The Porter-Whitesides Discrepancy: Revisiting Odd-Even Effects in Wetting Properties of n-Alkanethiolate SAMs
Abstract
:1. Introduction

2. Hydrophobicity of SAMs
2.1. The Original Studies

2.2. Molecular Simulation of Odd-Even Effects in Wetting
2.3. “Odd-Even” Effect in SAMs
2.3.1. Odd-Even in Wetting (Pre-2014)

2.3.2. Odd-Even Effect in Other SAM Properties
Odd-Even Effect in Charge Transport

Odd-Even Effect in Capacitance
Odd-Even in Tribology
Odd-Even in SAM Packing Structure

3. Substrates
3.1. Substrates Preparation
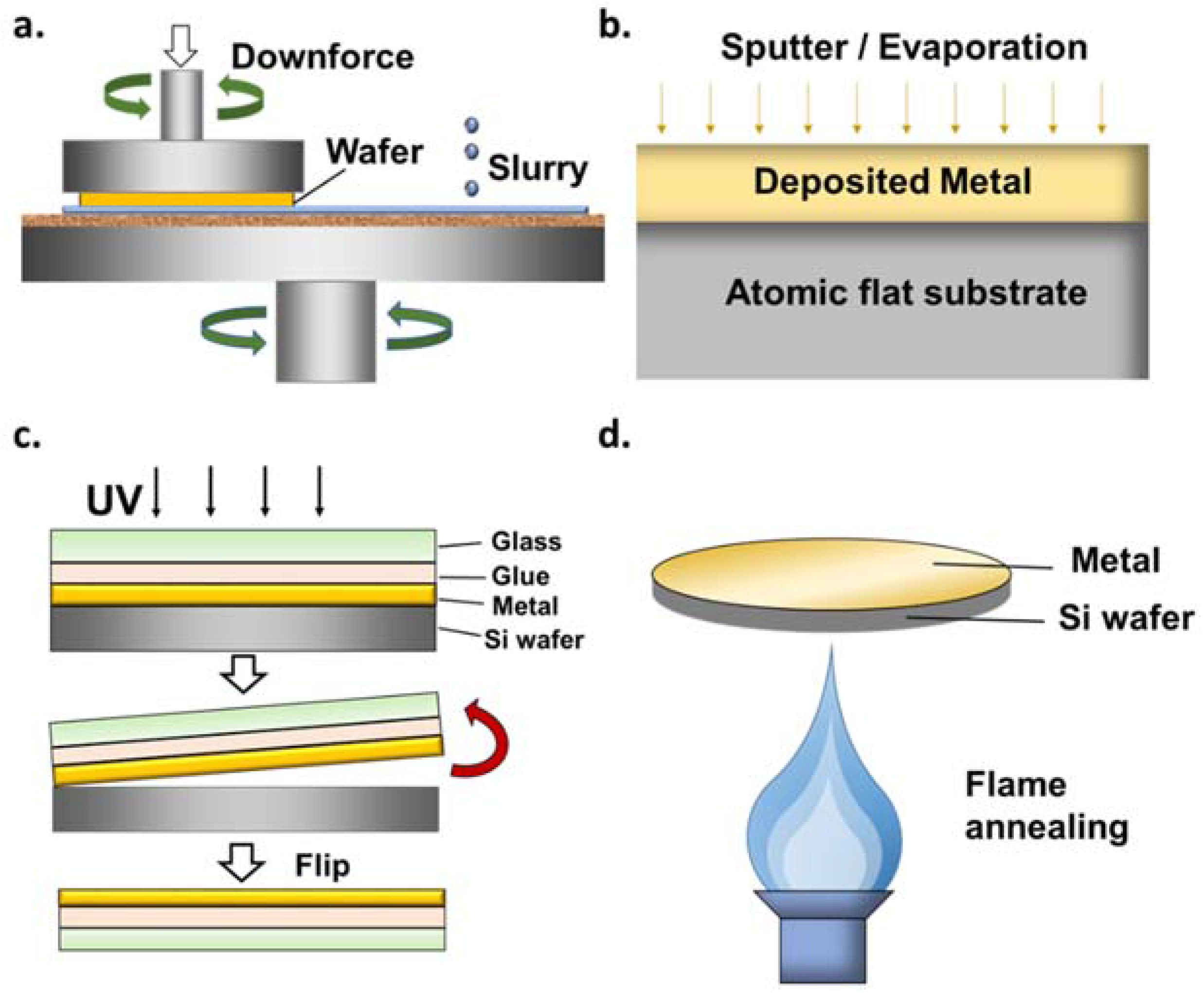
3.2. Substrates Roughness
| Methods | Metal | RMS Roughness (nm) | Reference |
|---|---|---|---|
| PVD | Ag | 5.13 ± 0.22 | [25] |
| Au | 2.03 | [50] | |
| Au | 2.27 ± 0.16 | [25] | |
| Au | 15 | [23] | |
| Pd | 0.8 | [12] | |
| Chemical mechanical polishing | Ag | 1.27 ± 0.16 | [51] |
| Ag | <5 | [52] | |
| Au | 0.38 ± 0.05 | [49] | |
| Template stripping | Ag | 0.6 ± 0.04 | [25] |
| Au | 0.6 | [32] | |
| Au | 0.36 ± 0.01 | [25] | |
| Au | ~0.3–0.7 | [26] | |
| Annealing-assisted template stripping | Au | 0.9 ± 0.2 | [24] |
| Au | 1.97 ± 0.12 | [53] |
3.3. Effect of Grain Size and/or Substrate Morphology
4. Effect of Substrate on Wetting: Resolution of the Whitesides and Porter Discrepancy
4.1. Odd-Even Effect in the Hydrophobicity of n-Alkanethiolate SAMs Depends on the Roughness of the Substrate

4.2. The Grain Size of the Substrate

4.3. The Surface Roughness Limit on the Odd-Even Effect in Hydrophobicity

5. Summary
Acknowledgments
Conflicts of Interest
References
- Franklin, B.; Brownrigg, W.; Farish, M. Of the stilling of waves by means of oil. Extracted from sundry letters between Benjamin Franklin, LL. D.F.R.S. William Brownrigg, M.D.F.R.S. and the reverend Mr. Farish. Phil. Trans. 1774, 64, 445–460. [Google Scholar] [CrossRef]
- Bigelow, W.C.; Pickett, D.L.; Zisman, W.A. Oleophobic monolayers: I. Films adsorbed from solution in non-polar liquids. J. Colloid Sci. 1946, 1, 513–538. [Google Scholar] [CrossRef]
- Kuhn, H.; Möbius, D. Systems of monomolecular layers—Assembling and physico-chemical behavior. Angew. Chem. Int. Ed. 1971, 10, 620–637. [Google Scholar] [CrossRef]
- Nuzzo, R.G.; Allara, D.L. Adsorption of bifunctional organic disulfides on gold surfaces. J. Amer. Chem. Soc. 1983, 105, 4481–4483. [Google Scholar] [CrossRef]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Atre, S.V.; Liedberg, B.; Allara, D.L. Chain length dependence of the structure and wetting properties in binary composition monolayers of OH-and CH3-terminated alkanethiolates on gold. Langmuir 1995, 11, 3882–3893. [Google Scholar] [CrossRef]
- Flynn, N.T.; Tran, T.N.T.; Cima, M.J.; Langer, R. Long-term stability of self-assembled monolayers in biological media. Langmuir 2003, 19, 10909–10915. [Google Scholar] [CrossRef]
- Graupe, M.; Takenaga, M.; Koini, T.; Colorado, R., Jr.; Lee, T.R. Oriented surface dipoles strongly influence interfacial wettabilities. J. Amer. Chem. Soc. 1999, 121, 3222–3223. [Google Scholar] [CrossRef]
