Graphite–Cement Paste: A New Coating of Reinforced Concrete Structural Elements for the Application of Electrochemical Anti-Corrosion Treatments
Abstract
:1. Introduction
2. Materials and Methods
- Case Study 1: Competency of a GCP anode system versus a reference anode system (mesh of Ti-RuO2), in applying ECE on pillar-size structural reinforced concrete elements.
- Case Study 2: Differences in the shape effect of the structural element during ECE application between both abovementioned types of anode systems.
- Case Study 3: Ability of GCP anode system to apply CP, CPrev, and combined ECE-CP treatments.
2.1. Materials and Methods Used in Case Studies 1 and 2
2.1.1. Reinforced Concrete Laboratory Specimens
2.1.2. Experimental Details of the ECE Tests
2.2. Materials and Methods Used in Case Study 3
2.2.1. Reinforced Concrete Laboratory Specimens
2.2.2. Common Experimental Details of the Electrochemical Tests in Case Study 3
2.2.3. ECE Treatments in Case Study 3
2.2.4. Application of CP, CPrev, and the Combined Treatments ECE+CP and ECE+CPrev
3. Results and Discussion
3.1. Case Study 1: Competency of a GCP Anode System versus a Reference Anode System (Mesh of Ti-RuO2) in Applying ECE on Pillar-Size Structural Reinforced Concrete Elements
3.2. Case Study 2: Differences in the Shape Effect Structural Elements during ECE Application in Both Types of Anode Systems
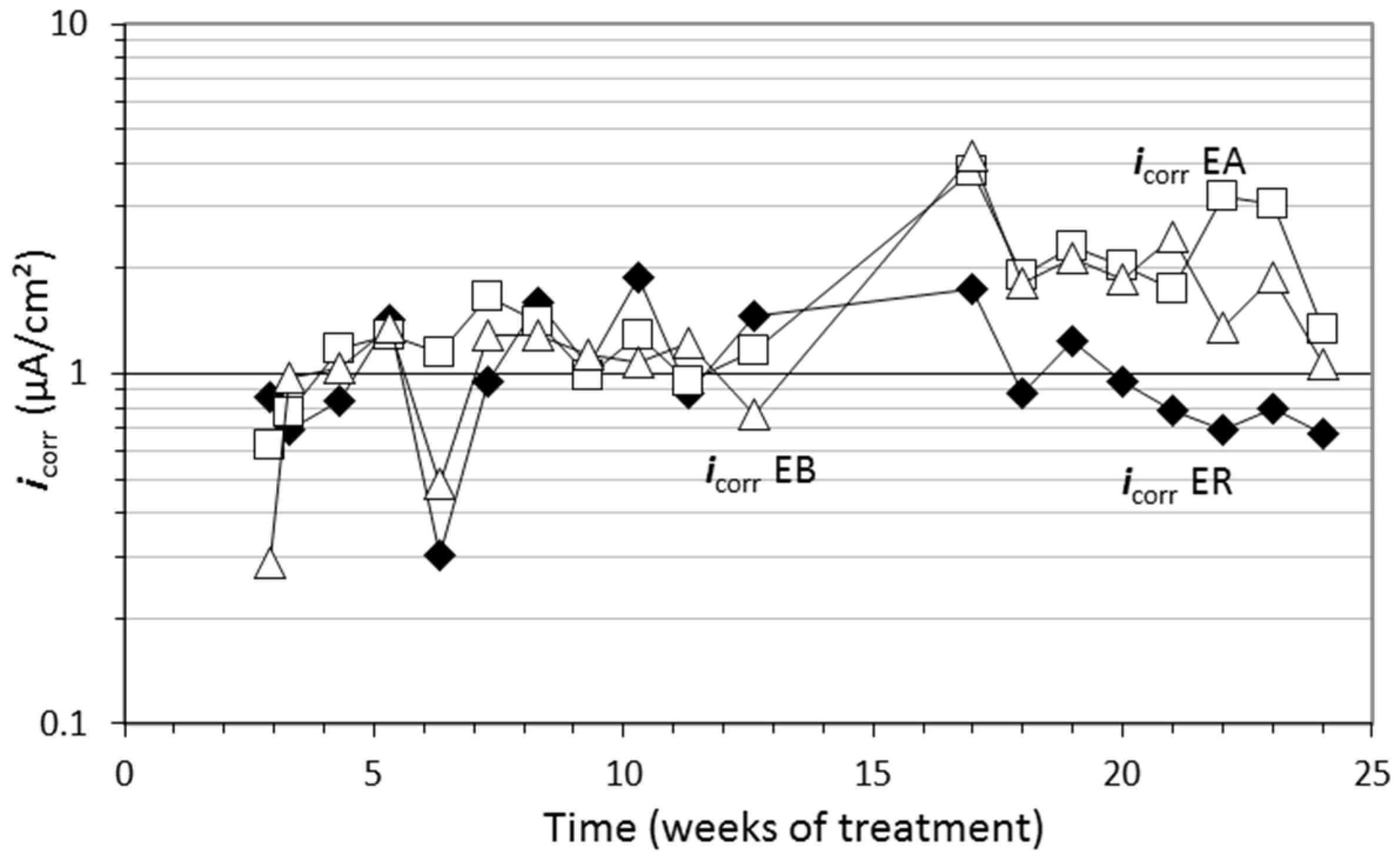
3.3. Case Study 3: Ability of the GCP Anode System to Apply CP, CPrev, and Combined ECE-CP Treatments
4. Conclusions
- For ECE treatments on structural concrete elements, including vertical or those of complex shape, the use of a GCP sprayed coating as anode is feasible, and achieves a similar efficiency (about 80%) to a conventional reference anode (Ti-RuO2 mesh) when passing a typical electric charge density of 5 MC/m2. Also, a substantial proportion of Cl− was removed with a low applied charge density, achieving 51% ECE efficiency at 1.5 MC/m2.
