Review of Antibacterial Activity of Titanium-Based Implants’ Surfaces Fabricated by Micro-Arc Oxidation
Abstract
:1. Introduction
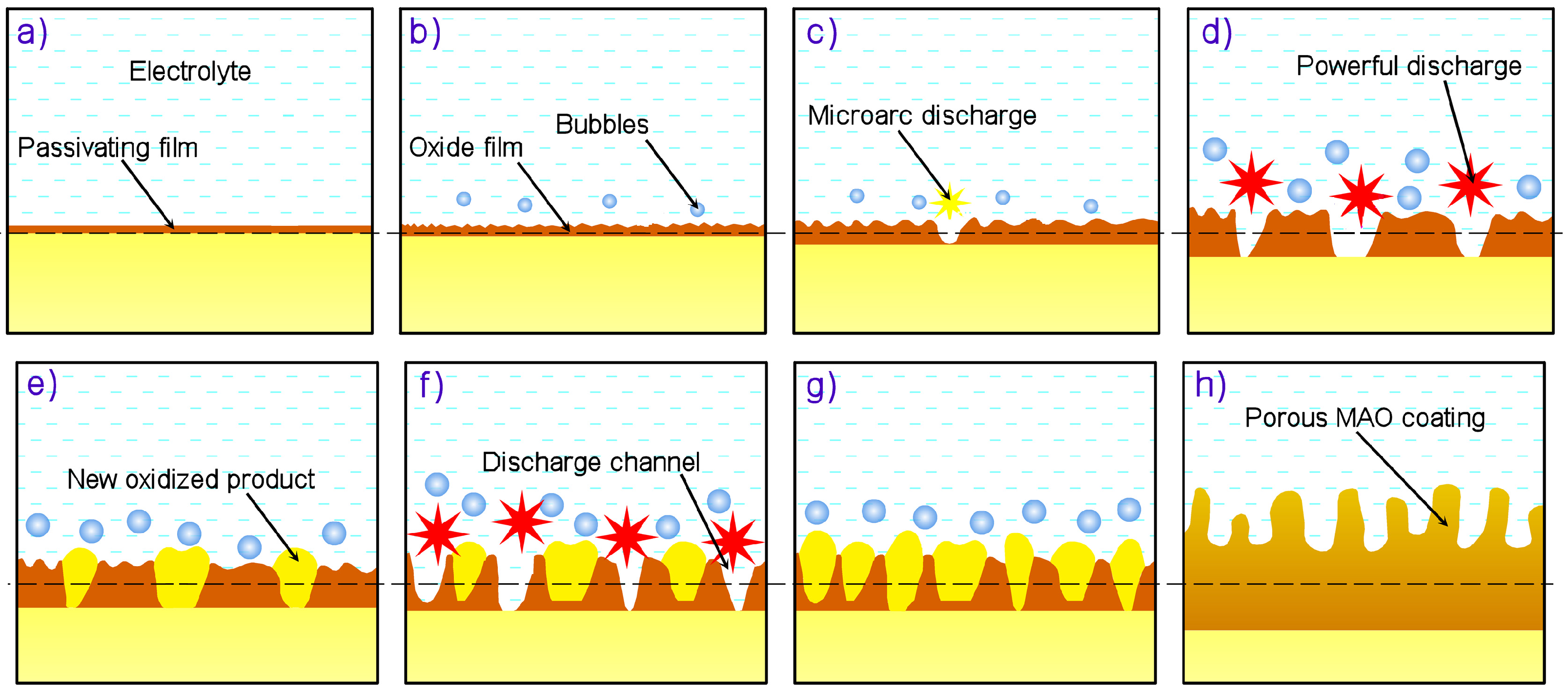
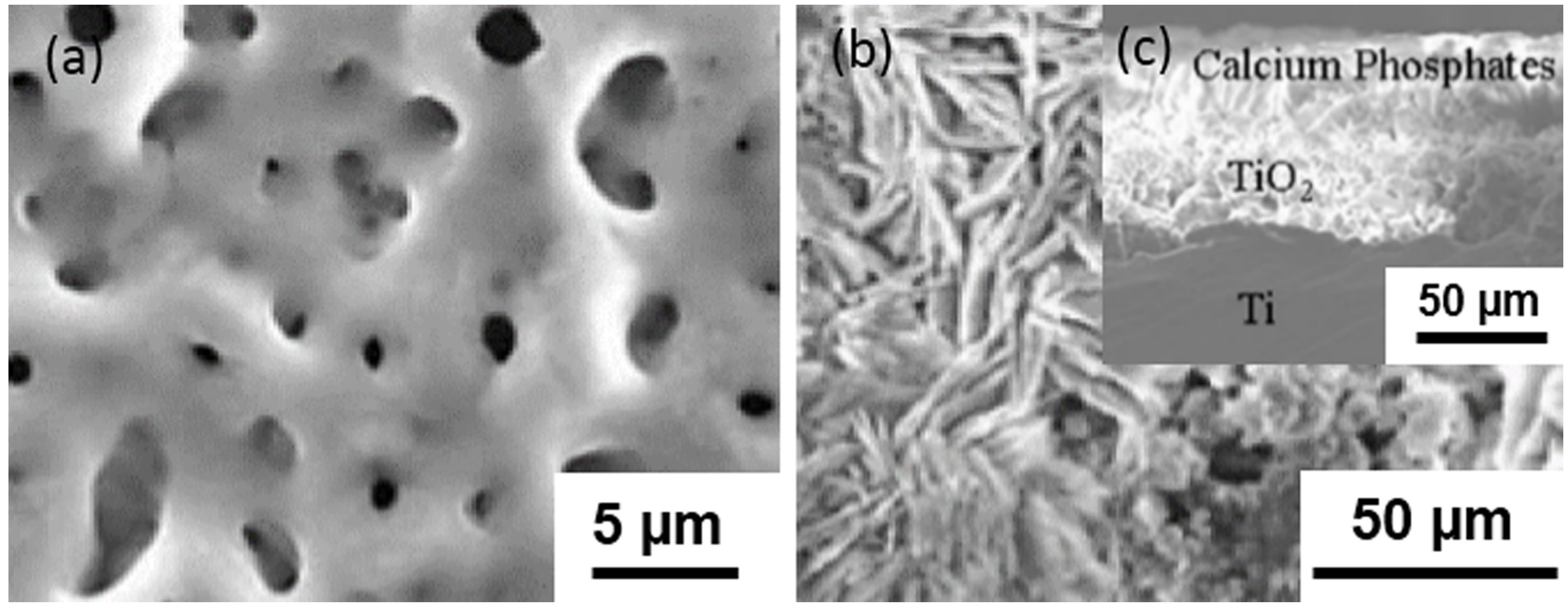
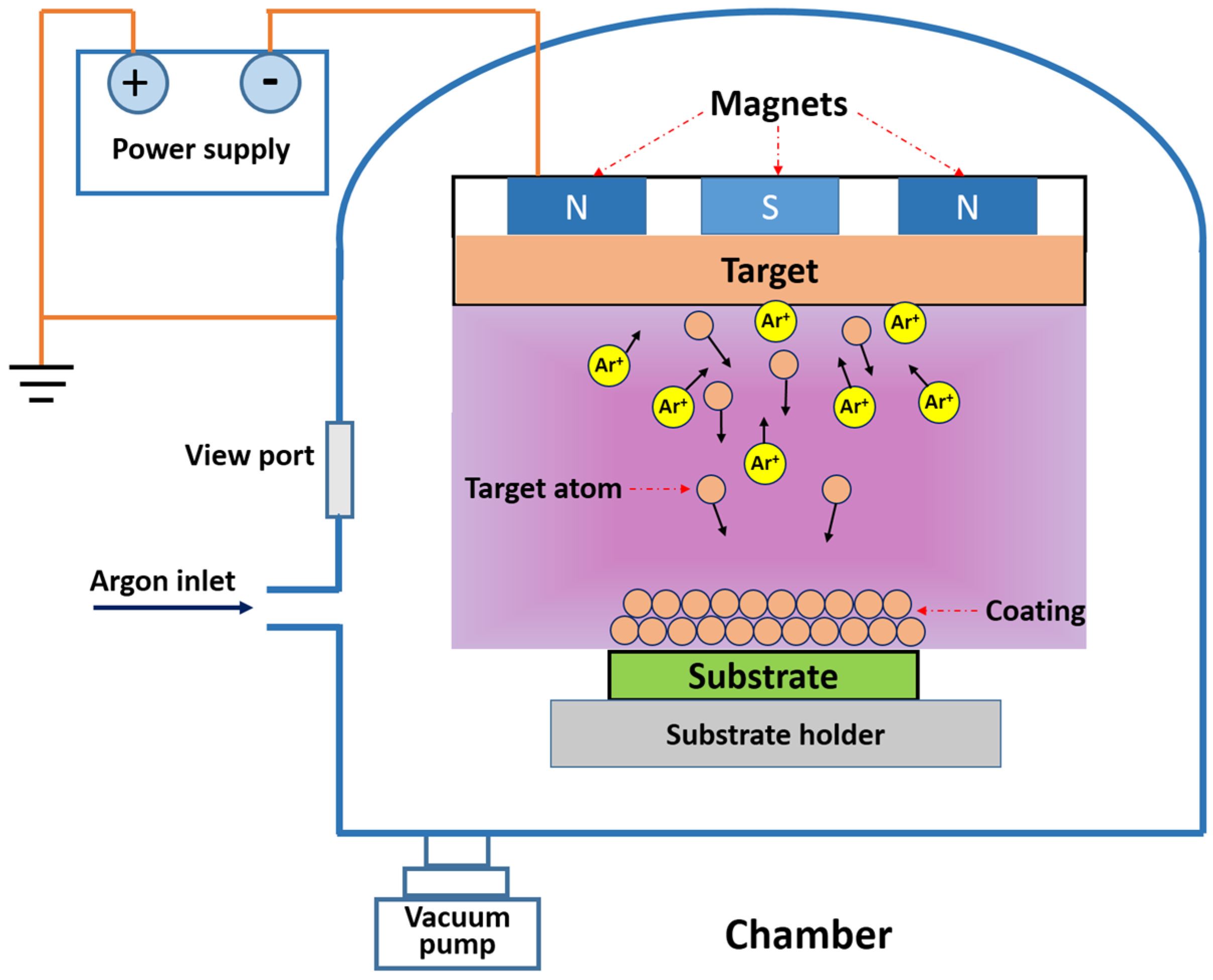
2. Micro-Arc Oxidation Method
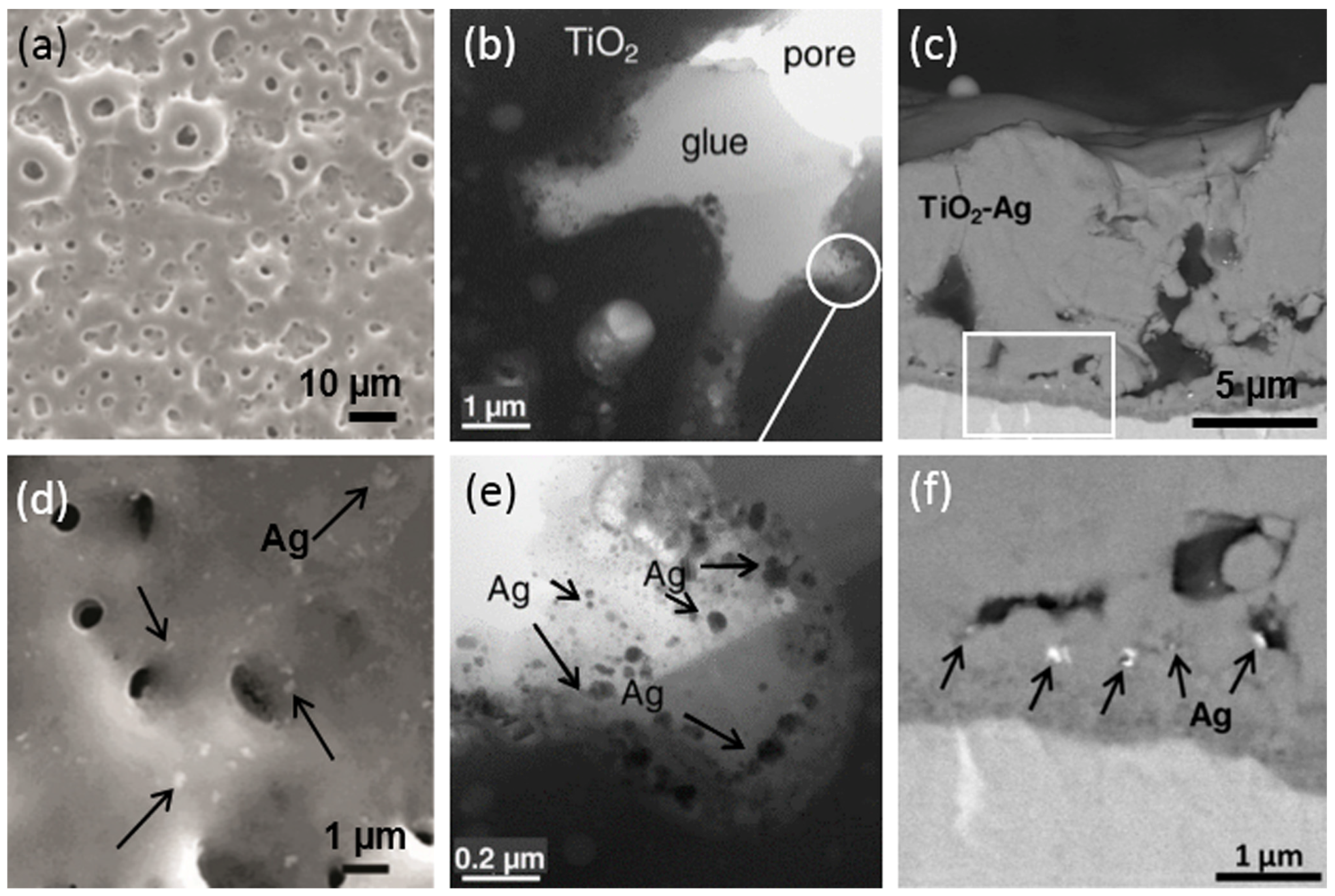
2.1. Introduction of Metal Nanoparticles into MAO Electrolyte
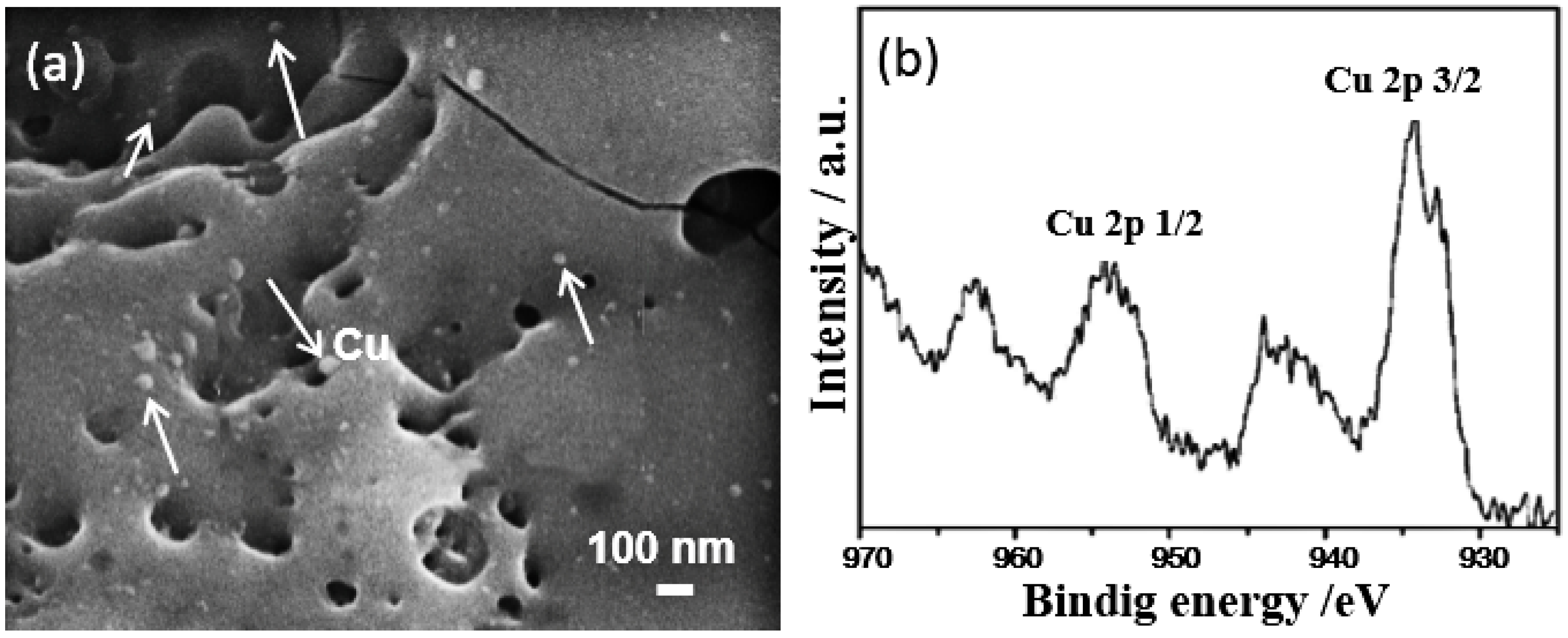
2.2. Introduction of Metallic Compounds into MAO Electrolyte
2.3. Introduction of Both Metal Nanoparticles and Metallic Compound into MAO Electrolyte
2.4. MAO Assisted by MS
3. Conclusions and Future Outlook
Acknowledgment
Conflict of Interest
References
- Wang, K. The use of titanium for medical applications in the USA. Mater. Sci. Eng. C 1996, A213, 134–137. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Pohrelyuk, I.; Yaskiv, O.; Tkachuk, O.; Lee, D.B. Formation of oxynitride layers on titanium alloys by gas diffusion treatment. Met. Mater. Int. 2009, 15, 949–953. [Google Scholar] [CrossRef]
- Glinel, K.; Thebault, P.; Humblot, V.; Pradier, C.M.; Jouenne, T. Antibacterial surfaces developed from bio-inspired approaches. Acta Biomater. 2012, 8, 1670–1684. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Huang, R.; Han, Y.; Lu, S. Enhanced osteoblast functions and bactericidal effect of Ca and Ag dual-ion implanted surface layers on nanograined titanium alloys. J. Mater. Chem. B 2014, 2, 4531–4543. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, W.; Qiao, Y.; Jiang, X.; Liu, X.; Ding, C. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2012, 8, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Leone, S. Prosthetic joint infections: Microbiology, diagnosis, management and prevention. Int. J. Antimicrob. Agents 2008, 32, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W. Infection and musculoskeletal conditions: Prosthetic-joint-associated infections. Best Pract. Res. Clin. Rheumatol. 2006, 20, 1045–1063. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.T.; Chang, Y.Y.; Huang, H.L.; Hsu, J.T.; Chen, Y.C.; Wu, A.Y.J. Characterization and antibacterial performance of bioactive Ti–Zn–O coatings deposited on titanium implants. Thin Solid Films 2013, 528, 143–150. [Google Scholar] [CrossRef]
- Bai, X.; Sandukas, S.; Appleford, M.; Ong, J.L.; Rabiei, A. Antibacterial effect and cytotoxicity of Ag-doped functionally graded hydroxyapatite coatings. J Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.N.; Teo, E.Y.; Ho, B.; Tay, B.Y.; Thian, E.S. Effect of silver content on the antibacterial and bioactive properties of silver-substituted hydroxyapatite. J Biomed. Mater. Res. A 2013, 101, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.; Wang, D.; Zhang, S.; Xie, Y.; Huang, C.; Zhang, Z. Synthesis and bactericidal ability of Ag/TiO2 composite films deposited on titanium plate. Appl. Surf. Sci. 2010, 257, 974–978. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, L.; Lv, Y.; Liu, H.; Wang, G.; Ren, N.; Liu, D.; Wang, J.; Boughton, R.I. Chemical assembly of silver nanoparticles on stainless steel for antimicrobial applications. Surf. Coat. Technol. 2010, 204, 3871–3875. [Google Scholar] [CrossRef]
- Alarcon, E.I.; Udekwu, K.; Skog, M.; Pacioni, N.L.; Stamplecoskie, K.G.; Gonzalez-Bejar, M.; Polisetti, N.; Wickham, A.; Richter-Dahlfors, A.; Griffith, M.; et al. The biocompatibility and antibacterial properties of collagen-stabilized, photochemically prepared silver nanoparticles. Biomaterials 2012, 33, 4947–4956. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Xiu, P.; Li, M.; Xu, X.; Shi, Y.; Cheng, Y.; Wei, S.; Zheng, Y.; Xi, T.; Cai, H.; et al. Bioinspired anchoring agnps onto micro-nanoporous TiO2 orthopedic coatings: Trap-killing of bacteria, surface-regulated osteoblast functions and host responses. Biomaterials 2016, 75, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Wang, H.; Wang, W.; Tong, L.; Pan, H.; Ruan, C.; Ma, Q.; Liu, M.; Yang, H.; Zhang, L.; et al. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials 2014, 35, 4255–4265. [Google Scholar] [CrossRef] [PubMed]
