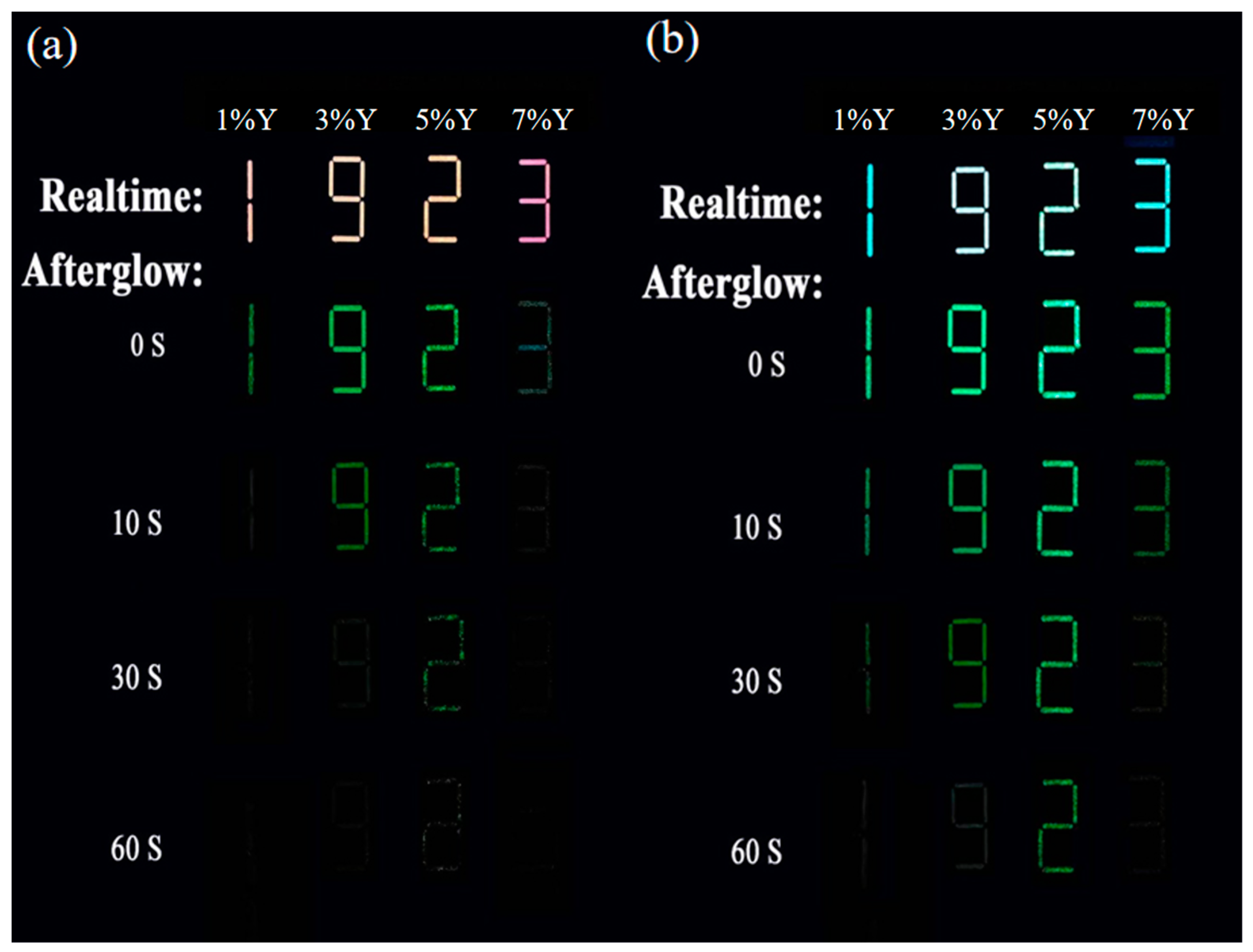
3.4. Multimode Anti-Counterfeiting Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Si, T.; Zhu, Q.; Zhang, T.; Sun, X.; Li, J.-G. Co-doping Mn2+/Cr3+ in ZnGa2O4 to fabricate chameleon-like phosphors for multi-mode dynamic anti-counterfeiting. Chem. Eng. J. 2021, 426, 131744. [Google Scholar] [CrossRef]
- Mitrokotsa, A.; Rieback, R.M.; Tanenbaum, A.S. Classifying RFID attacks and defenses. Inf. Syst. Front. 2010, 12, 491–505. [Google Scholar] [CrossRef]
- Li, Z.; Wang, M.; Zhang, X.; Wang, D.; Xu, W.; Yin, Y. Magnetic Assembly of Nanocubes for Orientation-Dependent Photonic Responses. Nano Lett. 2019, 19, 6673–6680. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ni, M.; Peng, H.; Huang, F.; Liao, Y.; Wang, M.; Zhu, J.; Roy, V.A.; Xie, X. Photoinitiation and Inhibition under Monochromatic Green Light for Storage of Colored 3D Images in Holographic Polymer-Dispersed Liquid Crystals. ACS Appl. Mater. Interfaces 2017, 9, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, Z.; Ma, Z.; Fan, F.; Liu, W. Achieving Multimodal Emission in Zn4B6O13:Tb3+, Yb3+ for Information Encryption and Anti-counterfeiting. Inorg. Chem. 2020, 59, 15681–15689. [Google Scholar] [CrossRef]
- Gu, L.; Shi, H.; Bian, L.; Gu, M.; Ling, K.; Wang, X.; Ma, H.; Cai, S.; Ning, W.; Fu, L.; et al. Colour-tunable ultra-long organic phosphorescence of a single-component molecular crystal. Nat. Photonics 2019, 13, 406–411. [Google Scholar] [CrossRef]
- Song, Z.; Lin, T.; Lin, L.; Lin, S.; Fu, F.; Wang, X.; Guo, L. Invisible security ink based on water-soluble graphitic carbon nitride quantum dots. Angew. Chem. Int. Ed. 2016, 55, 2773–2777. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Lü, J. Citric acid-assisted hydrothermal synthesis and luminescence of monodisperse NdPO4 nanorods. Appl. Surf. Sci. 2012, 259, 671–673. [Google Scholar] [CrossRef]
- Jiang, K.; Zhang, L.; Lu, J.; Xu, C.; Cai, C.; Lin, H. Triple-mode emission of carbon dots: Applications for advanced anti-counterfeiting. Angew. Chem. Int. Ed. 2016, 55, 7231–7235. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Huang, J.; Yan, L.; He, L.; Zhou, B. Multichannel control of PersL/upconversion/down-shifting luminescence in a single core–shell nanoparticle for information encryption. J. Phys. Chem. Lett. 2022, 13, 9007–9013. