Combustion Synthesis during Flame Spraying (“CAFSY”) for the Production of Catalysts on Substrates
Abstract
:1. Introduction
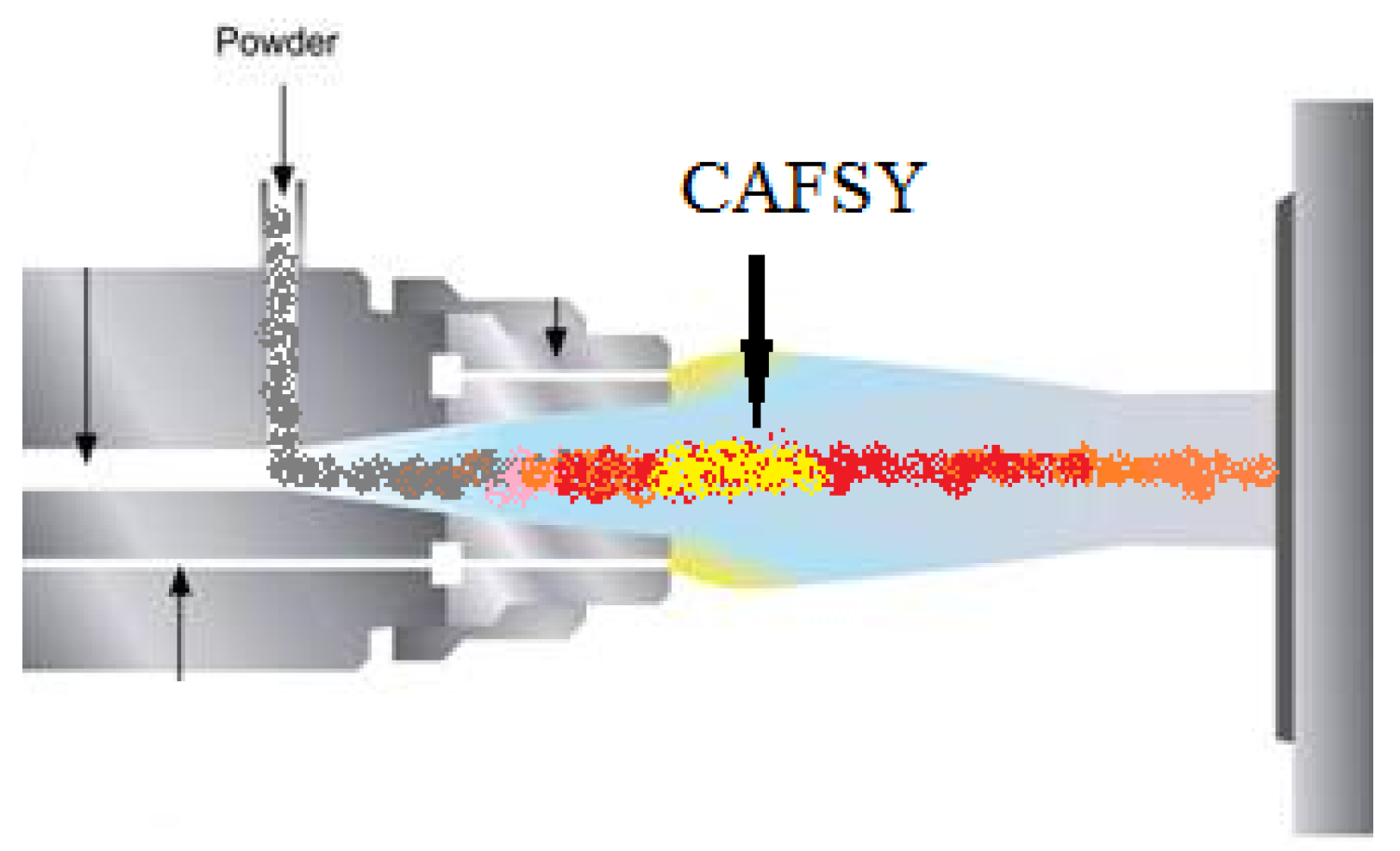
The CAFSY Method for Spraying Catalytically Active Coatings
2. Materials and Methods
2.1. Materials
2.2. CAFSY Process
2.3. Catalyst Characterisation
2.4. Conditions for Catalytic Dry Reforming of Methane
3. Results andDiscussion
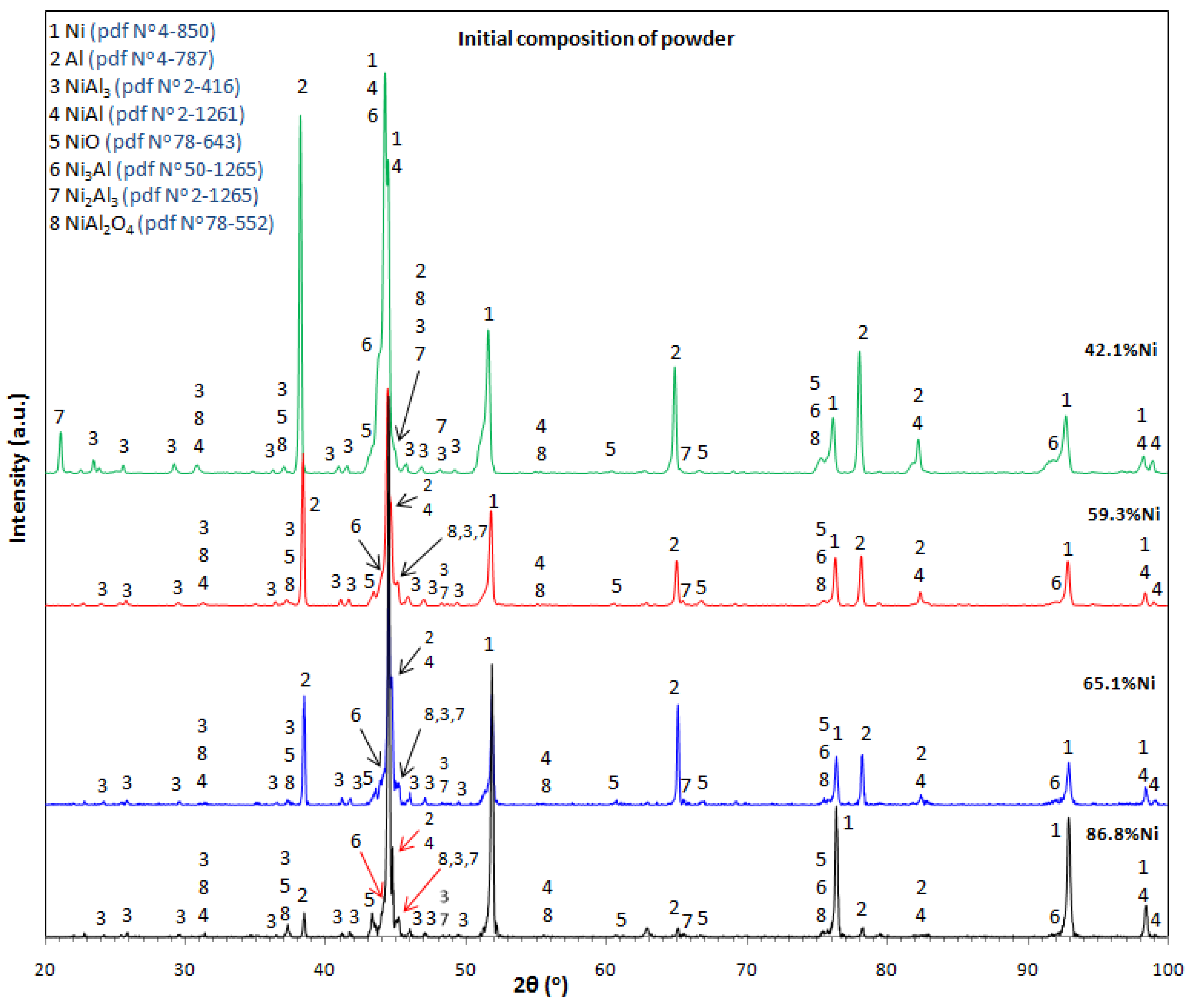
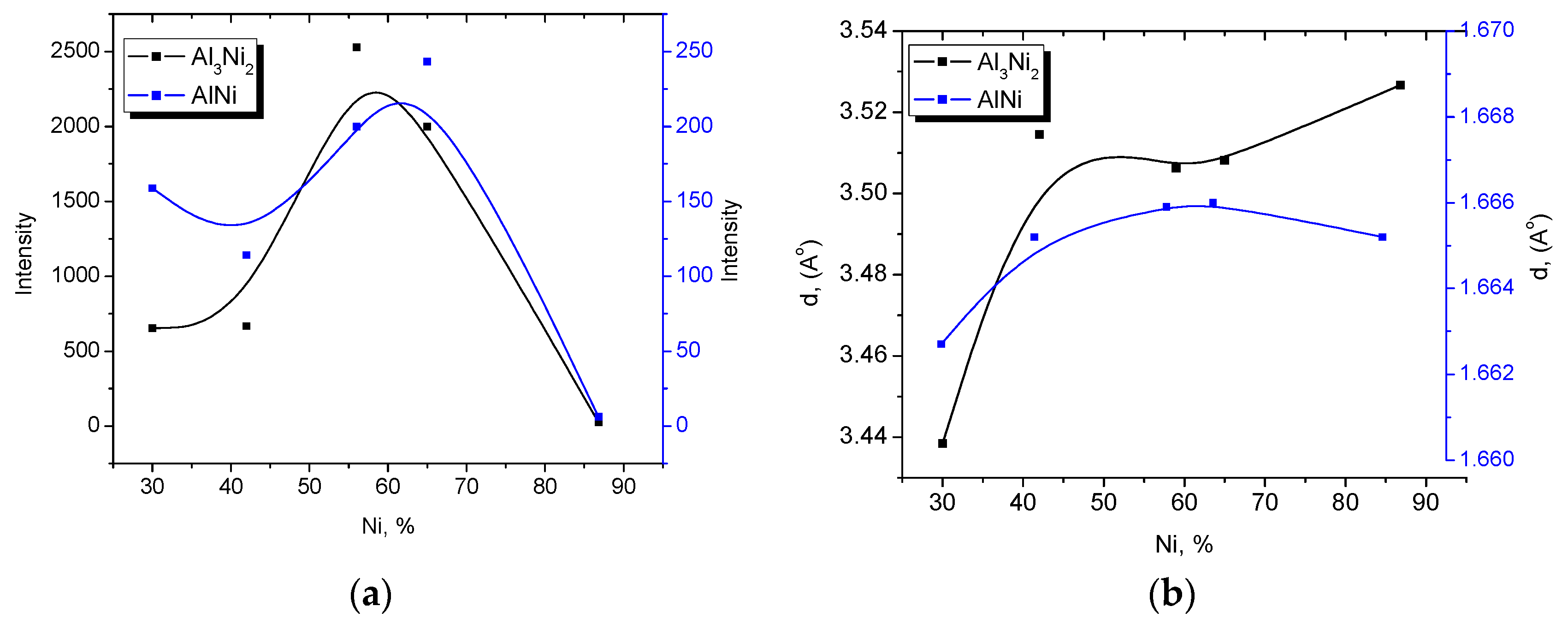
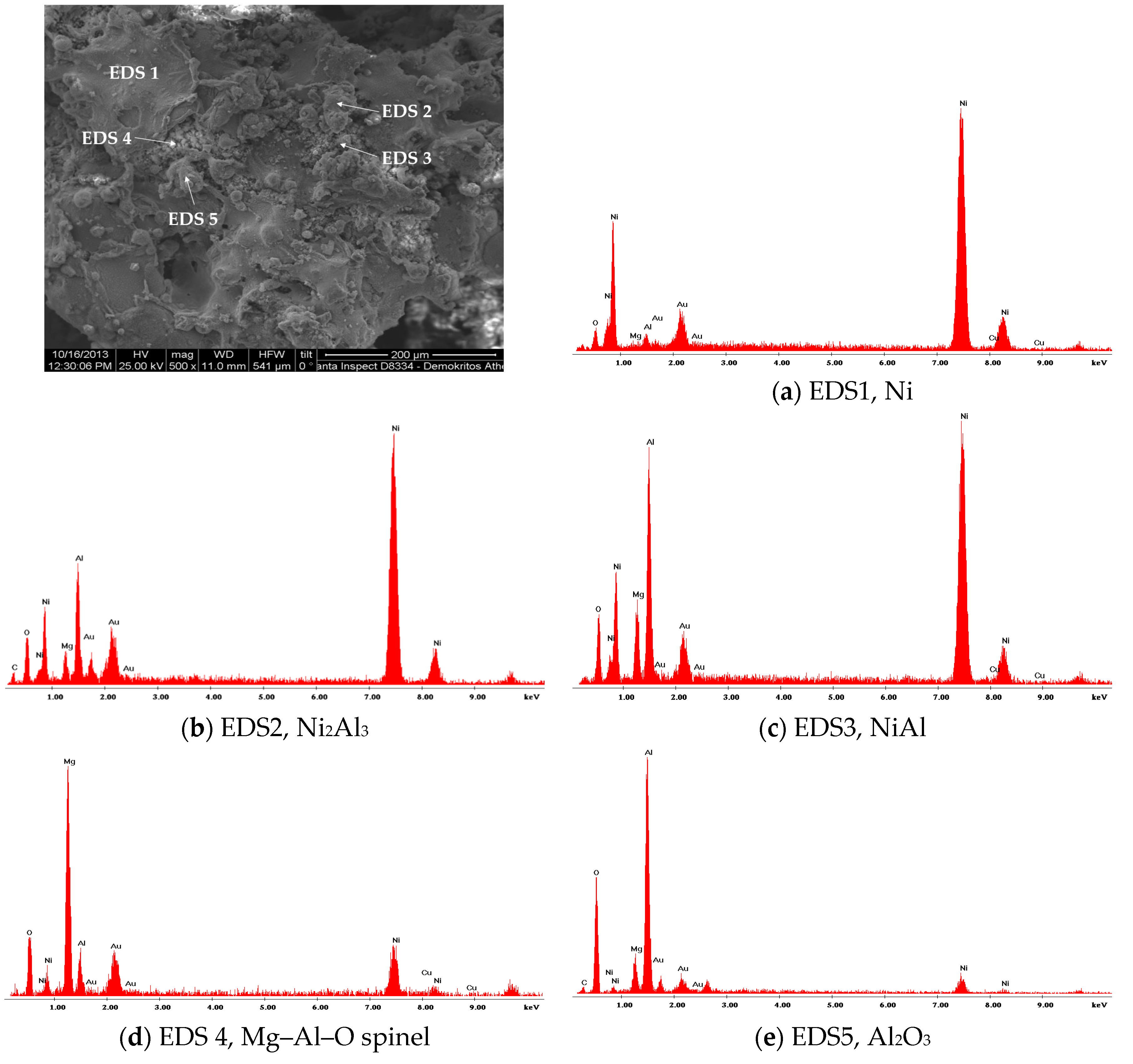
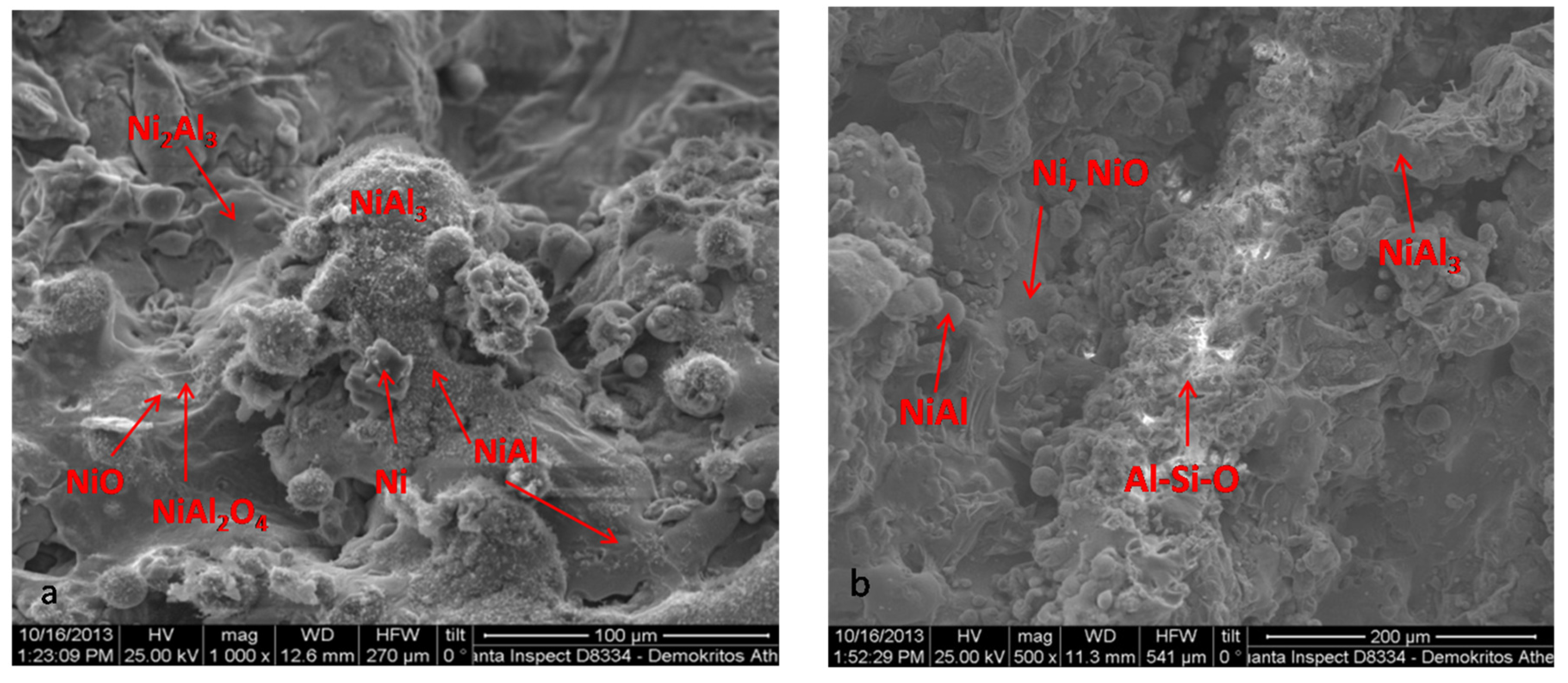
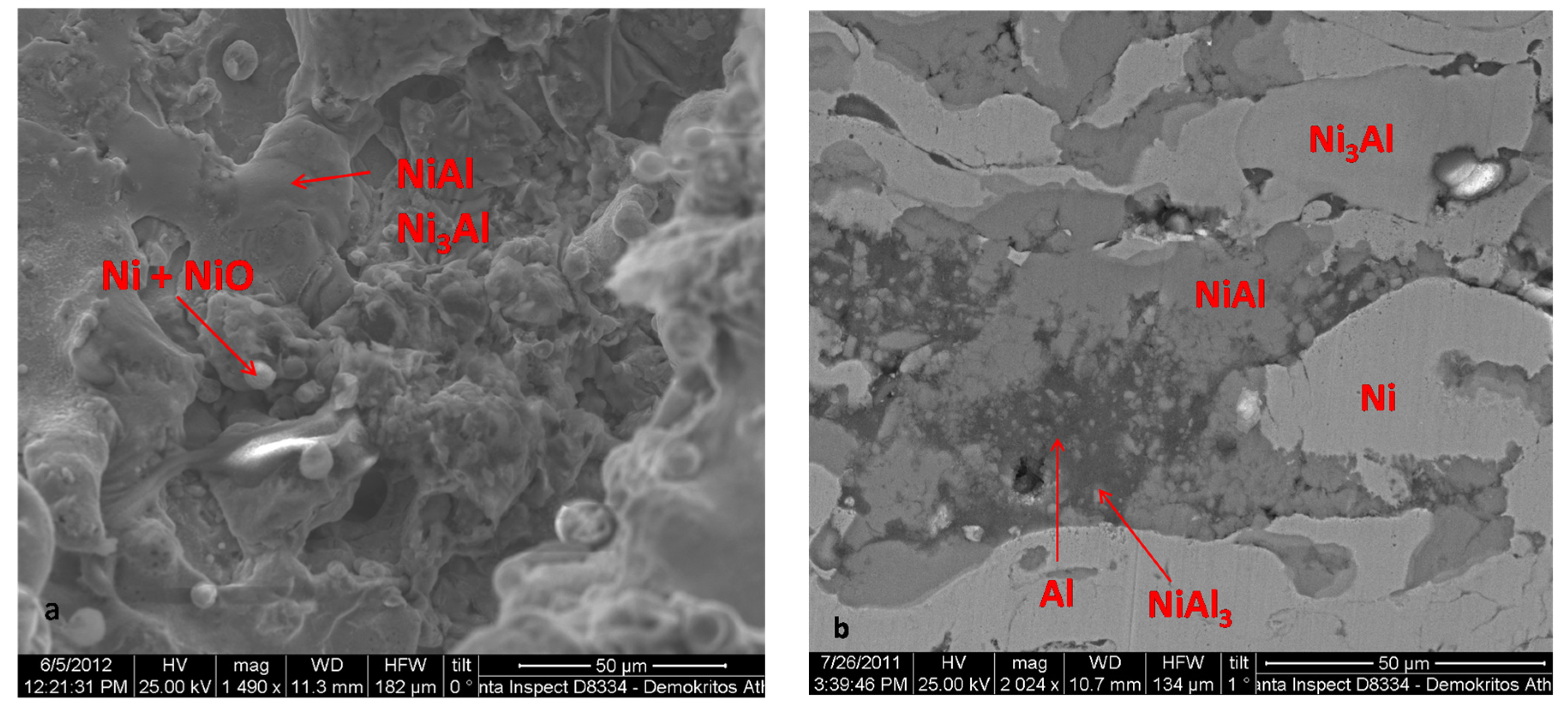
3.1. Catalyst Characterisation
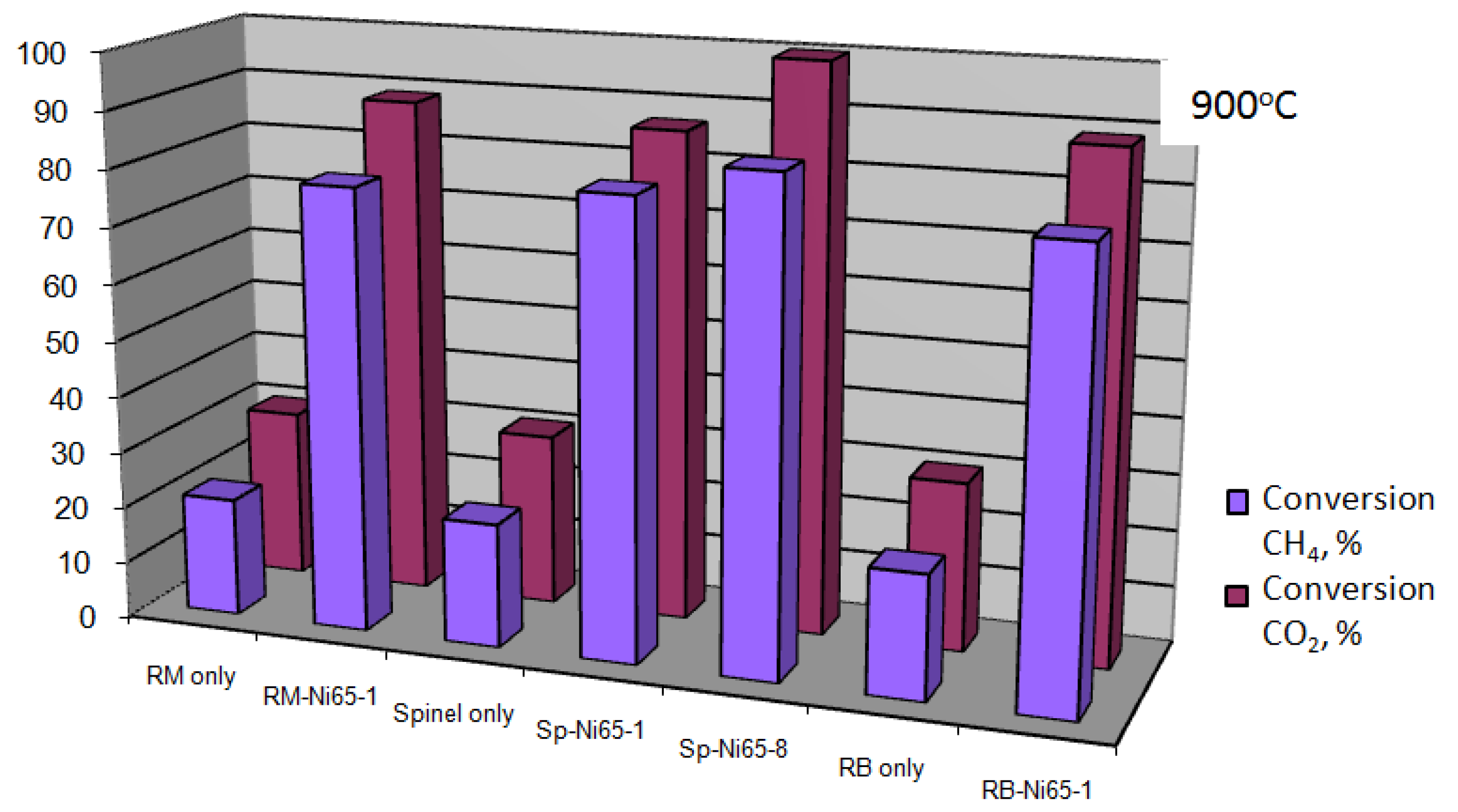
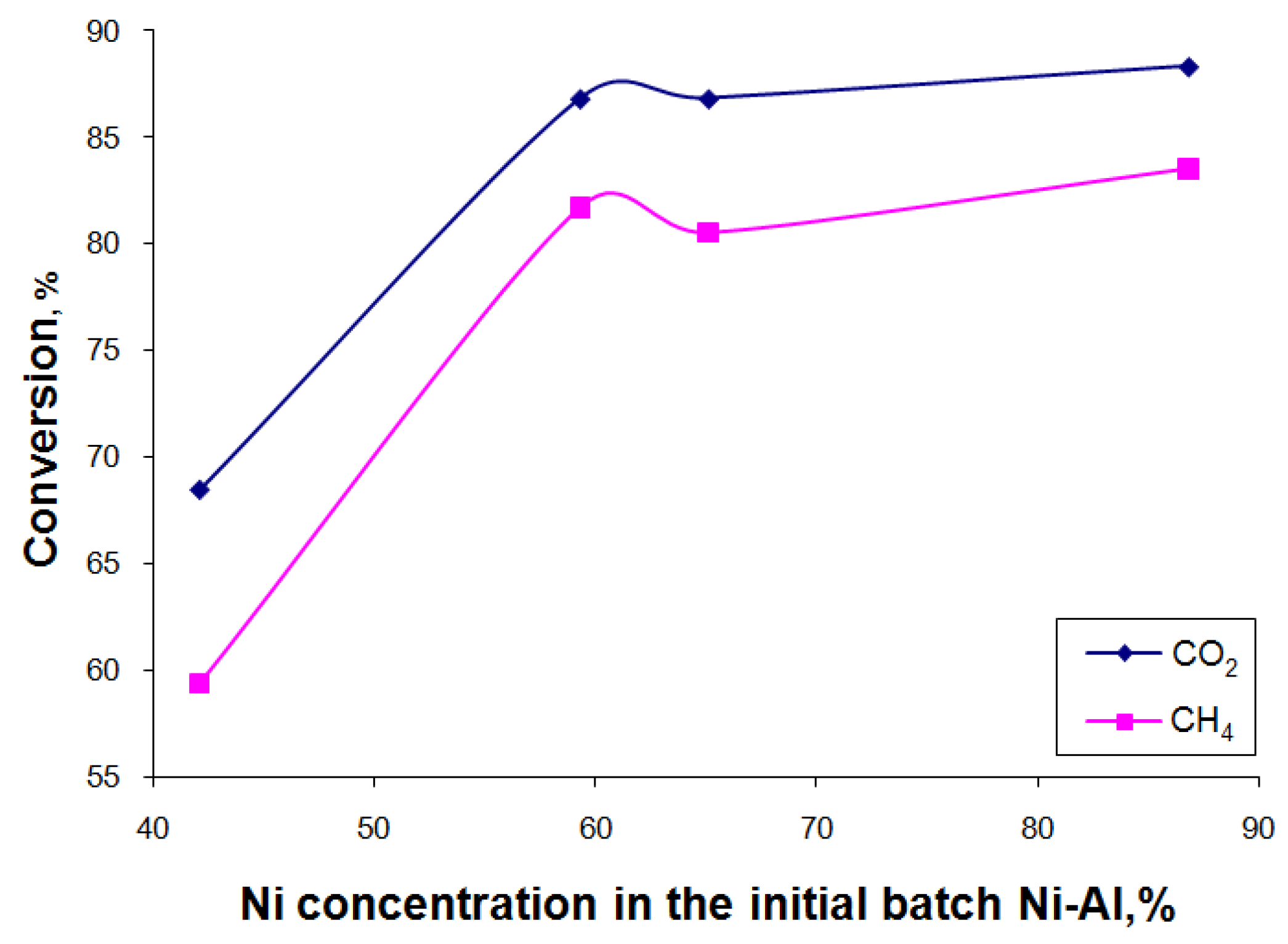
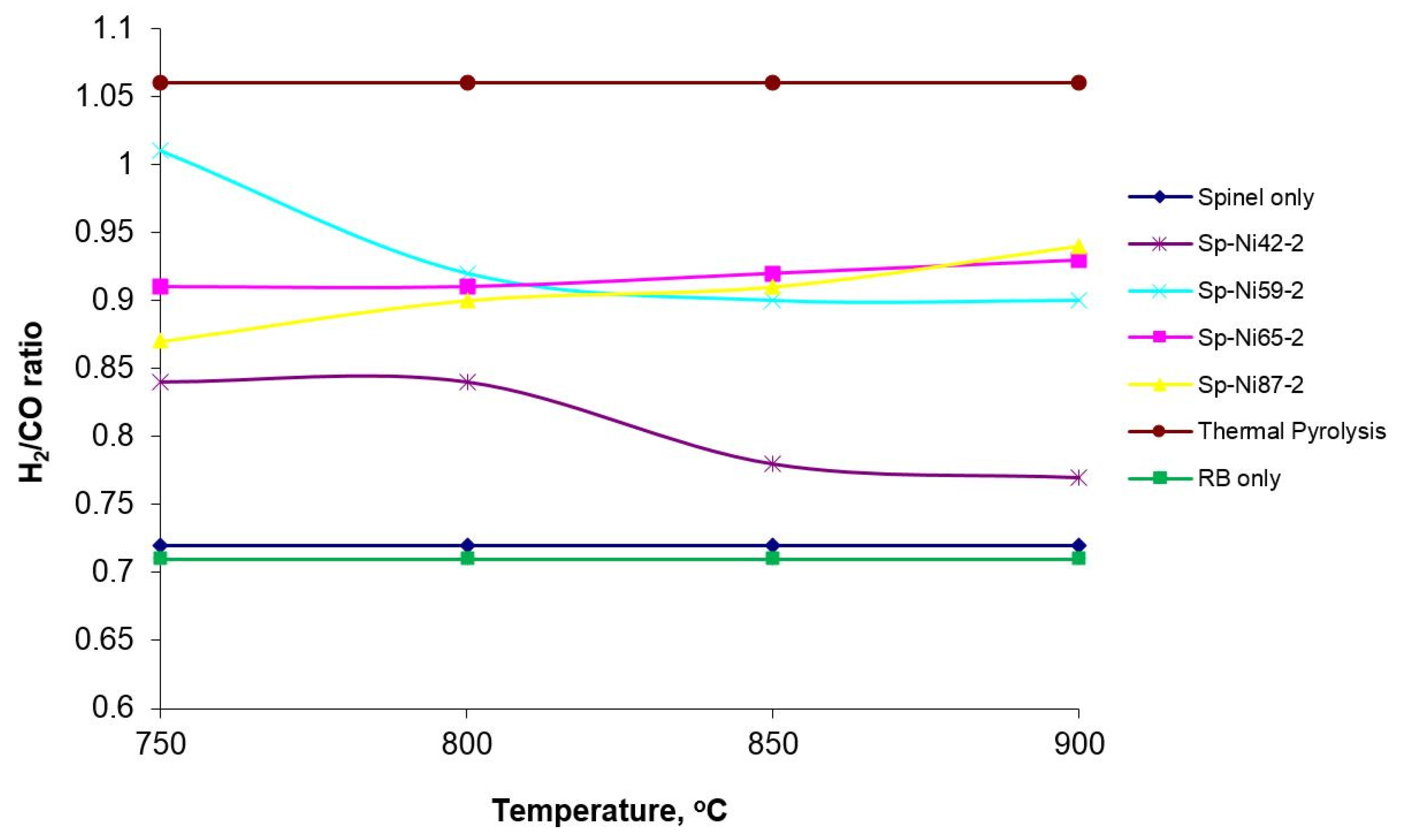
3.2. Catalytic Activity for Dry Reforming of Methane
Influence of Substrate Composition
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marinou, A.; Xanthopoulou, G.; Vekinis, G.; Lekatou, A.; Vardavoulias, M. Synthesis and heat treatment of sprayed high-temperature NiAl-Ni3Al coatings by in-flight combustion synthesis (CAFSY). Int. J. SHS 2015, 24, 192–201. [Google Scholar] [CrossRef]