- The results provide confirmation regarding the shape effect of the structural elements on the ECE efficiency. The typical spatial distribution of the steel rebar within certain concrete elements, for example vertical columns of the rectangular section, gives rise to an anisotropic configuration of the electric flow lines. This leads to variations in the local ECE efficiencies found at different sampling points for such types of reinforced concrete elements. The use of GCP anodes, rather than the more commonly employed Ti-RuO2 mesh embedded between polymeric layers, appears to reduce the differences found in the local ECE efficiencies due to the shape effect. This may be due to the nature of the electrolytic contact between the GCP coating and the concrete surface, which may avoid possible zones of high electric resistance due to defective contact between the somewhat rigid and springy metal mesh anodic system and the concrete surface.
- According to the results of the CP applied with a GCP coating as anode, this electrochemical technique is able to provide protection to steel, despite severe internal and external chloride contamination, provided an appropriate current density value is selected based on the Cl− content present. Its ability to recover the lost protection conditions through an increase of current density has also been demonstrated.
- Continuously applied CPrev (at a current density of 2 mA/m2) with a GCP coating as anode is able to maintain a conventional, initially Cl−-free concrete in a protection condition despite being exposed to a severe Cl− load. However, subject to the same experimental external chloride load, it is unable to recover protection conditions if the initial Cl− content is equal to or higher than 1% relative to cement mass.
- A GCP coating allows the application of a combined treatment of ECE followed by CP, changing only the current density provided by the DC power source. No damage was observed in this anode after the full combined process. This combination has been shown to be capable of maintaining or recovering protection conditions if the cathodic current density is set to the appropriate value according to the Cl− content present.