- Reithofer, M.R.; Lakshmanan, A.; Ping, A.T.; Chin, J.M.; Hauser, C.A. In situ synthesis of size-controlled, stable silver nanoparticles within ultrashort peptide hydrogels and their anti-bacterial properties. Biomaterials 2014, 35, 7535–7542. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, X.; Chen, Y.; Guo, M.; Zhang, Y.; Guo, X.; Gu, H. Antibiotic-loaded, silver core-embedded mesoporous silica nanovehicles as a synergistic antibacterial agent for the treatment of drug-resistant infections. Biomaterials 2016, 101, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, X.; He, X.; Li, M.; Huang, X.; Hang, R.; Tang, B. Wear and corrosion resistance of anti-bacterial Ti–Cu–N coatings on titanium implants. Appl. Surf. Sci. 2014, 317, 614–621. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, X.; Jiang, L.; Ma, Y.; Fan, A.; Tang, B. Surface microstructures and antimicrobial properties of copper plasma alloyed stainless steel. Appl. Surf. Sci. 2011, 258, 1399–1404. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, X.; Ma, Y.; Lin, N.; Fan, A.; Tang, B. Bactericidal behavior of cu-containing stainless steel surfaces. Appl. Surf. Sci. 2012, 258, 10058–10063. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Lin, N.; Huang, X.; Hang, R.; Fan, A.; Tang, B. Microstructure, antibacterial properties and wear resistance of plasma Cu–Ni surface modified titanium. Surf. Coat. Technol. 2013, 232, 515–520. [Google Scholar] [CrossRef]
- Huo, K.; Zhang, X.; Wang, H.; Zhao, L.; Liu, X.; Chu, P.K. Osteogenic activity and antibacterial effects on titanium surfaces modified with Zn-incorporated nanotube arrays. Biomaterials 2013, 34, 3467–3478. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Qin, H.; Cao, H.; Qian, S.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X.; Chu, P.K. Synergistic effects of dual Zn/Ag ion implantation in osteogenic activity and antibacterial ability of titanium. Biomaterials 2014, 35, 7699–7713. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Qin, H.; Cao, H.; Qiao, Y.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X.; Chu, P.K. Zn/Ag micro-galvanic couples formed on titanium and osseointegration effects in the presence of S. Aureus. Biomaterials 2015, 65, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surf. B Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, M.; Wang, H.; Zhang, X.; Tang, B.I.N. Preparation of copper and chromium alloyed layers on pure titanium by plasma surface alloying technology. Surf. Rev. Lett. 2015, 22, 1550056. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Z.; Raman, S.; He, F.; Huang, Y. Surface modification of medical metals by ion implantation of silver and copper. Vacuum 2007, 81, 1114–1118. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Xiong, G.Y.; Liang, H.; Raman, S.; He, F.; Huang, Y. Modification of medical metals by ion implantation of copper. Appl. Surf. Sci. 2007, 253, 9426–9429. [Google Scholar] [CrossRef]
- Cao, H.; Qiao, Y.; Liu, X.; Lu, T.; Cui, T.; Meng, F.; Chu, P.K. Electron storage mediated dark antibacterial action of bound silver nanoparticles: Smaller is not always better. Acta Biomater. 2013, 9, 5100–5110. [Google Scholar] [CrossRef] [PubMed]