- Xanthopoulou, G.; Marinou, A.; Vekinis, G.; Lekatou, A.; Vardavoulias, M. NiAl and NiO-Al composite coatings by combustion-assisted flame spraying. Coatings 2014, 4, 231–252. [Google Scholar] [CrossRef]
- Xanthopoulou, G. Self-propagating high-temperature synthesis as method of catalysts production. Sci. Cent. Asia 2010, 4, 35–56. [Google Scholar]
- Xanthopoulou, G.; Vekinis, G. Catalytic oxidation of CO over a Cu-Cr-oxide catalyst made by self-propagating high-temperature synthesis. Appl. Catal. B Environ. 1998, 19, 37–44. [Google Scholar] [CrossRef]
- Xanthopoulou, G. Oxide catalysts for pyrolysis of diesel fuel made by self-propagating high-temperature synthesis. Part I: Cobalt-modified Mg-Al spinel catalysts. Appl. Catal. A Gen. 1999, 182, 285–295. [Google Scholar] [CrossRef]
- Xanthopoulou, G. Oxide catalysts for pyrolysis of diesel fuel made by self-propagating high-temperature synthesis(SHS): Part II: Fe-Cr oxide catalysts based on chromite concentrates. Appl. Catal. A Gen. 1999, 187, 79–88. [Google Scholar] [CrossRef]