- Kudelski, A. Structures of monolayers formed from different HS–(CH2)2–x thiols on gold, silver and copper: Comparitive studies by surface-enhanced raman scattering. J. Raman Spectrosc. 2003, 34, 853–862. [Google Scholar] [CrossRef]
- Laibinis, P.E.; Whitesides, G.M.; Allara, D.L.; Tao, Y.T.; Parikh, A.N.; Nuzzo, R.G. Comparison of the structures and wetting properties of self-assembled monolayers of n-alkanethiols on the coinage metal surfaces, copper, silver, and gold. J. Amer. Chem. Soc. 1991, 113, 7152–7167. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef] [PubMed]
- Love, J.C.; Wolfe, D.B.; Haasch, R.; Chabinyc, M.L.; Paul, K.E.; Whitesides, G.M.; Nuzzo, R.G. Formation and structure of self-assembled monolayers of alkanethiolates on palladium. J. Amer. Chem. Soc. 2003, 125, 2597–2609. [Google Scholar] [CrossRef] [PubMed]
- Rusu, P.C.; Giovannetti, G.; Brocks, G. Dipole formation at interfaces of alkanethiolate self-assembled monolayers and Ag (111). J. Phys. Chem. C 2007, 111, 14448–14456. [Google Scholar] [CrossRef]
- Tao, F.; Bernasek, S.L. Understanding odd-even effects in organic self-assembled monolayers. Chem. Rev. 2007, 107, 1408–1453. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.-S.; Takano, H.; Porter, M.D. Mapping orientation differences of terminal functional groups by friction force microscopy. Anal. Chem. 1998, 70, 5209–5212. [Google Scholar] [CrossRef]
- Stoliar, P.; Kshirsagar, R.; Massi, M.; Annibale, P.; Albonetti, C.; de Leeuw, D.M.; Biscarini, F. Charge injection across self-assembly monolayers in organic field-effect transistors: Odd-even effects. J. Amer. Chem. Soc. 2007, 129, 6477–6484. [Google Scholar] [CrossRef] [PubMed]
- Thuo, M.M.; Reus, W.F.; Nijhuis, C.A.; Barber, J.R.; Kim, C.; Schulz, M.D.; Whitesides, G.M. Odd-even effects in charge transport across self-assembled monolayers. J. Amer. Chem. Soc. 2011, 133, 2962–2975. [Google Scholar] [CrossRef] [PubMed]
- Baghbanzadeh, M.; Simeone, F.C.; Bowers, C.M.; Liao, K.-C.; Thuo, M.; Baghbanzadeh, M.; Miller, M.S.; Carmichael, T.B.; Whitesides, G.M. Odd–even effects in charge transport across n-alkanethiolate-based sams. J. Amer. Chem. Soc. 2014, 136, 16919–16925. [Google Scholar] [CrossRef] [PubMed]
- Dubi, Y. Transport through self-assembled monolayer molecular junctions: Role of in-plane dephasing. J. Phys. Chem. C 2014, 118, 21119–21127. [Google Scholar] [CrossRef]
- Walczak, M.M.; Chung, C.; Stole, S.M.; Widrig, C.A.; Porter, M.D. Structure and interfacial properties of spontaneously adsorbed n-alkanethiolate monolayers on evaporated silver surfaces. J. Amer. Chem. Soc. 1991, 113, 2370–2378. [Google Scholar] [CrossRef]
- Biebuyck, H.A.; Bain, C.D.; Whitesides, G.M. Comparison of organic monolayers on polycrystalline gold spontaneously assembled from solutions containing dialkyl disulfides or alkanethiols. Langmuir 1994, 10, 1825–1831. [Google Scholar] [CrossRef]