- Necula, B.S.; van Leeuwen, J.P.T.M.; Fratila-Apachitei, L.E.; Zaat, S.A.J.; Apachitei, I.; Duszczyk, J. In vitro cytotoxicity evaluation of porous TiO2–Ag antibacterial coatings for human fetal osteoblasts. Acta Biomater. 2012, 8, 4191–4197. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-B.; Otgonbayar, U.; Lee, J.-H.; Kim, K.-M.; Kim, K.-N. Silver ion-exchanged sodium titanate and resulting effect on antibacterial efficacy. Surf. Coat. Technol. 2010, 205, S172–S176. [Google Scholar] [CrossRef]
- García-Serrano, J.; Gómez-Hernández, E.; Ocampo-Fernández, M.; Pal, U. Effect of Ag doping on the crystallization and phase transition of TiO2 nanoparticles. Curr. Appl. Phys. 2009, 9, 1097–1105. [Google Scholar] [CrossRef]
- Meng, F.; Sun, Z. A mechanism for enhanced hydrophilicity of silver nanoparticles modified TiO2 thin films deposited by RF magnetron sputtering. Appl. Surf. Sci. 2009, 255, 6715–6720. [Google Scholar] [CrossRef]
- Tian, X.B.; Wang, Z.M.; Yang, S.Q.; Luo, Z.J.; Fu, R.K.Y.; Chu, P.K. Antibacterial copper-containing titanium nitride films produced by dual magnetron sputtering. Surf. Coat. Technol. 2007, 201, 8606–8609. [Google Scholar] [CrossRef]
- Li, B.; Liu, X.; Meng, F.; Chang, J.; Ding, C. Preparation and antibacterial properties of plasma sprayed nano-titania/silver coatings. Mater. Chem. Phys. 2009, 118, 99–104. [Google Scholar] [CrossRef]
- Mungkalasiri, J.; Bedel, L.; Emieux, F.; Doré, J.; Renaud, F.N.R.; Maury, F. Dli-CVD of TiO2–Cu antibacterial thin films: Growth and characterization. Surf. Coat. Technol. 2009, 204, 887–892. [Google Scholar] [CrossRef]
- Sul, Y.T.; Kang, B.S.; Johansson, C.; Um, H.S.; Park, C.J.; Albrektsson, T. The roles of surface chemistry and topography in the strength and rate of osseointegration of titanium implants in bone. J. Biomed. Mater. Res. A 2009, 89, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Zhou, Y.; Yang, C. Structure, cell response and biomimetic apatite induction of gradient TiO2-based/nano-scale hydrophilic amorphous titanium oxide containing ca composite coatings before and after crystallization. Colloids Surf. B Biointerfaces 2009, 74, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Sun, J.F.; Chu, P.K.; Han, Y.; Zhang, Y.M. Bone integration capability of a series of strontium-containing hydroxyapatite coatings formed by micro-arc oxidation. J Biomed. Mater. Res. A 2013, 101, 2465–2480. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.H.; Lee, I.S.; Han, I.H.; Park, J.C.; Chung, S.M. Effects of surface morphology on human osteosarcoma cell response. Curr. Appl. Phys. 2007, 7, e6–e10. [Google Scholar] [CrossRef]
- Sun, J.; Han, Y.; Cui, K. Microstructure and apatite-forming ability of the mao-treated porous titanium. Surf. Coat. Technol. 2008, 202, 4248–4256. [Google Scholar] [CrossRef]
- Miao, S.; Cheng, K.; Weng, W.; Du, P.; Shen, G.; Han, G.; Yan, W.; Zhang, S. Fabrication and evaluation of Zn containing fluoridated hydroxyapatite layer with zn release ability. Acta Biomater. 2008, 4, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, H.; Chen, C.; Zhao, Z. Review of the biocompatibility of micro-arc oxidation coated titanium alloys. Mater. Des. 2015, 85, 640–652. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.E. Transmission electron microscopy of coatings formed by plasma electrolytic oxidation of titanium. Acta Biomater. 2009, 5, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Li, J.F.; Feng, J.Y.; Sun, W.; Mao, Z.Q. Anatase TiO2 films with 2.2 eV band gap prepared by micro-arc oxidation. Mater. Sci. Eng. B 2007, 139, 216–220. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Wu, X.Q.; Xue, Z.G.; Matykina, E.; Skeldon, P.; Thompson, G.E. Microstructure, corrosion and wear performance of plasma electrolytic oxidation coatings formed on Ti–6Al–4V alloy in silicate-hexametaphosphate electrolyte. Surf. Coat. Technol. 2013, 217, 129–139. [Google Scholar] [CrossRef]