- Xanthopoulou, G. Oxidative dehydrodimerization of methane using lead and samarium based catalysts made by self-propagating high-temperature synthesis. Appl. Catal. A Gen. 1999, 185, L185–L192. [Google Scholar] [CrossRef]
- Xanthopoulou, G.; Vekinis, G. Deep oxidation of methane using catalysts and carriers produced by self-propagating high temperature synthesis. Appl. Catal. A Gen. 2000, 199, 227–238. [Google Scholar] [CrossRef]
- Xanthopoulou, G.; Vekinis, G. An overview of some environmental applications of the self-propagating high-temperature synthesis. Adv. Environ. Res. 2001, 5, 117–128. [Google Scholar] [CrossRef]
- Xanthopoulou, G.; Vekinis, G. Catalytic pyrolysis of naphtha on the SHS catalysts. Eurasian Chem. Technol. J. 2010, 12, 17–21. [Google Scholar] [CrossRef]
- Dinka, P.; Mukasyan, A.S. Solution combustion catalysts for steam reforming of JP-8 surrogate. J. Power Sources 2007, 167, 472–481. [Google Scholar] [CrossRef]
- Hirano, T.; Purwanto, H.; Akiyama, T.W.T. Self-propagating high-temperature synthesis with post-heat treatment of La1−xSrxFeO3 (x = 0–1) perovskite as catalyst for soot combustion. J. Alloy. Compd. 2009, 470, 245–249. [Google Scholar] [CrossRef]