- Yang, J.; Han, J.; Isaacson, K.; Kwok, D.Y. Effects of surface defects, polycrystallinity, and nanostructure of self-assembled monolayers for octadecanethiol adsorbed onto au on wetting and its surface energetic interpretation. Langmuir 2003, 19, 9231–9238. [Google Scholar] [CrossRef]
- Godin, M.; Williams, P.J.; Tabard-Cossa, V.; Laroche, O.; Beaulieu, L.; Lennox, R.B.; Grütter, P. Surface stress, kinetics, and structure of alkanethiol self-assembled monolayers. Langmuir 2004, 20, 7090–7096. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, L.B.; Tevis, I.D.; Atkinson, M.B.; Gathiaka, S.M.; Luna, R.E.; Thuo, M. Odd-even effect in the hydrophobicity of n-alkanethiolate self-assembled monolayers depends upon the roughness of the substrate and the orientation of the terminal moiety. Langmuir 2014, 30, 11985–11992. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Z.; Oyola-Reynoso, S.; Gathiaka, S.M.; Thuo, M. Limits to the effect of substrate roughness or smoothness on the odd–even effect in wetting properties of n-alkanethiolate monolayers. Langmuir 2015, 31, 7047–7054. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Puck, A.; Graupe, M.; Colorado, R., Jr.; Shon, Y.-S.; Lee, T.R.; Perry, S.S. Structure, wettability, and frictional properties of phenyl-terminated self-assembled monolayers on gold. Langmuir 2001, 17, 7364–7370. [Google Scholar] [CrossRef]
- Srivastava, P.; Chapman, W.G.; Laibinis, P.E. Odd-even variations in the wettability of n-alkanethiolate monolayers on gold by water and hexadecane: A molecular dynamics simulation study. Langmuir 2005, 21, 12171–12178. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.-T.; Frey, S.; Yang, Y.-J.; Zharnikov, M.; Buck, M.; Wühn, M.; Wöll, C.; Helmchen, G. On the importance of the headgroup substrate bond in thiol monolayers: A study of biphenyl-based thiols on gold and silver. Langmuir 2001, 17, 1582–1593. [Google Scholar] [CrossRef]
- Thuo, M.M.; Reus, W.F.; Simeone, F.C.; Kim, C.; Schulz, M.D.; Yoon, H.J.; Whitesides, G.M. Replacing-CH2CH2-with-CONH-does not significantly change rates of charge transport through AgTS-SAM//Ga2O3/egain junctions. J. Am. Chem. Soc. 2012, 134, 10876–10884. [Google Scholar] [CrossRef] [PubMed]
- Nurbawono, A.; Liu, S.; Nijhuis, C.A.; Zhang, C. Odd-even effects in charge transport through self-assembled monolayer of alkanethiolates. J. Phys. Chem. C 2015, 119, 5657–5662. [Google Scholar] [CrossRef]
- Jiang, L.; Sangeeth, C.S.; Nijhuis, C.A. The origin of the odd-even effect in the tunneling rates across egain junctions with self-assembled monolayers (SAMs) of n-alkanethiolates. J. Amer. Chem. Soc. 2015, 137, 10659–10667. [Google Scholar] [CrossRef] [PubMed]
- Nerngchamnong, N.; Yuan, L.; Qi, D.-C.; Li, J.; Thompson, D.; Nijhuis, C.A. The role of van der waals forces in the performance of molecular diodes. Nat. nanotechnol. 2013, 8, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Thompson, D.; Cao, L.; Nerngchangnong, N.; Nijhuis, C.A. One carbon matters: The origin and reversal of odd-even effects in molecular diodes with self-assembled monolayers of ferrocenyl-alkanethiolates. J. Phys. Chem. C 2015, 119, 17910–17919. [Google Scholar] [CrossRef]