- Necula, B.S.; Fratila-Apachitei, L.E.; Zaat, S.A.; Apachitei, I.; Duszczyk, J. In vitro antibacterial activity of porous TiO2-Ag composite layers against methicillin-resistant staphylococcus aureus. Acta Biomater. 2009, 5, 3573–3580. [Google Scholar] [CrossRef] [PubMed]
- Necula, B.S.; Apachitei, I.; Tichelaar, F.D.; Fratila-Apachitei, L.E.; Duszczyk, J. An electron microscopical study on the growth of TiO2-Ag antibacterial coatings on Ti6Al7Nb biomedical alloy. Acta Biomater. 2011, 7, 2751–2757. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhang, X.; Wu, H.; Tian, L.; Ma, Y.; Tang, B. Microstructure and antibacterial properties of Cu-doped TiO2 coating on titanium by micro-arc oxidation. Appl. Surf. Sci. 2014, 292, 944–947. [Google Scholar] [CrossRef]
- Song, W.H.; Ryu, H.S.; Hong, S.H. Antibacterial properties of Ag (or Pt)-containing calcium phosphate coatings formed by micro-arc oxidation. J. Biomed. Mater. Res. A 2009, 88, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Muhaffel, F.; Cempura, G.; Menekse, M.; Czyrska-Filemonowicz, A.; Karaguler, N.; Cimenoglu, H. Characteristics of multi-layer coatings synthesized on Ti6Al4V alloy by micro-arc oxidation in silver nitrate added electrolytes. Surf. Coat. Technol. 2016, 307, 308–315. [Google Scholar] [CrossRef]
- Teker, D.; Muhaffel, F.; Menekse, M.; Karaguler, N.G.; Baydogan, M.; Cimenoglu, H. Characteristics of multi-layer coating formed on commercially pure titanium for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Huang, X.; Liu, Y.; Bai, L.; Yang, X.; Hang, R.; Tang, B.; Chu, P.K. High-current anodization: A novel strategy to functionalize titanium-based biomaterials. Electrochim. Acta 2015, 173, 345–353. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Yu, H.; Yang, X.; Wang, Y.; Hang, R.; Tang, B. One-step fabrication of cytocompatible micro/nano-textured surface with TiO2 mesoporous arrays on titanium by high current anodization. Electrochim. Acta 2016, 199, 116–125. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Z.; Gu, B.; Sun, J.; Zhu, L. Biological activity and antibacterial property of nano-structured TiO2 coating incorporated with Cu prepared by micro-arc oxidation. J. Mater. Sci. Technol. 2013, 29, 237–244. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, J.; Huang, X.; Zhang, Y.; Han, Y. The dual function of Cu-doped TiO2 coatings on titanium for application in percutaneous implants. J. Mater. Chem. B 2016, 4, 3788–3800. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Li, J.; He, X.; Hang, R.; Huang, X.; Tian, L.; Tang, B. Corrosion behavior of Zn-incorporated antibacterial TiO2 porous coating on titanium. Ceram. Int. 2016, 42, 17095–17100. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Q.; Han, Y. Zn and Ag co-doped anti-microbial TiO2 coatings on Ti by micro-arc oxidation. J. Mater. Sci. Technol. 2016, 32, 919–924. [Google Scholar] [CrossRef]
- Yeo, S.Y.; Jeong, S.H. Preparation and characterization of polypropylene/silver nanocomposite fibers. Polym. Int. 2003, 52, 1053–1057. [Google Scholar] [CrossRef]
- Zinjarde, S. Bio-inspired nanomaterials and their applications as antimicrobial agents. Chron. Young Sci. 2012, 3, 74–81. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. Coli as a model for gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Kim, J.D.; Choi, H.C.; Lee, C.W. Antibacterial activity of CNT-Ag and Go-Ag nanocomposites against gram-negative and gram-positive bacteria. B Korean Chem. Soc. 2013, 34, 3261–3264. [Google Scholar] [CrossRef]