- Xanthopoulou, G. Catalytic properties of the SHS products—Review. Adv. Sci. Technol. 2010, 63, 287–296. [Google Scholar] [CrossRef]
- Karanasios, K.; Xanthopoulou, G.; Vekinis, G.; Zoumpoulakis, L. Co-Al oxide SHS catalysts for dry reforming of methane. Int. J. Self-Propag. High-Temp. Synth. 2014, 23, 222–231. [Google Scholar] [CrossRef]
- Kim, W.Y.; Lee, Y.H.; Park, H.; Choi, Y.H.; Lee, M.H.; Lee, J.S. Coke tolerance of Ni/Al2O3 nanosheet catalyst for dry reforming of methane. Catal. Sci. Technol. 2016, 6, 2060–2064. [Google Scholar] [CrossRef]
- Xu, S.; Wang, X. Highly active and coking resistant Ni/CeO2–ZrO2 catalyst for partial oxidation of methane. J. Fuel 2005, 84, 563–567. [Google Scholar] [CrossRef]
- Pengpanich, S.; Meeyoo, V.; Riksomboon, T. Methane partial oxidation over Ni/CeO2–ZrO2 mixed oxide solid solution catalysts. J. Catal. Today 2004, 93–95, 95–105. [Google Scholar] [CrossRef]
- Larimi, S.; Alavi, S.M. Partial oxidation of methane over Ni/CeZrO2 mixed oxide solid solution catalysts. Int. J. Chem. Eng. Appl. 2012, 3, 6–9. [Google Scholar]