- Given the novel nature of the GCP coating and its relatively short track record, it is recommended that further research is carried out to assess the GCP coating in a range of operating conditions to fully ascertain its suitability for practical applications.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lankard, D.R.; Slater, J.E.; Hedden, W.A.; Niesz, D.E. Neutralization of Chloride in Concrete. In Battelle Columbus Laboratories; Report No. FHWA-RD-76-60; Federal Highway Administration: Washington, DC, USA, 1975; p. 143. [Google Scholar]
- Slater, J.E.; Lankard, D.R.; Moreland, P.J. Electrochemical removal of chlorides from concrete bridge decks. Mater. Perform. 1976, 15, 21–26. [Google Scholar]
- Tritthart, J. Electrochemical Chloride Removal: An Overview and Scientific Aspects; The American Ceramic Society: Westerville, OH, USA, 1998; pp. 401–441. [Google Scholar]
- Mietz, J. Electrochemical Rehabilitation Methods for Reinforced Concrete Structures: A State of the Art Report. In Publication Number 24 of the European Federation of Corrosion; IOM Communications Ltd.: London, UK, 1998; p. 57. [Google Scholar]
- Andrade, C.; Castellote, M.; Alonso, C. An overview of electrochemical realkalisation and chloride extraction. In Rehabilitation of Structures, Proceedings of 2nd International RILEM/CSIRO/ACRA Conference, Melbourne, Australia, 21–23 September 1998; Ho, D.W.S., Godson, I., Collins, F., Eds.; RILEM: Melbourne, Australia, 1998; pp. 1–12. [Google Scholar]
- Pedeferri, P.; Bertolini, L. La Durabilità del Calcestruzzo Armato; McGraw-Hill: Milano, Italy, 2000. (In Italian) [Google Scholar]
- Bertolini, L.; Elsener, B.; Pedeferri, P.; Polder, R. Corrosion of Steel in Concrete; Wiley-Vch.: Weinheim, Germany, 2004. [Google Scholar]
- Climent, M.A.; Sánchez de Rojas, M.J.; de Vera, G.; Garcés, P. Effect of type of anodic arrangements on efficiency of electrochemical chloride removal from concrete. ACI Mater. J. 2006, 103, 243–250. [Google Scholar]
- Glass, G.K.; Chadwick, J.R. An investigation into the mechanisms of protection afforded by a cathodic current and the implications for advances in the field of cathodic protection. Corros. Sci. 1994, 36, 2193–2209. [Google Scholar] [CrossRef]
- Christodoulou, C.; Glass, G.; Webb, J.; Austin, S.; Goodier, C. Assessing the long term benefits of impressed current cathodic protection. Corros. Sci. 2010, 52, 2671–2679. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Chung, D.D.L. Carbon fiber reinforced mortar as an electrical contact material for cathodic protection. Cem. Concr. Res. 1995, 25, 689–694. [Google Scholar] [CrossRef]
- Hou, J.; Chung, D.D.L. Cathodic protection of steel reinforced concrete facilitated by using carbon fiber reinforced mortar or concrete. Cem. Concr. Res. 1997, 27, 649–656. [Google Scholar] [CrossRef]
- DePeuter, F.; Lazzari, L. New conductive overlay for CP in concrete: Results of long term testing. In Proceedings of Corrosion/93-National Association of Corrosion Engineers Annual Conference, New Orleans, LA, USA, 7–12 March 1993. no. 325.
- Bertolini, L.; Bolzoni, F.; Pastore, T.; Pedeferri, P. Effectiveness of a conductive cementitious mortar anode for cathodic protection of steel in concrete. Cem. Concr. Res. 2004, 34, 681–694. [Google Scholar] [CrossRef]
- Chung, D.D.L. Electrically conductive cement-based materials. Adv. Cem. Res. 2004, 16, 167–176. [Google Scholar] [CrossRef]
- Garcés, P.; Fraile, J.; Vilaplana-Ortego, E.; Cazorla, D.; Alcocel, E.G.; Andión, L.G. Effects of carbon fibres on the mechanical properties and corrosion levels of reinforced Portland cement mortars. Cem. Concr. Res. 2005, 35, 324–331. [Google Scholar] [CrossRef]
- Garcés, P.; Andión, L.G.; de la Varga, I.; Catalá, G.; Zornoza, E. Corrosion of steel reinforcement in structural concrete with carbon material addition. Corros. Sci. 2007, 49, 2557–2566. [Google Scholar] [CrossRef]