- Iavicoli, I.; Fontana, L.; Leso, V.; Bergamaschi, A. The effects of nanomaterials as endocrine disruptors. Int. J. Mol. Sci. 2013, 14, 16732–16801. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.K.; Kim, B.H.; Jung, G. Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis. 2009, 93, 1037–1043. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X. Activating titanium oxide coatings for orthopedic implants. Surf. Coat. Technol. 2013, 233, 57–64. [Google Scholar] [CrossRef]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef] [PubMed]
- Raffi, S.M.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W.; Hasan, M.M. Investigations into the antibacterial behavior of copper nanoparticles against escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Usman, M.S.; El Zowalaty, M.E.; Shameli, K.; Zainuddin, N.; Salama, M.; Ibrahim, N.A. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomed. 2013, 8, 4467–4479. [Google Scholar] [PubMed]
- Le Pape, H.; Solano-Serena, F.; Contini, P.; Devillers, C.; Maftah, A.; Leprat, P. Evaluation of the anti-microbial properties of an activated carbon fibre supporting silver using a dynamic method. Carbon 2002, 40, 2947–2954. [Google Scholar] [CrossRef]
- Mahapatra, O.; Bhagat, M.; Gopalakrishnan, C.; Arunachalam, K.D. Ultrafine dispersed CuO nanoparticles and their antibacterial activity. J. Exp. Nanosci. 2008, 3, 185–193. [Google Scholar] [CrossRef]
- Li, L.H.; Kong, Y.M.; Kim, H.W.; Kim, Y.W.; Kim, H.E.; Heo, S.J.; Koak, J.Y. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials 2004, 25, 2867–2875. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, X.; Chen, A.; Shen, M.; Wen, S. Synthesis of bioactive ceramic on the titanium substrate by micro-arc oxidation. J. Biomed. Mater. Res. A 2009, 90, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Song, W.H.; Jun, Y.K.; Han, Y.; Hong, S.H. Biomimetic apatite coatings on micro-arc oxidized titania. Biomaterials 2004, 25, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Han, Y.; Huang, X. Hydroxyapatite coatings prepared by micro-arc oxidation in Ca- and P-containing electrolyte. Surf. Coat. Technol. 2007, 201, 5655–5658. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Courtney, H.S.; Bettenga, M.; Agrawal, C.M.; Bumgardner, J.D.; Ong, J.L. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 2006, 27, 5512–5517. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Batzer, R.; Kieswetter, K.; Liu, Y.; Cochran, D.L.; Szmuckler-Moncler, S.; Dean, D.D.; Schwartz, Z. Titanium surface roughness alters responsiveness of MG63 osteoblast-like cells to 1α,25-(OH)2D3. J. Biomed. Mater. 1998, 39, 77–85. [Google Scholar] [CrossRef]
- Naveena, N.; Venugopal, J.; Rajeswari, R.; Sundarrajan, S.; Sridhar, R.; Shayanti, M.; Narayanan, S.; Ramakrishna, S. Biomimetic composites and stem cells interaction for bone and cartilage tissue regeneration. J. Mater. Chem. 2012, 22, 5239–5253. [Google Scholar] [CrossRef]
- Jamuna-Thevi, K.; Bakar, S.A.; Ibrahim, S.; Shahab, N.; Toff, M.R.M. Quantification of silver ion release, in vitro cytotoxicity and antibacterial properties of nanostuctured Ag doped TiO2 coatings on stainless steel deposited by rf magnetron sputtering. Vacuum 2011, 86, 235–241. [Google Scholar] [CrossRef]