- Kornilovitch, P.; Bratkovsky, A.; Williams, R.S. Current rectification by molecules with asymmetric tunneling barriers. Phys. Rev. B 2002, 66, 165436. [Google Scholar] [CrossRef]
- Nijhuis, C.A.; Reus, W.F.; Whitesides, G.M. Molecular rectification in metal-SAM-metal oxide-metal junctions. J. Am. Chem. Soc. 2009, 131, 17814–17827. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, C.A.; Reus, W.F.; Barber, J.R.; Dickey, M.D.; Whitesides, G.M. Charge transport and rectification in arrays of sam-based tunneling junctions. Nano lett. 2010, 10, 3611–3619. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, C.A.; Reus, W.F.; Whitesides, G.M. Mechanism of rectification in tunneling junctions based on molecules with asymmetric potential drops. J. Amer. Chem. Soc. 2010, 132, 18386–18401. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Tsai, T.-K.; Lin, C.-M.; Chen, C.-h.; Chan, Y.-C.; Chen, H.-W. Structures of self-assembled monolayers of n-alkanoic acids on gold surfaces modified by underpotential deposition of silver and copper: Odd-even effect. Langmuir 2002, 18, 5473–5478. [Google Scholar] [CrossRef]
- Mikulski, P.T.; Herman, L.A.; Harrison, J.A. Odd and even model self-assembled monolayers: Links between friction and structure. Langmuir 2005, 21, 12197–12206. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jamison, A.C.; Barriet, D.; Lee, T.R.; Ruths, M. Odd–even effects in the friction of self-assembled monolayers of phenyl-terminated alkanethiols in contacts of different adhesion strengths. J. Adhes. Sci. Technol. 2010, 24, 2511–2529. [Google Scholar] [CrossRef]
- Ramin, L.; Jabbarzadeh, A. Effect of compression on self-assembled monolayers: A molecular dynamics study. Modell. Simul. Mater. Sci. Eng. 2012, 20, 085010. [Google Scholar] [CrossRef]
- Ramin, L.; Jabbarzadeh, A. Effect of load on structural and frictional properties of alkanethiol self-assembled monolayers on gold: Some odd-even effects. Langmuir 2012, 28, 4102–4112. [Google Scholar] [CrossRef] [PubMed]
- Ramin, L.; Jabbarzadeh, A. Effect of water on structural and frictional properties of self assembled monolayers. Langmuir 2013, 29, 13367–13378. [Google Scholar] [CrossRef] [PubMed]
- Heister, K.; Rong, H.-T.; Buck, M.; Zharnikov, M.; Grunze, M.; Johansson, L. Odd-even effects at the s-metal interface and in the aromatic matrix of biphenyl-substituted alkanethiol self-assembled monolayers. J. Phys. Chem. B 2001, 105, 6888–6894. [Google Scholar] [CrossRef]
- Shaporenko, A.; Brunnbauer, M.; Terfort, A.; Grunze, M.; Zharnikov, M. Structural forces in self-assembled monolayers: Terphenyl-substituted alkanethiols on noble metal substrates. J. Phys. Chem. B 2004, 108, 14462–14469. [Google Scholar] [CrossRef]
- Azzam, W.; Bashir, A.; Terfort, A.; Strunskus, T.; Wöll, C. Combined stm and ftir characterization of terphenylalkanethiol monolayers on Au (111): Effect of alkyl chain length and deposition temperature. Langmuir 2006, 22, 3647–3655. [Google Scholar] [CrossRef] [PubMed]