- Zornoza, E.; Catalá, G.; Jiménez, F.; Andión, L.G.; Garcés, P. Electromagnetic interference shielding with Portland cement paste containing carbon materials and processed fly ash. Mater. Constr. 2010, 60, 21–32. [Google Scholar]
- Garcés, P.; Zornoza, E.; Alcocel, E.G.; Galao, Ó.; Andión, L.G. Mechanical properties and corrosion of CAC mortars with carbon fibers. Constr. Build. Mater. 2012, 34, 91–96. [Google Scholar] [CrossRef]
- Han, B.; Yu, X.; Ou, J. Multifunctional and Smart Carbon Nanotube Reinforced Cement-Based Materials. In Nanotechnology in Civil Infrastructure: A Paradigm Shift; Gopalakrishnan, K., Birgisson, B., Taylor, P., Attoh-Okine, N.O., Eds.; Springer: Berlin, Heidelberg, Germany, 2011; pp. 1–47. [Google Scholar]
- Pérez, A.; Climent, M.A.; Garcés, P. Electrochemical extraction of chlorides from reinforced concrete using a conductive cement paste as the anode. Corros. Sci. 2010, 52, 1576–1581. [Google Scholar] [CrossRef]
- Cañón, A.; Garcés, P.; Climent, M.A.; Carmona, J.; Zornoza, E. Feasibility of electrochemical chloride extraction from structural reinforced concrete using a sprayed conductive graphite powder-cement as anode. Corros. Sci. 2013, 77, 128–134. [Google Scholar] [CrossRef]
- Garcés, P.; Sánchez de Rojas, M.J.; Climent, M.A. Effect of the reinforcement bar arrangement on the efficiency of electrochemical chloride removal technique applied to the reinforced concrete structures. Corros. Sci. 2006, 48, 531–545. [Google Scholar] [CrossRef]
- Carmona, J.; Climent, M.A.; Antón, C.; de Vera, G.; Garcés, P. Shape effect of electrochemical chloride extraction in structural reinforced concrete elements using a new cement-based anodic system. Materials 2015, 8, 2901–2917. [Google Scholar] [CrossRef] [Green Version]
- Pedeferri, P. Cathodic protection and cathodic prevention. Constr. Build. Mat. 1996, 10, 391–402. [Google Scholar] [CrossRef]
- Bertolini, L.; Bolzoni, F.; Pedeferri, P.; Lazzari, L.; Pastore, T. Cathodic protection and cathodic prevention in concrete principles and applications. J. Appl. Electrochem. 1998, 28, 1321–1331. [Google Scholar] [CrossRef]
- Carmona, J.; Garcés, P.; Climent, M.A. Efficiency of a conductive cement-based anodic system for the application of cathodic protection, cathodic prevention and electrochemical chloride extraction to control corrosion in reinforced concrete structures. Corros. Sci. 2015, 96, 102–111. [Google Scholar] [CrossRef]
- Comisión Permanente del Hormigón. Instrucción de Hormigón Estructural EHE-08 (Spanish Code on Structural Concrete EHE-08); Ministerio de Fomento: Madrid, Spain, 2008. (In Spanish) [Google Scholar]
- UNE EN 12390–3:2009 Ensayos de Hormigón Endurecido. Parte 3: Determinación de la Resistencia a Compresión de Probetas (Testing Hardened Concrete–Part 3: Compressive Strength of Test Specimens); Asociación Española de Normalización y Certificación AENOR: Madrid, Spain, 2009. (In Spanish)
- UNE 83980:2014 Durabilidad del Hormigón. Métodos de Ensayo. Determinación de la Absorción de Agua, la Densidad y la Porosidad Accesible al Agua del Hormigón (Concrete Durability. Test Methods. Determination of the Water Absorption, Density and Accessible Porosity for Water in Concrete); Asociación Española de Normalización y Certificación AENOR: Madrid, Spain, 2014. (In Spanish)
- UNE EN 12390–7:2009 Ensayos de Hormigón Endurecido. Parte 7: Densidad del Hormigón Endurecido (Testing Hardened Concrete–Part 7: Density of Hardened Concrete); Asociación Española de Normalización y Certificación AENOR: Madrid, Spain, 2009. (In Spanish)
- CEN/TS 14038–2 Technical Specification. Electrochemical Re-alkalization and Chloride Extraction Treatments for Reinforced Concrete–Part 2: Chloride Extraction; European Committee of Standardization CEN: Brussels, Belgium, 2011.