- Brammer, K.S.; Oh, S.; Cobb, C.J.; Bjursten, L.M.; van der Heyde, H.; Jin, S. Improved bone-forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater. 2009, 5, 3215–3223. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Daraio, C.; Chen, L.H.; Pisanic, T.R.; Finones, R.R.; Jin, S. Significantly accelerated osteoblast cell growth on aligned TiO2 nanotubes. J. Biomed. Mater. Res. A 2006, 78, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Von Wilmowsky, C.; Bauer, S.; Lutz, R.; Meisel, M.; Neukam, F.W.; Toyoshima, T.; Schmuki, P.; Nkenke, E.; Schlegel, K.A. In vivo evaluation of anodic TiO2 nanotubes: An experimental study in the pig. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 89, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Hang, R.; Huang, X.; Zhao, L.; Zhang, X.; Wang, L.; Tang, B.; Ma, S.; Chu, P.K. The effects of titania nanotubes with embedded silver oxide nanoparticles on bacteria and osteoblasts. Biomaterials 2014, 35, 4223–4235. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Ryu, J.J.; Sung, Y.M. One-step approach for nano-crystalline hydroxyapatite coating on titanium via micro-arc oxidation. Electrochem. Commun. 2007, 9, 1886–1891. [Google Scholar] [CrossRef]
- Ni, J.H.; Shi, Y.L.; Yan, F.Y.; Chen, J.Z.; Wang, L. Preparation of hydroxyapatite-containing titania coating on titanium substrate by micro-arc oxidation. Mater. Res. Bull. 2008, 43, 45–53. [Google Scholar] [CrossRef]
- Han, Y.; Chen, D.; Sun, J.; Zhang, Y.; Xu, K. UV-enhanced bioactivity and cell response of micro-arc oxidized titania coatings. Acta Biomater. 2008, 4, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Hang, R.; Gao, A.; Huang, X.; Wang, X.; Zhang, X.; Qin, L.; Tang, B. Antibacterial activity and cytocompatibility of Cu-Ti-O nanotubes. J. Biomed. Mater. Res. A 2014, 102, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Zong, M.; Bai, L.; Liu, Y.; Wang, X.; Zhang, X.; Huang, X.; Hang, R.; Tang, B. Antibacterial ability and angiogenic activity of Cu-Ti-O nanotube arrays. Mater. Sci. Eng. C 2017, 71, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, I.; Luthen, F.; Prinz, C.; Kreikemeyer, B.; Zietz, C.; Neumann, H.G.; Rychly, J. A dual function of copper in designing regenerative implants. Biomaterials 2015, 44, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, Y.; Wu, C.; Zhou, H.; Zreiqat, H. Biological response of human bone cells to zinc-modified Ca-Si-based ceramics. Acta Biomater. 2008, 4, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.; Li, H.; Tanner, D.A.; Towler, M.R.; Wall, J.G. The antibacterial effects of zinc ion migration from zinc-based glass polyalkenoate cements. J. Mater. Sci. Mater. Med. 2006, 17, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ramaswamy, Y.; Chang, J.; Woods, J.; Chen, Y.; Zreiqat, H. The effect of Zn contents on phase composition, chemical stability and cellular bioactivity in Zn-Ca-Si system ceramics. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Ibănescu, M.; Muşat, V.; Textor, T.; Badilita, V.; Mahltig, B. Photocatalytic and antimicrobial Ag/ZnO nanocomposites for functionalization of textile fabrics. J. Alloys Compd. 2014, 610, 244–249. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Qiao, Y.; Zhu, H.; Ding, C. Antimicrobial activity and cytocompatibility of Ag plasma-modified hierarchical TiO2 film on titanium surface. Colloids Surf. B Biointerfaces 2014, 113, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S. Tunable surface plasmon resonance of silver nanoclusters in ion exchanged soda lime glass. J. Alloys Compd. 2014, 598, 11–15. [Google Scholar] [CrossRef]
- Kim, T.; Yoshitake, M.; Yagyu, S.; Nemsak, S.; Nagata, T.; Chikyow, T. XPS study on band alignment at Pt-O-terminated ZnO(0001) interface. Surf. Interface Anal. 2010, 42, 1528–1531. [Google Scholar] [CrossRef]