- Soloviev, S.O.; Kapran, A.Y.; Orlyk, S.N.; Gubareni, E.V. Carbon dioxide reforming of methane on monolithic Ni/Al2O3-based catalysts. J. Natur. Gas Chem. 2011, 20, 184–190. [Google Scholar] [CrossRef]
- Fidalgo, B.; Arenillas, A.; Menindez, J.A. Synergetic effect of a mixture of activated carbon + Ni/Al2O3 used as catalysts for the CO2 reforming of CH4. Appl. Catal. Ser. A 2010, 390, 78–83. [Google Scholar] [CrossRef]
- Moniri, A.; Alavi, S.M.; Rezaei, M. Syngas production by combined carbon dioxide reforming and partial oxidation of methane over Ni/α-Al2O3 catalysts. J. Nat. Gas Chem. 2010, 19, 638–641. [Google Scholar] [CrossRef]
- ALuna, E.C.; Iriarte, M.E. Carbon dioxide reforming of methane over a metal modified Ni–Al2O3 catalyst. Appl. Catal. Ser. A 2008, 343, 10–15. [Google Scholar]
- Meshkani, F.; Rezaei, M. Nanocrystalline MgO supported nickel-based bimetallic catalysts for carbon dioxide reforming of methane. Int. J. Hydrog. Energy 2010, 35, 10295–10301. [Google Scholar] [CrossRef]
