Mn-Promoted Growth and Photoluminescence of Molybdenum Disulphide Monolayer
Abstract
:1. Introduction
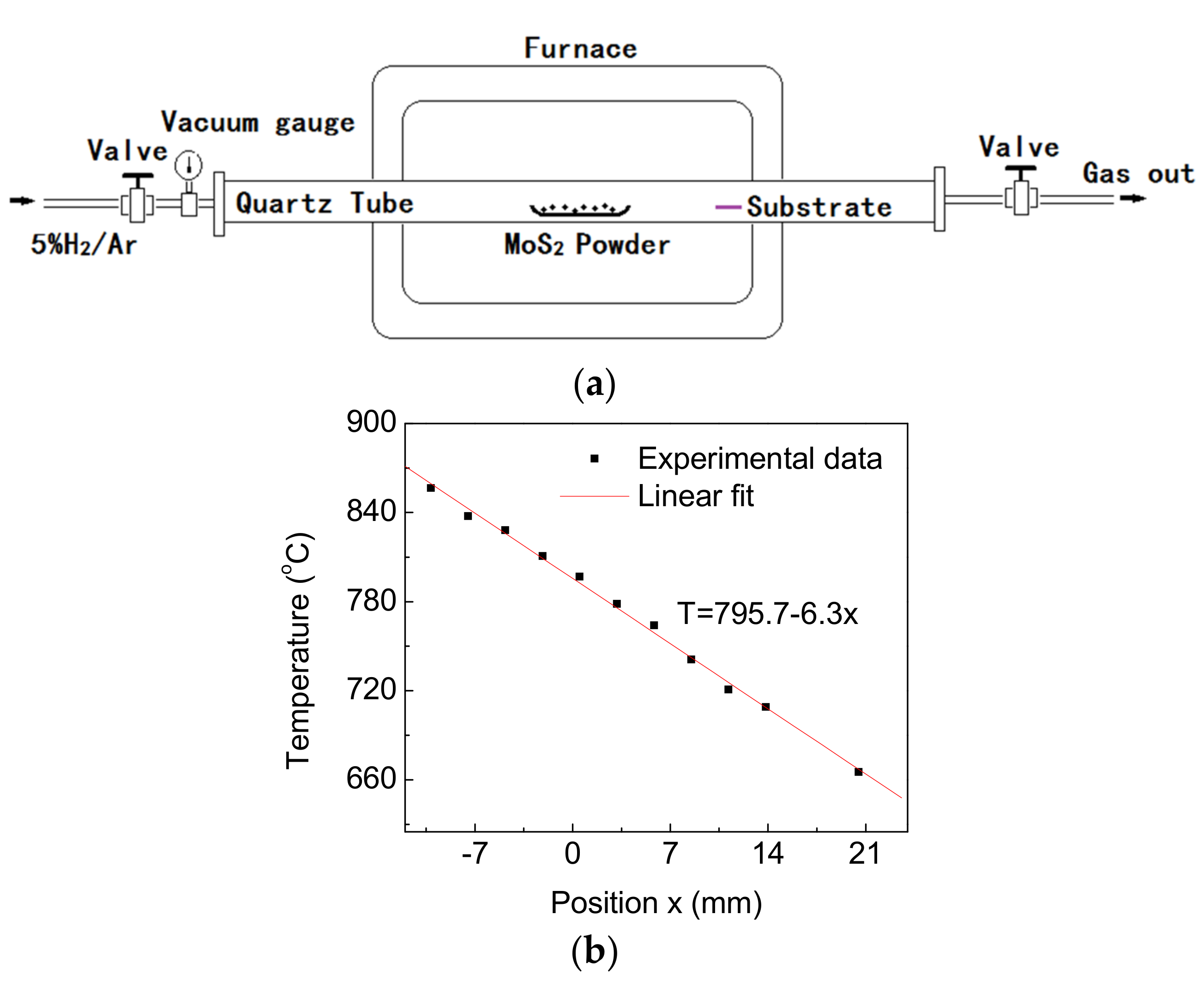
2. Materials and Methods