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hai, J.; Li, S.; Wang, B.; Yang, Z. Luminescent magnetic nanoparticles encapsulated in MOFs for highly selective and sensitive detection of ClO−/SCN− and anti-counterfeiting. Nanoscale 2018, 10, 8667–8676. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, B.; Jia, L.; Fan, Y.; Chen, R.; Zhu, T.; Liu, B. Dual-mode, color-tunable, lanthanide-doped core–shell nanoarchitectures for anti-counterfeiting inks and latent fingerprint recognition. ACS Appl. Mater. Interfaces 2019, 11, 35294–35304. [Google Scholar] [CrossRef] [PubMed]
- Hai, O.; Li, J.; Pei, M.; Ren, Q.; Wu, X.; Qin, B.; Xiao, X.; He, X.; Li, T.; Chen, Y. Perovskite-Based Nanostructures for Fluorescence Afterglow Anticounterfeiting. ACS Appl. Nano Mater. 2023, 6, 8990–8998. [Google Scholar] [CrossRef]
- Stockman, A.; Lindsay; Sharpe, T. The spectral sensitivities of the middle- and long-wavelength-sensitive cones derived from measurements in observers of known genotype. Vis. Res. 2000, 40, 1711–1737. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Delgado, T.; Richard, C.; Viana, B. ZGSO spinel nanoparticles with dual emission of NIR persistent luminescence for anti-counterfeiting applications. Materials 2023, 16, 1132. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, F.; Zhang, Z.; Huang, C.; Lin, Y. Luminescent properties of SrAl2O4: Eu, Dy material prepared by the gel method. J. Eur. Ceram. Soc. 2000, 20, 2129–2132. [Google Scholar] [CrossRef]
- Blasse, G.; Grabmaier, B. A General Introduction to Luminescent Materials; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar] [CrossRef]
- Wang, H.; Lin, C.K.; Liu, X.M.; Lin, J.; Yu, M. Monodisperse spherical core-shell-structured phosphors obtained by functionalization of silica spheres with Y2O3: Eu3+ layers for field emission displays. Appl. Phys. Lett. 2005, 87, 181907. [Google Scholar] [CrossRef]
- Chen, X.J.; Li, W.; Hu, S.S.; Akhmedov, N.G.; Reed, D.; Li, X.L.; Liu, X.B. Polyvinyl alcohol coating induced preferred crystallographic orientation in aqueous zinc battery anodes. Nano Energy 2022, 98, 107269. [Google Scholar] [CrossRef]
- Buijs, M.; Meyerink, A.; Blasse, G. Energy transfer between Eu3+ ions in a lattice with two different crystallographic sites: Y2O3: Eu3+, Gd2O3: Eu3+ and Eu2O3. J. Lumin. 1987, 37, 9–20. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Lin, C.K.; Liu, X.M.; Lin, J. Synthesis and Luminescence Properties of Monodisperse Spherical Y2O3: Eu3+@ SiO2 Particles with Core−shell Structure. J. Phys. Chem. C 2007, 111, 11223–11230. [Google Scholar] [CrossRef]