- Elsener, B.; Molina, M.; Böhni, H. The electrochemical removal of chlorides from reinforced concrete. Corros. Sci. 1993, 35, 1563–1570. [Google Scholar] [CrossRef]
- Elsener, B.; Angst, U. Mechanism of electrochemical chloride removal. Corros. Sci. 2007, 49, 4504–4522. [Google Scholar] [CrossRef]
- Sánchez de Rojas, M.J.; Garcés, P.; Climent, M.A. Electrochemical extraction of chlorides from reinforced concrete: variables affecting treatment efficiency. Mater. Constr. 2006, 56, 17–26. [Google Scholar]
- Vennesland, Ø.; Climent, M.A.; Andrade, C. Recommendation of RILEM TC 178-TMC: Testing and modeling chloride penetration in concrete. Methods for obtaining dust samples by means of grinding concrete in order to determine the chloride concentration profile. Mater. Struct. 2013, 46, 337–344. [Google Scholar]
- Climent, M.A.; Viqueira, E.; de Vera, G.; López, M.M. Analysis of acid-soluble chloride in cement, mortar and concrete by potentiometric titration without filtration steps. Cem. Concr. Res. 1999, 29, 893–898. [Google Scholar] [CrossRef]
- Climent, M.A.; de Vera, G.; Viqueira, E.; López, M.M. Generalization of the possibility of eliminating the filtration step in the determination of acid-soluble chloride content in cement and concrete by potentiometric titration. Cem. Concr. Res. 2004, 34, 2291–2295. [Google Scholar] [CrossRef]
- Galao, O.; Baeza, F.J.; Zornoza, E.; Garcés, P. Strain and damage sensing properties on multifunctional cement composites with CNF. Cem. Concr. Comp. 2014, 46, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Andrade, C.; Alonso, C.; Gulikers, J.; Polder, R.; Cigna, R.; Vennesland, Ø.; Salta, M. Test methods for on-site reinforcement corrosion rate measurement of steel reinforcement in concrete by means of the Polarization Resistance method. RILEM Recommendation of TC-154. Electrochemical Techniques for measuring metallic corrosion. Mater. Struct. 2004, 37, 623–643. [Google Scholar] [CrossRef]
- ISO 12696: 2012(en) Cathodic Protection of Steel in Concrete; ISO: Geneva, Switzerland, 2012.
- Glass, G.K.; Hassanein, A.M.; Buenfeld, N.R. Cathodic protection afforded by an intermittent current applied to reinforced concrete. Corros. Sci. 2001, 43, 1111–1131. [Google Scholar] [CrossRef]
- Pellegrini-Cervantes, M.J.; Barrios-Durstewitz, C.P.; Núñez-Jaquez, R.E.; Almeraya-Calderón, F.; Rodríguez-Rodríguez, M.; Castorena-González, J.H.; Garcés-Velázquez, E.; Maldonado-Bandala, E.E.; Nieves-Mendoza, D.; García-Contreras, J.P. Conductive cement pastes with carbon fibers as anodes in the electrochemical chloride extraction. Int. J. Electrochem. Sci. 2015, 10, 3830–3840. [Google Scholar]
- Miranda, J.M.; González, J.A.; Cobo, A.; Otero, E. Several questions about electrochemical rehabilitation methods for reinforced concrete structures. Corros. Sci. 2006, 48, 2172–2188. [Google Scholar] [CrossRef]
- Miranda, J.M.; Cobo, A.; Otero, E.; González, J.A. Limitations and advantages of electrochemical chloride removal in corroded reinforced concrete structures. Cem. Concr. Res. 2007, 37, 596–603. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, X. Modeling cathodic prevention for unconventional concrete in salt-laden environment. Anti-Corros. Methods Mater. 2012, 59, 121–131. [Google Scholar] [CrossRef]
- Dugarte, M.; Sagüés, A.A.; Williams, K. Cathodic prevention for reinforcing steel in cracked concrete of chloride contaminated structures. In Proceedings of CORROSION 2015 Conference, NACE-2015-6102, NACE International, Houston, TX, USA, 15–19 March 2015; p. 11.

