- Xanthopoulou, G.; Varitis, S.; Karanasios, K.; Vekinis, G. SHS-Produced Ni-Co-Al-Mg-O catalysts for dry reforming of methane. SHS J. 2014, 23, 92–100. [Google Scholar] [CrossRef]
- Sikka, S.K.; Nair, G.J.; Roy, F.; Kakodkar, A.; Chidambaram, R. The recent Indian nuclear tests: A seismic overview. Curr. Sci. 2000, 79, 1359–1366. [Google Scholar]
- Itin, V.I.; Naiborodenko, Y.S. High Temperature Synthesis of Intermetallic Compounds; Tomsk University Publisher: Tomsk, Russia, 1989. [Google Scholar]
- Morsi, K. Review: Reaction synthesis processing of Ni-Al intermetallic materials. Mater. Sci. Eng. A 2001, 299, 1–15. [Google Scholar] [CrossRef]
- Pidria, M.; Merlone, E.; Rostagno, M.; Tabone, L.; Bechis, F.; Vallauri, D.; Deorsola, F.A.; Amato, I.; Rodriguez, M.A. SHS production, processing and evaluation of advanced materials for wear-resistant cutting tools. Mater. Sci. Forum 2003, 426, 4373–4378. [Google Scholar] [CrossRef]
- Xanthopoulou, G.; Marinou, A.; Karanasios, K.; Vekinis, G. Catalytic Activity of NiAl Composite Coatings Produced by In-Flight SHS during Thermal Spraying. In Proceedings of the SHS 2013 International Symposium, South Padre Island, TX, USA, 21–24 October 2013; pp. 277–279.