- Wu, X.; Tao, Y.; Gao, F.; Dong, L.; Hu, Z. Preparation and photoluminescence of yttrium hydroxide and yttrium oxide doped with europium nanowires. J. Cryst. Growth 2005, 277, 643–649. [Google Scholar] [CrossRef]
- Goldner, P.; Auzel, F. Comparison Between Standard and Modified Judd-Ofelt Theories in a Pr3+-Doped Fluoride Glass. Acta Phys. Pol. A 1996, 90, 191–196. [Google Scholar] [CrossRef]
- Walsh, B.M. Judd-Ofelt theory: Principles and practices. In Advances in Spectroscopy for Lasers and Sensing; Springer: Berlin/Heidelberg, Germany, 2006; pp. 403–433. [Google Scholar] [CrossRef]
- Li, Y.; Gecevicius, M.; Qiu, J. Long persistent phosphors—From fundamentals to applications. Chem. Soc. Rev. 2016, 45, 2090–2136. [Google Scholar] [CrossRef] [PubMed]
- Clabau, F.; Rocquefelte, X.; Jobic, S.; Deniard, P.; Whangbo, M.-H.; Garcia, A.; Le Mercier, T. Mechanism of phosphorescence appropriate for the long-lasting phosphors Eu2+-doped SrAl2O4 with codopants Dy3+ and B3+. Chem. Mater. 2005, 17, 3904–3912. [Google Scholar] [CrossRef]
- Jiang, H.Z.; Zhang, L.; Huang, Y.D.; Jia, D.Z.; Guo, Z.P. Synthesis and optical properties of ultra-fine Sr5Al2O8: Eu3+ nanorod phosphor from a low-heating-temperature solid-state precursor method. Mater. Sci. Eng. B 2007, 145, 23–27. [Google Scholar] [CrossRef]
- Li, C.; Imai, Y.; Adachi, Y.; Yamada, H.; Nishikubo, K.; Xu, C. One-step synthesis of luminescent nanoparticles of complex oxide, strontium aluminate. J. Am. Ceram. Soc. 2007, 90, 2273–2275. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Chen, B.; Zhu, Q. Coating Red Phosphor on Green Luminescent Material for Multi-Mode Luminescence and Advanced Anti-Counterfeit Applications. Coatings 2024, 14, 509. https://doi.org/10.3390/coatings14040509
Liu J, Chen B, Zhu Q. Coating Red Phosphor on Green Luminescent Material for Multi-Mode Luminescence and Advanced Anti-Counterfeit Applications. Coatings. 2024; 14(4):509. https://doi.org/10.3390/coatings14040509
Chicago/Turabian StyleLiu, Jiale, Bo Chen, and Qi Zhu. 2024. "Coating Red Phosphor on Green Luminescent Material for Multi-Mode Luminescence and Advanced Anti-Counterfeit Applications" Coatings 14, no. 4: 509. https://doi.org/10.3390/coatings14040509