- Carvalho, P.; Sampaio, P.; Azevedo, S.; Vaz, C.; Espinós, J.P.; Teixeira, V.; Carneiro, J.O. Influence of thickness and coatings morphology in the antimicrobial performance of zinc oxide coatings. Appl. Surf. Sci. 2014, 307, 548–557. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; Meng, F.; Chu, P.K. Biological actions of silver nanoparticles embedded in titanium controlled by micro-galvanic effects. Biomaterials 2011, 32, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Hang, R.; Gao, A.; Zhang, X.; Huang, X.; Wang, Y.; Tang, B.; Zhao, L.; Chu, P.K. Nanostructured titanium–silver coatings with good antibacterial activity and cytocompatibility fabricated by one-step magnetron sputtering. Appl. Surf. Sci. 2015, 355, 32–44. [Google Scholar] [CrossRef]
- Gao, A.; Hang, R.; Chu, P.K. Recent advances in anti-infection surfaces fabricated on biomedical implants by plasma-based technology. Surf. Coat. Technol. 2016, 312, 2–6. [Google Scholar] [CrossRef]
- Zhang, X.; Hang, R.; Wu, H.; Huang, X.; Ma, Y.; Lin, N.; Yao, X.; Tian, L.; Tang, B. Synthesis and antibacterial property of Ag-containing TiO2 coatings by combining magnetron sputtering with micro-arc oxidation. Surf. Coat. Technol. 2013, 235, 748–754. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, H.; Geng, Z.; Huang, X.; Hang, R.; Ma, Y.; Yao, X.; Tang, B. Microstructure and cytotoxicity evaluation of duplex-treated silver-containing antibacterial TiO2 coatings. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 402–410. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, X.; Bai, L.; Hang, R.; Huang, X.; Qin, L.; Yao, X.; Tang, B. Antibacterial ability and osteogenic activity of porous Sr/Ag-containing TiO2 coatings. Biomed. Mater. 2016, 11, 045008. [Google Scholar] [CrossRef] [PubMed]

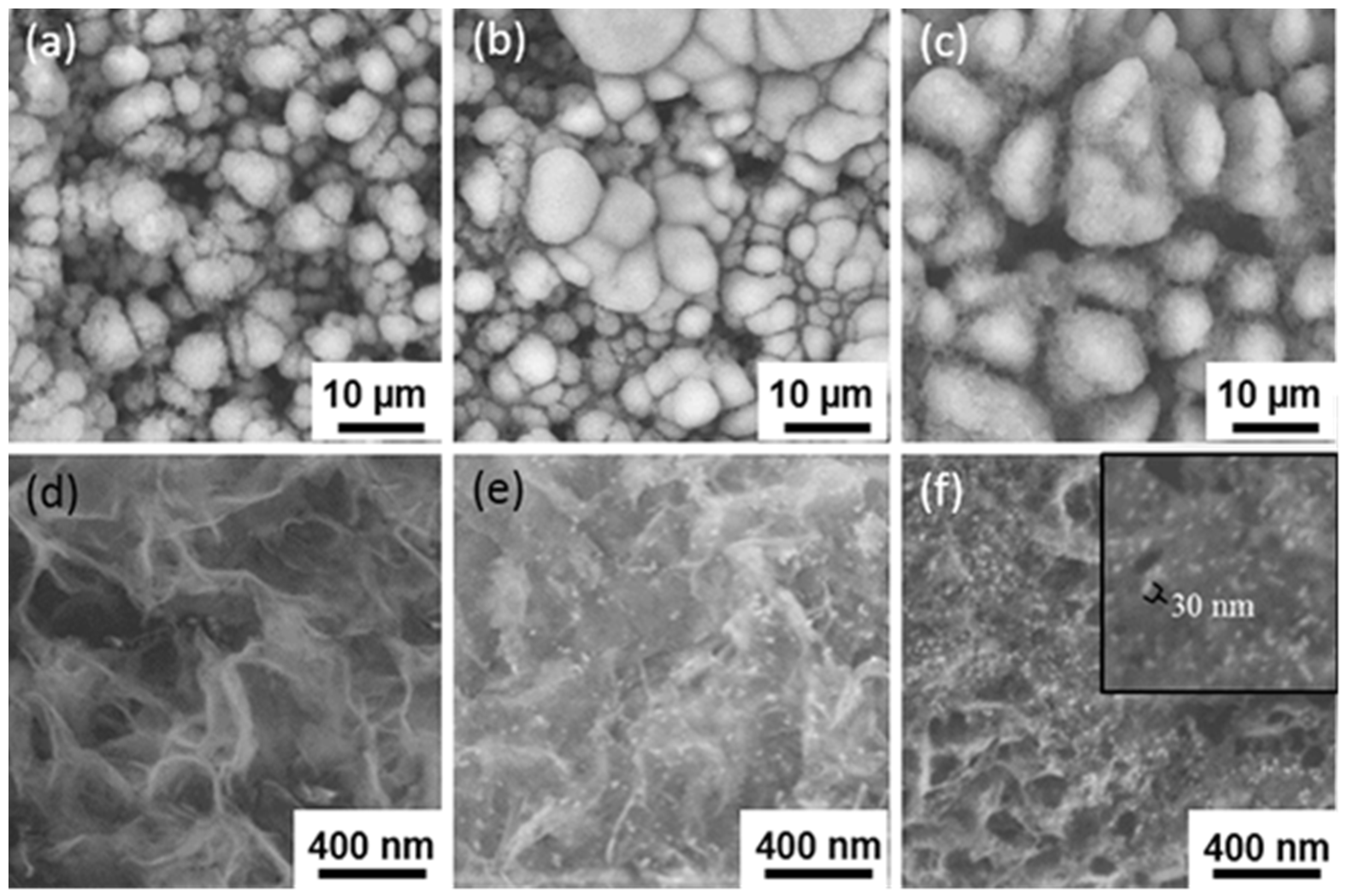
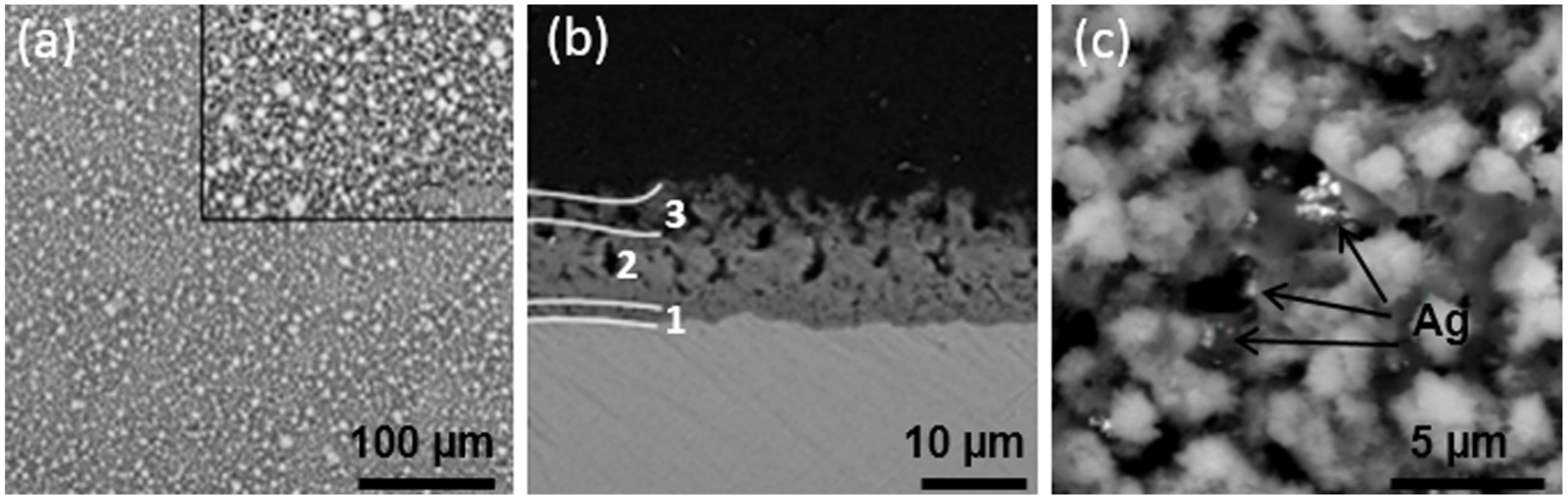
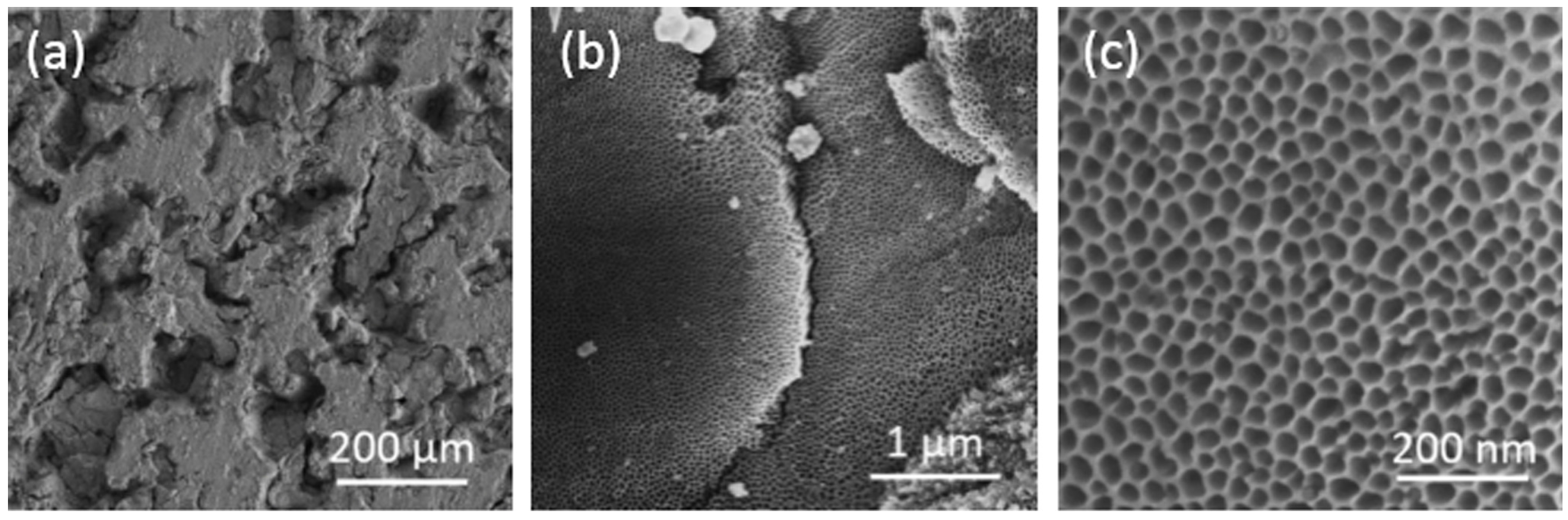
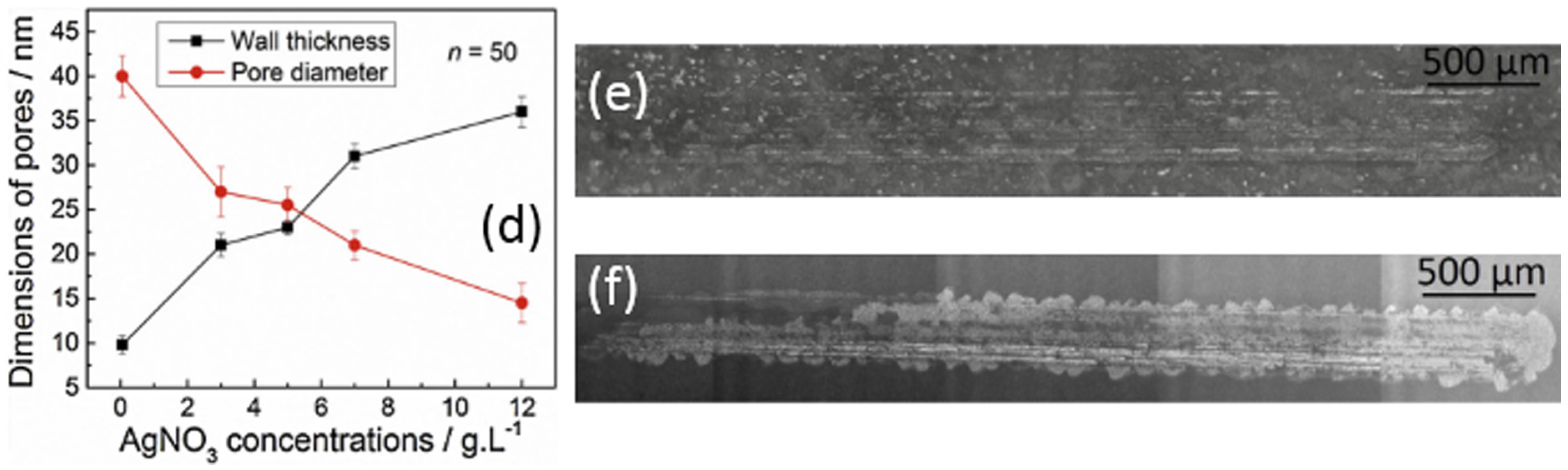
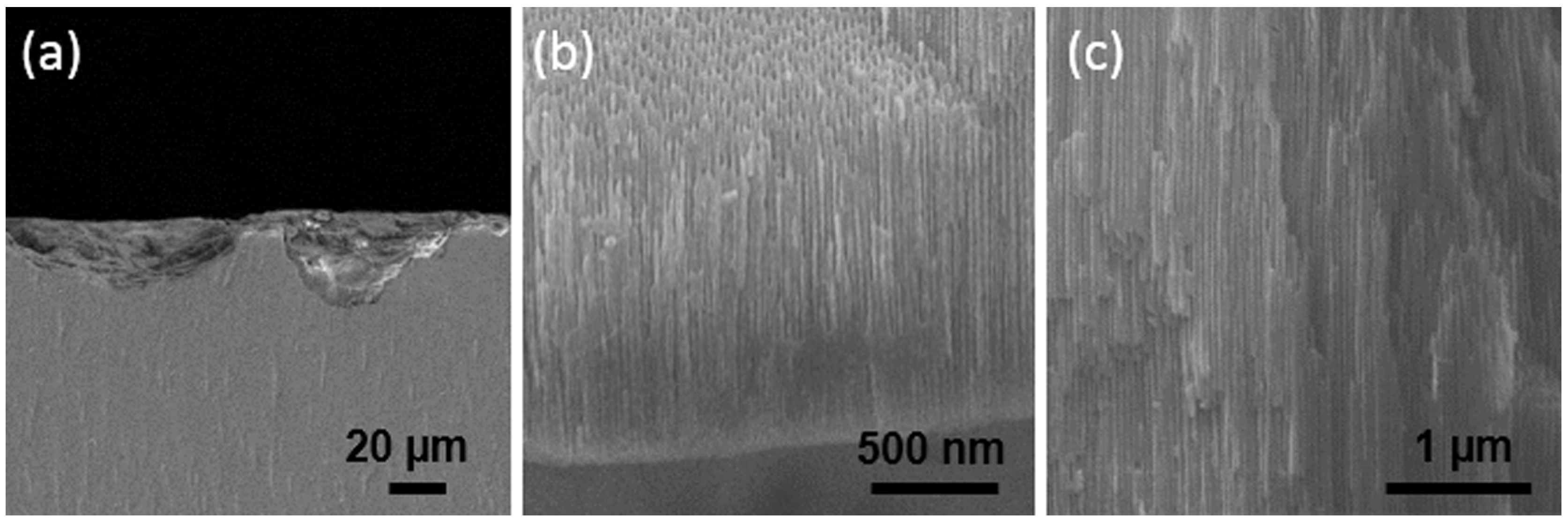
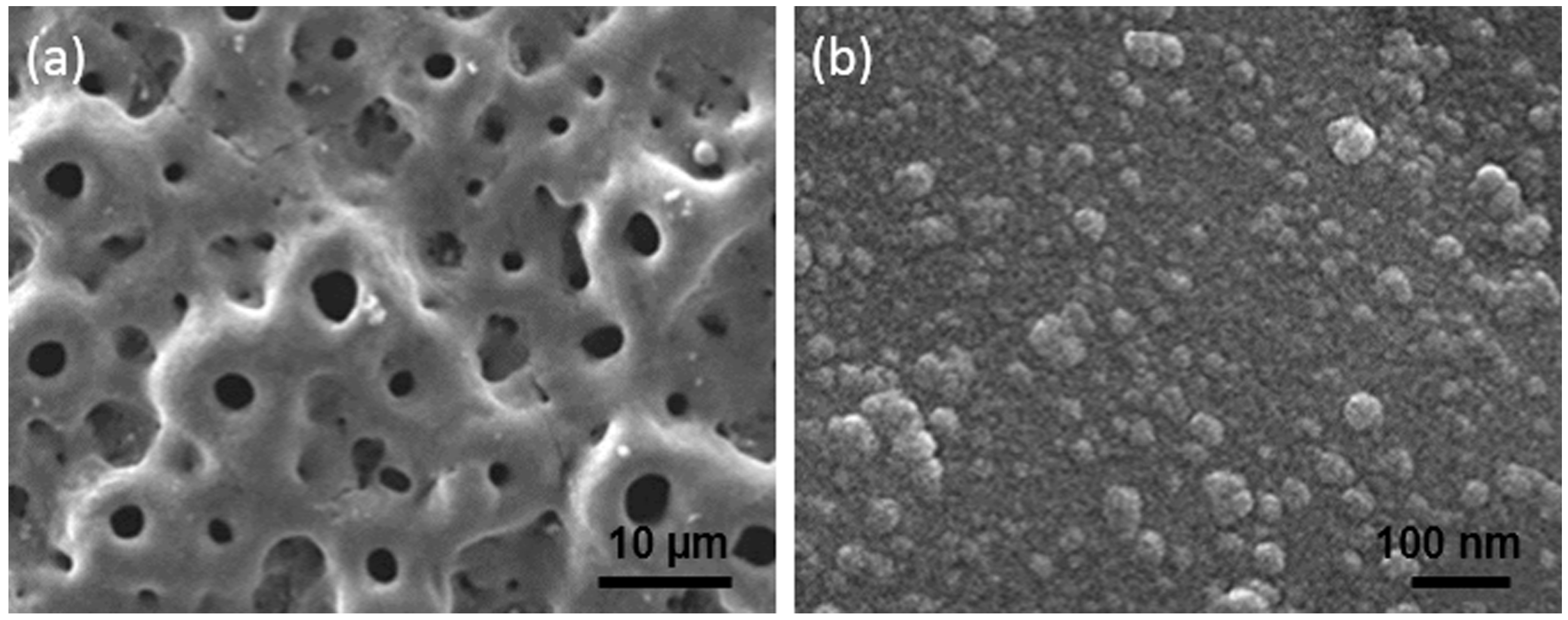
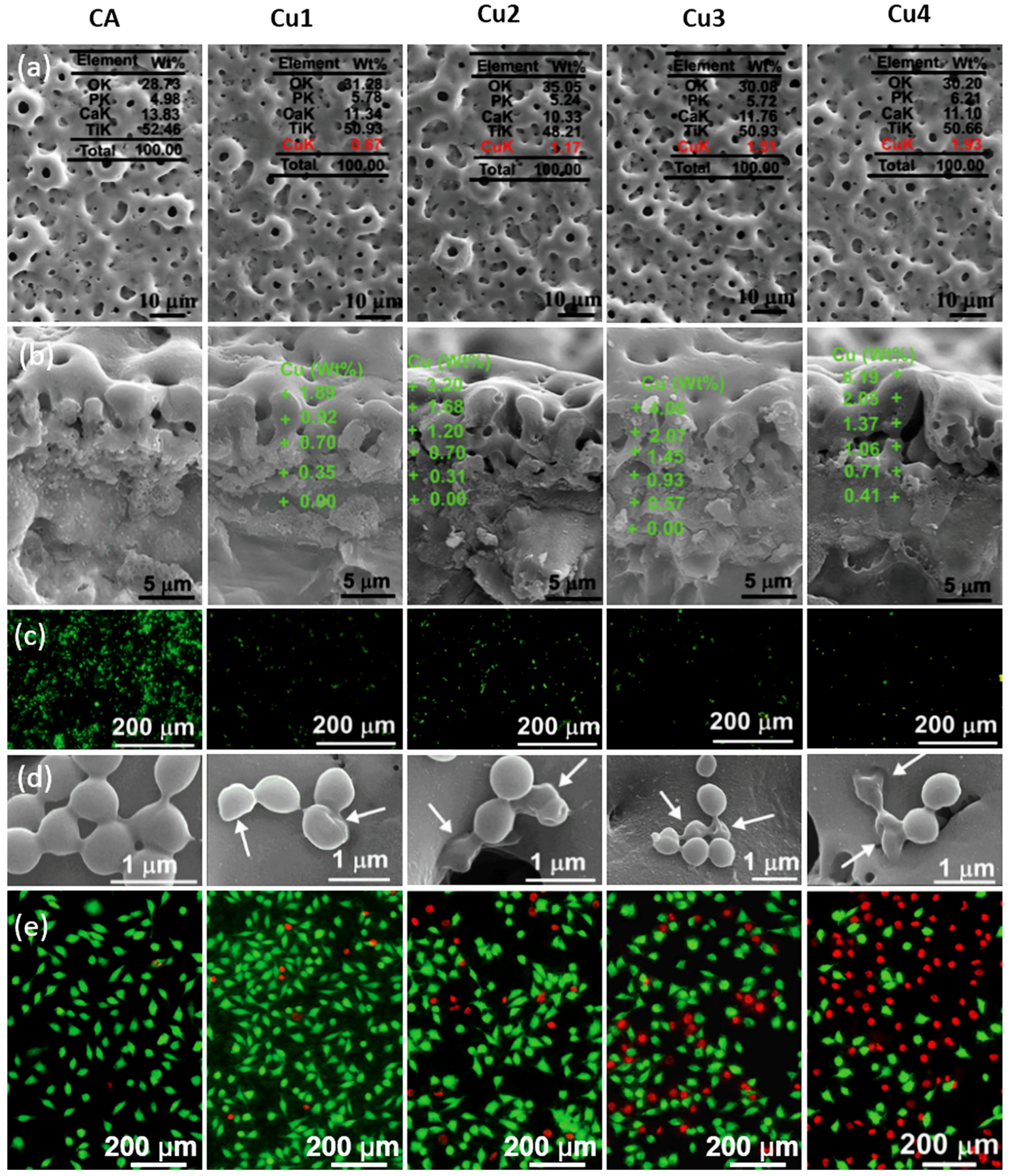
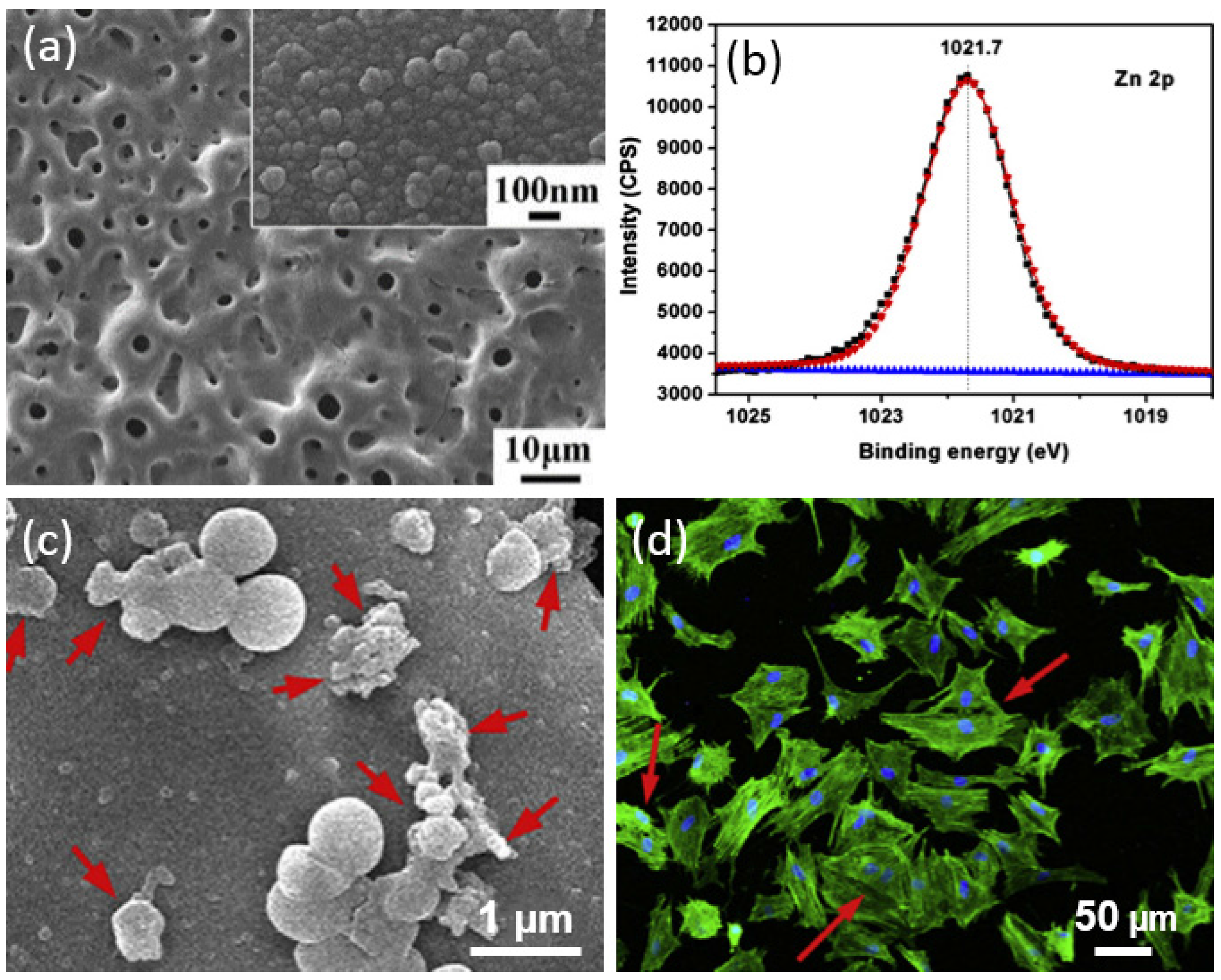

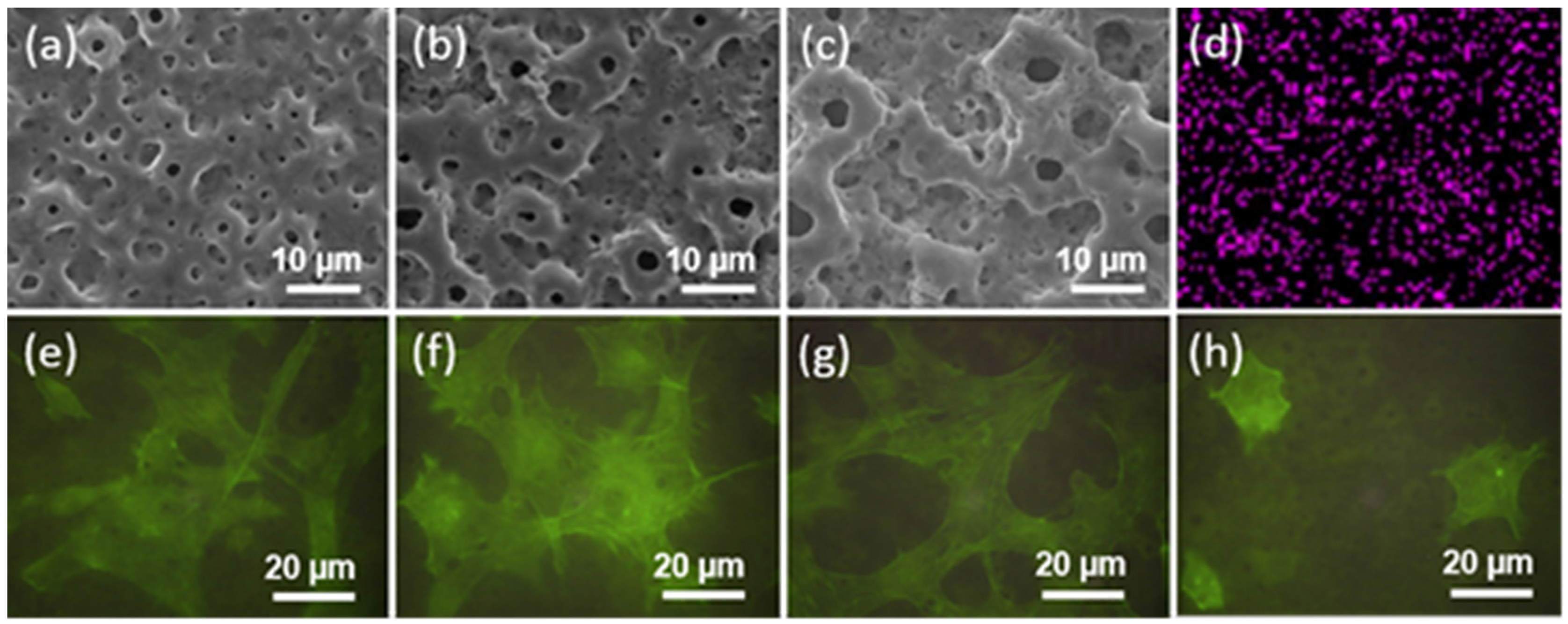
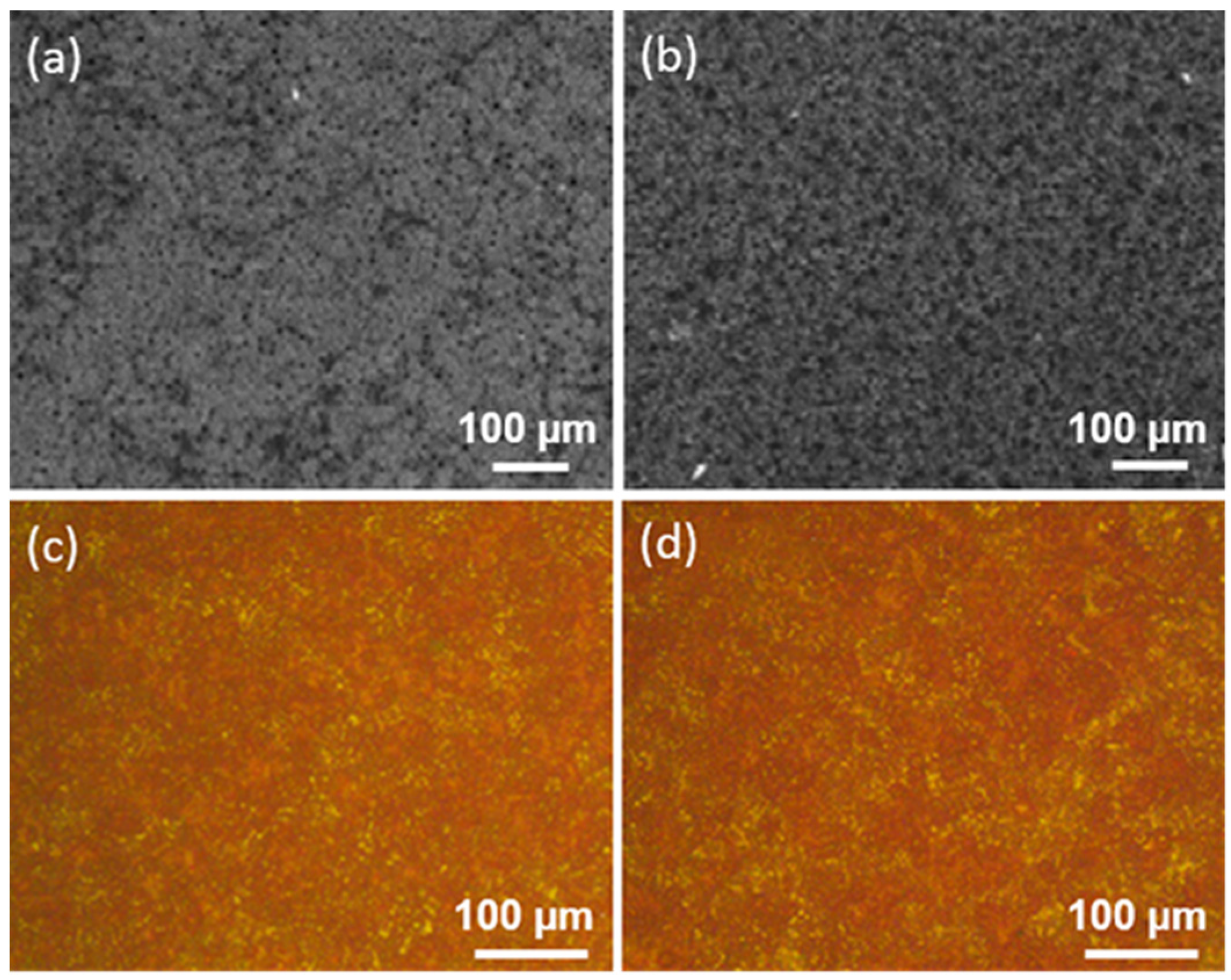
| Ti Alloy | Electrolyte | Electrical Parameter | Surface Topography (Pore Size) | XRD Detected Phase | Surface Content of Ag/Cu/Zn (wt %) | Release Amount of Ag/Cu/Zn (ppb) | Tested Bacteria | Biocompatibility | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voltage (V) | Current Density (A·dm−2) | Oxidation Time (s) | ||||||||||||
| Ti-6Al-7Nb | 0.02 M Ca-GP, 0.15 M CA, and 3.0 g·L−1 Ag NPs | <250 | 20 | 300 | Porous structures (<3 μm) with Ag NP of 37 nm | Ti, rutile and anatase | 0.03 | – | S. aureus | – | [55] | |||
| Ti-6Al-7Nb | 0.02 M Ca-GP, 0.15 M CA, and 3.0 g·L−1 Ag NPs | <250 | 20 | 0–300 | Porous structures (<5 μm) with Ag NP of 7–25 nm | – | – | – | – | – | [56] | |||
| Ti-6Al-7Nb | 0.02 M Ca-GP, 0.15 M CA, and 0.3 g·L−1 Ag NPs | 234 ± 3 | 20 | 300 | Porous structures (<5 μm) | – | – | 12 | S. aureus | Human osteoblastic cell | [38] | |||
| Ti-6Al-7Nb | 0.02 M Ca-GP, 0.15 M CA, and 3.0 g·L−1 Ag NPs | 237 ± 2 | 20 | 300 | Porous structures (<5 μm) | – | – | 89 | S. aureus | Human osteoblastic cell | [38] | |||
| Cp-Ti | 2 g·L−1 NaOH, 15 g·L−1 NaH2PO4 and 3.0 g·L−1 Cu NPs | – | 20 | 300 | Porous structures (<5 μm) with Cu NP of 60 nm | – | – | – | Escherichia coli, S. aureus | – | [57] | |||
| Cp-Ti | 0.04 M β-GP, 0.4 M CA 2 and 0.004 M AgNO3 | 250–350 | – | 180 | Spherical pores (<3 μm) | Ti, rutile, and anatase | – | – | S. aureus | Human osteosarcoma (HOS) cell | [58] | |||
| Cp-Ti | 0.04 M β-GP, 0.4 M CA and 0.004 M AgNO3 | 420 | – | 180 | Irregular and rough pores with spherical particles and flake | Rutile, β-Ca2P2O7, α-TCP 3 and HA 4 | 0.21–0.45 | – | S. aureus | Human osteosarcoma (HOS) cell | [58] | |||
| Cp-Ti | 0.04 M β-GP, 0.4 M CA and 0.00006 M AgNO3 | 420 | – | 180 | Irregular and rough pores with spherical particles and flake | Rutile, β-Ca2P2O7, α-TCP and HA | 0.1 | – | S. aureus | Human osteosarcoma (HOS) cell | [58] | |||