- Ramin, L.; Jabbarzadeh, A. Odd-even effects on the structure, stability, and phase transition of alkanethiol self-assembled monolayers. Langmuir 2011, 27, 9748–9759. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.S.; Ferrato, M.-A.; Niec, A.; Biesinger, M.C.; Carmichael, T.B. Ultrasmooth gold surfaces prepared by chemical mechanical polishing for applications in nanoscience. Langmuir 2014, 30, 14171–14178. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-K.; Yu, H.; Lee, T.G.; Lee, N.; Bahng, J.H.; Song, N.W.; Chegal, W.; Shon, H.K.; Koo, J.-Y. Highly-ordered self-assembled monolayer of alkanethiol on thermally annealed polycrystalline gold films. Chem. Phys. 2014, 428, 105–110. [Google Scholar] [CrossRef]
- Tiani, D.J.; Yoo, H.; Mudalige, A.; Pemberton, J.E. Interfacial structure in thin water layers formed by forced dewetting on self-assembled monolayers of ω-terminated alkanethiols on Ag. Langmuir 2008, 24, 13483–13489. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Nania, S.L.; Shaw, S.K. Structure of aqueous water films on textured-OH-terminated self-assembled monolayers. Langmuir 2015, 31, 2382–2389. [Google Scholar] [CrossRef] [PubMed]
- Banner, L.T.; Richter, A.; Pinkhassik, E. Pinhole-free large-grained atomically smooth Au (111) substrates prepared by flame-annealed template stripping. Surf. Interface Anal. 2009, 41, 49–55. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Gathiaka, S.M.; Oyola-Reynoso, S.; Thuo, M. Wettability of n-alkanethiolate self-assembled monolayers correlates more with surface roughness than with the presence of large grains on the substrate. Langmuir. submitted.
- Semaltianos, N.G.; Wilson, E.G. Investigation of the surface morphology of thermally evaporated thin gold films on mica, glass, silicon and calcium fluoride substrates by scanning tunneling microscopy. Thin Solid Films 2000, 366, 111–116. [Google Scholar] [CrossRef]
- Yuan, L.; Jiang, L.; Thompson, D.; Nijhuis, C.A. On the remarkable role of surface topography of the bottom electrodes in blocking leakage currents in molecular diodes. J. Amer. Chem. Soc. 2014, 136, 6554–6557. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Chen, J.; Oyola-Reynoso, S.; Thuo, M. The Porter-Whitesides Discrepancy: Revisiting Odd-Even Effects in Wetting Properties of n-Alkanethiolate SAMs. Coatings 2015, 5, 1034-1055. https://doi.org/10.3390/coatings5041034
Wang Z, Chen J, Oyola-Reynoso S, Thuo M. The Porter-Whitesides Discrepancy: Revisiting Odd-Even Effects in Wetting Properties of n-Alkanethiolate SAMs. Coatings. 2015; 5(4):1034-1055. https://doi.org/10.3390/coatings5041034
Chicago/Turabian StyleWang, Zhengjia, Jiahao Chen, Stephanie Oyola-Reynoso, and Martin Thuo. 2015. "The Porter-Whitesides Discrepancy: Revisiting Odd-Even Effects in Wetting Properties of n-Alkanethiolate SAMs" Coatings 5, no. 4: 1034-1055. https://doi.org/10.3390/coatings5041034