| Material | Dosage |
|---|---|
| Portland cement | 350 kg/m3 |
| w/c Ratio | 0.6 |
| Distilled water | 210 kg/m3 |
| Limestone aggregate 4/6 | 466 kg/m3 |
| Limestone aggregate 6/12 | 679 kg/m3 |
| Limestone sand | 630 kg/m3 |
| NaCl | 3.3% (2% Cl− relative to cement mass) |
| Material | Dosage (for 5 kg of Paste) |
|---|---|
| Portland cement | 1.39 kg |
| Graphite powder | 1.39 kg |
| Distilled water | 2.22 kg |
| Studied Techniques | Initial % Cl− in Concrete | Reference Sample | Treated Samples |
|---|---|---|---|
| CP | 2 | R (no electrochemical treatment) | A (CP treated) |
| CPrev | 0 | P (no electrochemical treatment) | B (CPrev treated) |
| ECE+CP | 2 | ER (treated only with ECE) | EA (ECE+CP treated) |
| ECE+CPrev | 2 | EB (ECE+CPrev treated) |
| Material | Dosage |
|---|---|
| Portland cement CEM I 42.5 R | 250 kg/m3 |
| w/c Ratio | 0.65 |
| Limestone aggregate max. size 12 mm | 1890 kg/m3 |
| Superplasticizer | 2.50 kg/m3 |
| NaCl | Nil or 3.3% (2% Cl− relative to cement mass) |
| Initial % Cl− | Current Density | Initial ∆Efeed | Final ∆Efeed | Electric Charge Density |
|---|---|---|---|---|
| 2 % relative to cement mass | 2 A/m2 of concrete exposed surface (3.4 A/m2 of steel surface) | 16–24 V | 23–22 V | 1.5 MC/m2 of concrete exposed surface (2.6 MC/m2 of steel surface) |
| Trial | Horizontal Section of Specimen | Anode System | Core Sample Location | Average Efficiency (%) |
|---|---|---|---|---|
| 1 | Circular section | Ti-RuO2 | – | 82.8 |
| 2 | Circular section | GCP | – | 82.6 |
| 3 | Rectangular section | Ti-RuO2 | Center of the biggest face | 52.5 |
| 4 | Rectangular section | Ti-RuO2 | Concrete cover over steel | 85.2 |
| 5 | Rectangular section | GCP | Center of the biggest face | 64.4 |
| 6 | Rectangular section | GCP | Concrete cover over steel | 76.2 |
| Specimen | Initial Cl− Content (% ref. cem. mass) | Electrochemical Treatment Previous to the 24-week First Phase | Electrochemical Treatment during the 24-week First Phase | Final Averaged* Cl− Content (% ref. cem. mass) |
|---|---|---|---|---|
| P | 0 | – | – | 4.93 |
| R | 2 | – | – | 6.08 |
| ER | 2 | ECE | – | 4.26 |
| A | 2 | – | CP | 5.39 |
| B | 0 | – | CPrev | 3.94 |
| EA | 2 | ECE | CP | 3.41 |
| EB | 2 | ECE | CPrev | 3.42 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Climent, M.-Á.; Carmona, J.; Garcés, P. Graphite–Cement Paste: A New Coating of Reinforced Concrete Structural Elements for the Application of Electrochemical Anti-Corrosion Treatments. Coatings 2016, 6, 32. https://doi.org/10.3390/coatings6030032
Climent M-Á, Carmona J, Garcés P. Graphite–Cement Paste: A New Coating of Reinforced Concrete Structural Elements for the Application of Electrochemical Anti-Corrosion Treatments. Coatings. 2016; 6(3):32. https://doi.org/10.3390/coatings6030032
Chicago/Turabian StyleCliment, Miguel-Ángel, Jesús Carmona, and Pedro Garcés. 2016. "Graphite–Cement Paste: A New Coating of Reinforced Concrete Structural Elements for the Application of Electrochemical Anti-Corrosion Treatments" Coatings 6, no. 3: 32. https://doi.org/10.3390/coatings6030032