- Marinou, A. Synthesis of High Temperature Coatings by the New Method CAFSY. Ph.D. Thesis, University of Ioannina, Ioannina, Greece, 2015. [Google Scholar]
- Naiborodenko, Y.S.; Itin, V.I.; Savitskii, K.V. Exothermic effects during sintering of a mixture of nickel and aluminum powders. J. Sov. Phys. 1968, 11, 89–93. [Google Scholar] [CrossRef]
- Handbook of Thermal Spray Technology; ASM Thermal Spray Technology; ASM International: Materials Park, OH, USA, 2004.

| Specimen | Ni in Powder Mixture, wt.% | Number of Layers (Gun Passes) | Concentration of Catalyst on the Substrate *, % |
|---|---|---|---|
| Sp-Ni65-2 | 65.1 | 2 | 4.70 |
| Sp-Ni42-2 | 42.1 | 2 | 8.60 |
| Sp-Ni59-2 | 59.3 | 2 | 8.50 |
| Sp-Ni65-1 | 65.1 | 1 | 4.00 |
| Sp-Ni87-2 | 86.8 | 2 | 13.00 |
| Sp-Ni65-5 | 65.1 | 5 | 32.90 |
| Sp-Ni65-8 | 65.1 | 8 | 70.60 |
| Sp-Ni65-2 | 65.1 | 2 | 13.90 |
| RB-Ni65-1 | 65.1 | 1 | 4.00 |
| RM-Ni65-1 | 65.1 | 1 | 4.00 |
| Specimen | Initial Mixture Composition | Catalyst Composition |
|---|---|---|