| Ti6Al4V | β-GP, CA and 0.1 g·L−1 AgNO3 | 400 | – | 300 | Granular morphology with Ag NPs of 20–30 nm | Ti, rutile, anatase, CaTiO3 and HA | 0.6 | 2500 | E. coli | – | [59] | |||
| Ti6Al4V | β-GP, CA and 0.4 g·L−1 AgNO3 | 400 | – | 300 | Needle-like morphology with Ag NPs of 20–30 nm | Ti, rutile, anatase, CaTiO3 and HA | 2.1 | 8000 | E. coli | – | [59] | |||
| Cp-Ti | Na2HPO4, CA, and 0.0025 M CH3COOAg | 380 | – | 300 | Flake-like morphology with regional Ag particles less than 200 nm | Ti, rutile, anatase, CaTiO3 and HA | 4.6 | – | E. coli, S. aureus | – | [60] | |||
| Cp-Ti | 0.5–1.0 g·L−1 AgNO3 | – | 65 | 5–240 | Highly ordered nanopores with Ag NPs of 10–30 nm within micropits | – | – | 200–450 | S. aureus | Newborn mouse pre-osteoblast cells | [61] | |||
| Cp-Ti | 7.6 g·L−1 Na3PO4, 9.4 g·L−1 Ca(NO3)2 and 1.0 g·L−1 AgNO3 | – | 65 | 240 | Highly ordered nanopores with Ag NPs of 10–30 nm within micropits | – | – | – | S. aureus | Newborn mouse pre-osteoblast cells | [61] | |||
| Cp-Ti | 1.0~8.0 g·L−1 Cu(NO3)2 | – | 65 | 240 | Mesopores (20–40 nm) within micropits | Ti | – | – | – | Osteoblast cells | [62] | |||
| Cp-Ti | 3.8~7.6 g·L−1 Na3PO4 and 1.0 g·L−1 Cu(NO3)2 | – | 65 | 240 | Mesopores (20–40 nm) within micropits | Ti | – | – | – | Osteoblast cells | [62] | |||
| Cp-Ti | 0.05 M β-GP, 0.1 M CA and 0.05 M (CH3COO)2Cu | – | 16.5 | 240 | Micropores or crater structures (3–5 μm) with nano-grains of 30–50 nm | Ti and anatase | 1.43 | – | S. aureus | Human osteosarcoma cell | [63] | |||
| Cp-Ti | 0.02 M β-GP, 0.2 M CA and 0.00125~0.005 M Cu (CH3COO)2 | 450 | – | 90 | Micropore structures (1–4 μm) | Rutile and anatase | 0.67–1.93 | 2.8–60.2 | S. aureus | Mouse fibroblast cell | [64] | |||
| Cp-Ti | 0.15 M Ca-GP, 0.02 M CA and 0.06 M ZA | – | 30 | 300 | Porous structures (<5 μm) | Ti, rutile and anatase | 8.7 | – | E. coli, S. aureus | Osteoblast cells | [65] | |||
| Cp-Ti | 0.05 M β-GP, 0.1 M CA and 0.02~0.06 ZA | – | 16.5 | 240 | Microporous structures (<5 μm) | – | 4.6–9.3 | 1000–3620 | E. coli, S. aureus | Rat bone mesenchymal stem cells | [10] | |||
| Cp-Ti | 0.02 M β-GP, 0.1 M CA, 0.1 M ZA, and 6 g·L−1 Ag NPs | 390 | – | 30–90 | Microporous structures (1–4 μm) | Anatase and rutile | 1.06–1.42 (Ag), 22.19–26.93 (Zn) | – | S. aureus | – | [66] | |||
| Cp-Ti | 0.02 M β-GP, 0.1 M CA, 0.1 M ZA, and 6 g·L−1 Ag NPs | 390 | – | 120 | Microporous structures (1–4 μm) with Ag NPs of 5–10 nm | Anatase, rutile and ZnO | 1.56 (Ag), 29.38 (Zn) | 684 (Ag), 6880 (Zn) | S. aureus | – | [66] | |||
| Cp-Ti | 0.02 M β-GP, 0.1 M CA, 0.1 M ZA, and 6 g·L−1 Ag NPs | 390 | – | 240 | Microporous structures (1–4 μm) with some deposits | Anatase, rutile, ZnO and Zn2TiO4 | 1.58 (Ag), 31.27 (Zn) | – | S. aureus | – | [66] | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Zhang, X.; Wang, X.; Qin, L. Review of Antibacterial Activity of Titanium-Based Implants’ Surfaces Fabricated by Micro-Arc Oxidation. Coatings 2017, 7, 45. https://doi.org/10.3390/coatings7030045
He X, Zhang X, Wang X, Qin L. Review of Antibacterial Activity of Titanium-Based Implants’ Surfaces Fabricated by Micro-Arc Oxidation. Coatings. 2017; 7(3):45. https://doi.org/10.3390/coatings7030045
Chicago/Turabian StyleHe, Xiaojing, Xiangyu Zhang, Xin Wang, and Lin Qin. 2017. "Review of Antibacterial Activity of Titanium-Based Implants’ Surfaces Fabricated by Micro-Arc Oxidation" Coatings 7, no. 3: 45. https://doi.org/10.3390/coatings7030045
APA StyleHe, X., Zhang, X., Wang, X., & Qin, L. (2017). Review of Antibacterial Activity of Titanium-Based Implants’ Surfaces Fabricated by Micro-Arc Oxidation. Coatings, 7(3), 45. https://doi.org/10.3390/coatings7030045