| Sp-Ni42-2 | 42.1%Ni + 57.9%Al | Ni2Al3, NiAl, NiAl3 |
| Sp-Ni59-2 | 59.3%Ni + 40.7%Al | Ni2Al3, NiAl |
| Sp-Ni65-2 | 65.1%Ni + 34.9%Al | NiAl, Ni2Al3 |
| Sp-Ni87-2 | 86.8%Ni + 13.2%Al | Ni2Al3, NiAl, Ni3Al |
| RB-Ni65-2 | 65.1%Ni + 34.9%Al | NiAl, Ni2Al3, NiAl3, Ni3Al |
| RM-Ni65-2 | 65.1%Ni + 34.9%Al | NiAl, Ni2Al3, NiAl3, Ni3Al |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xanthopoulou, G.; Marinou, A.; Karanasios, K.; Vekinis, G. Combustion Synthesis during Flame Spraying (“CAFSY”) for the Production of Catalysts on Substrates. Coatings 2017, 7, 14. https://doi.org/10.3390/coatings7010014
Xanthopoulou G, Marinou A, Karanasios K, Vekinis G. Combustion Synthesis during Flame Spraying (“CAFSY”) for the Production of Catalysts on Substrates. Coatings. 2017; 7(1):14. https://doi.org/10.3390/coatings7010014
Chicago/Turabian StyleXanthopoulou, Galina, Amalia Marinou, Konstantinos Karanasios, and George Vekinis. 2017. "Combustion Synthesis during Flame Spraying (“CAFSY”) for the Production of Catalysts on Substrates" Coatings 7, no. 1: 14. https://doi.org/10.3390/coatings7010014