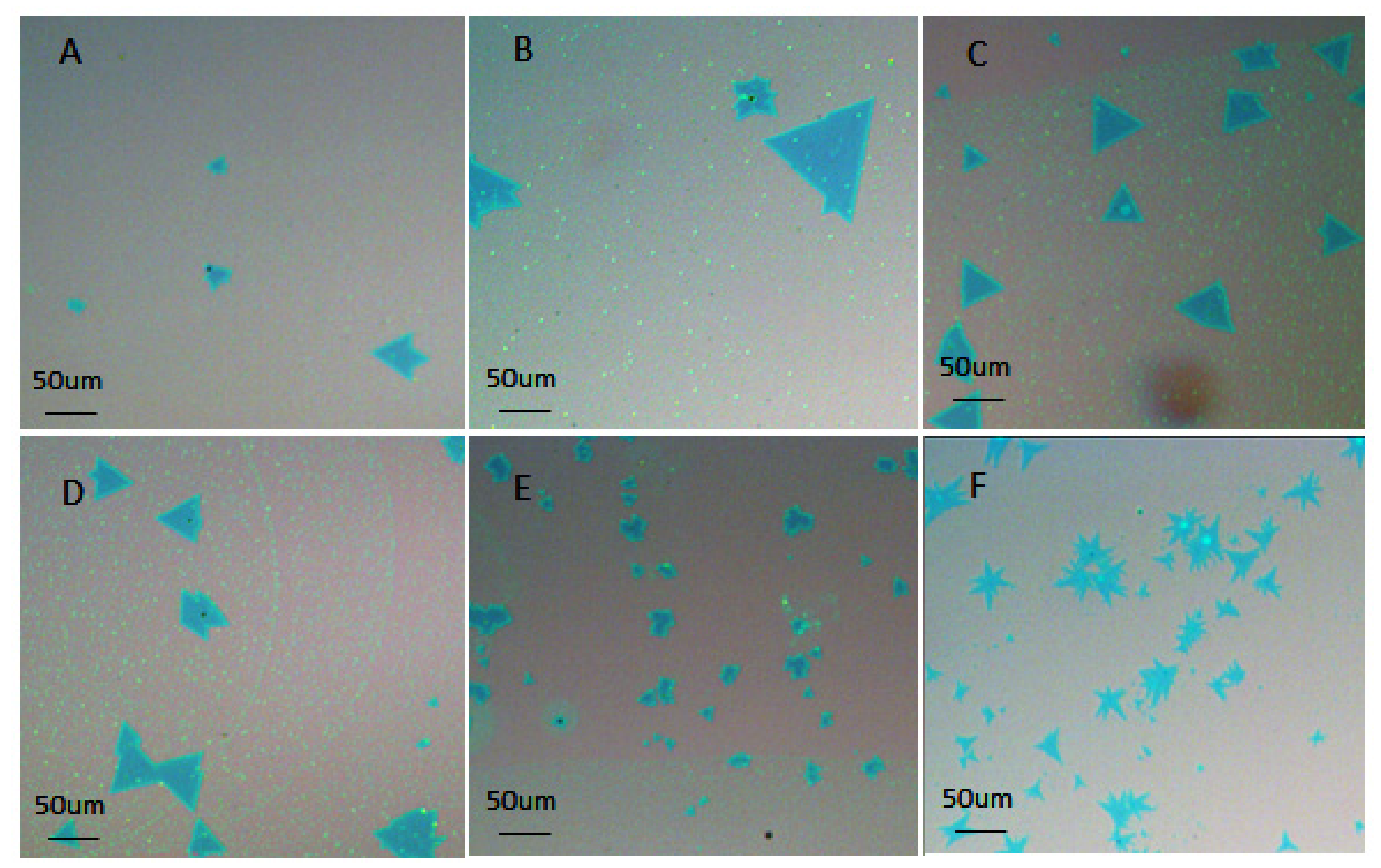
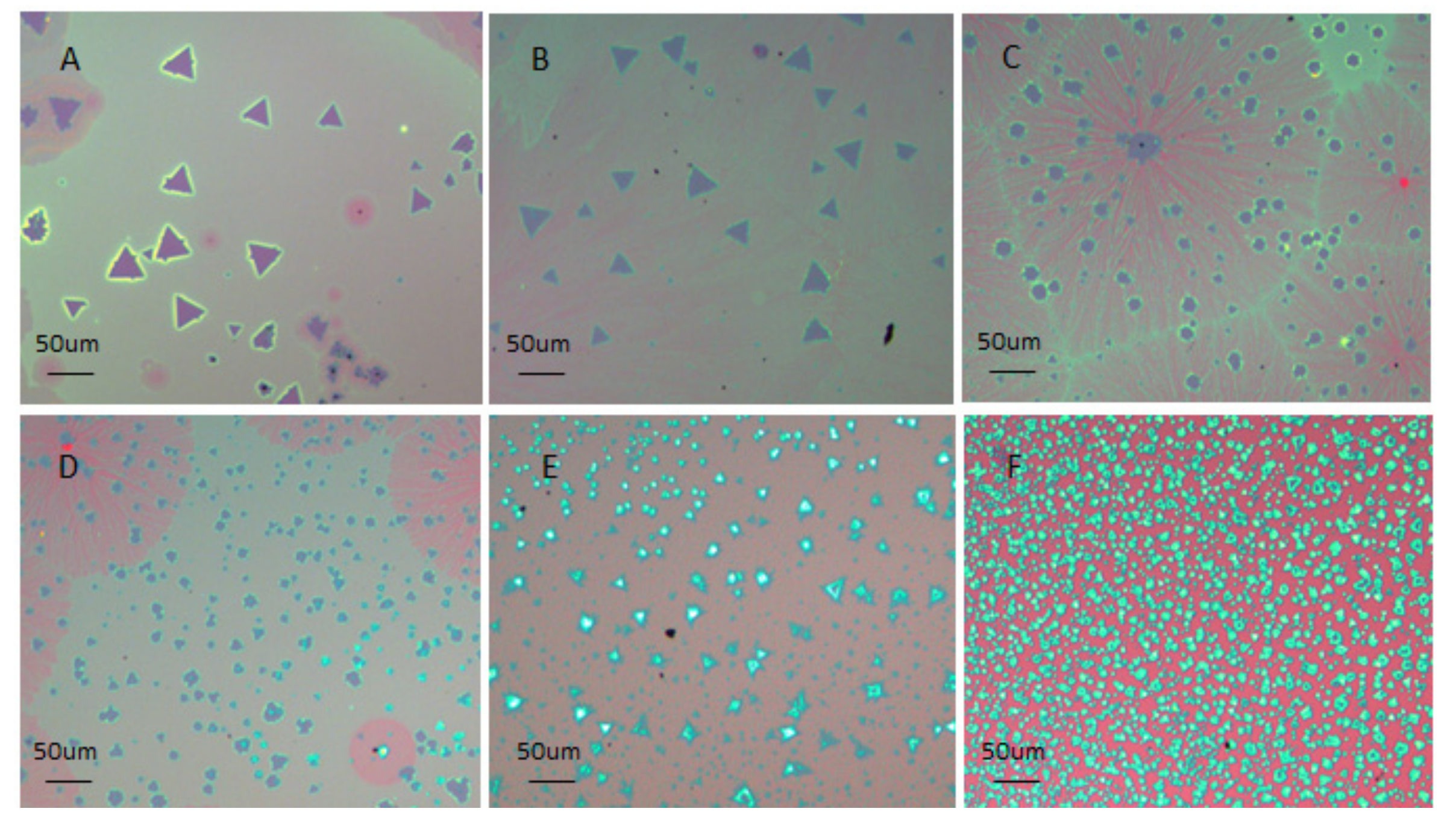
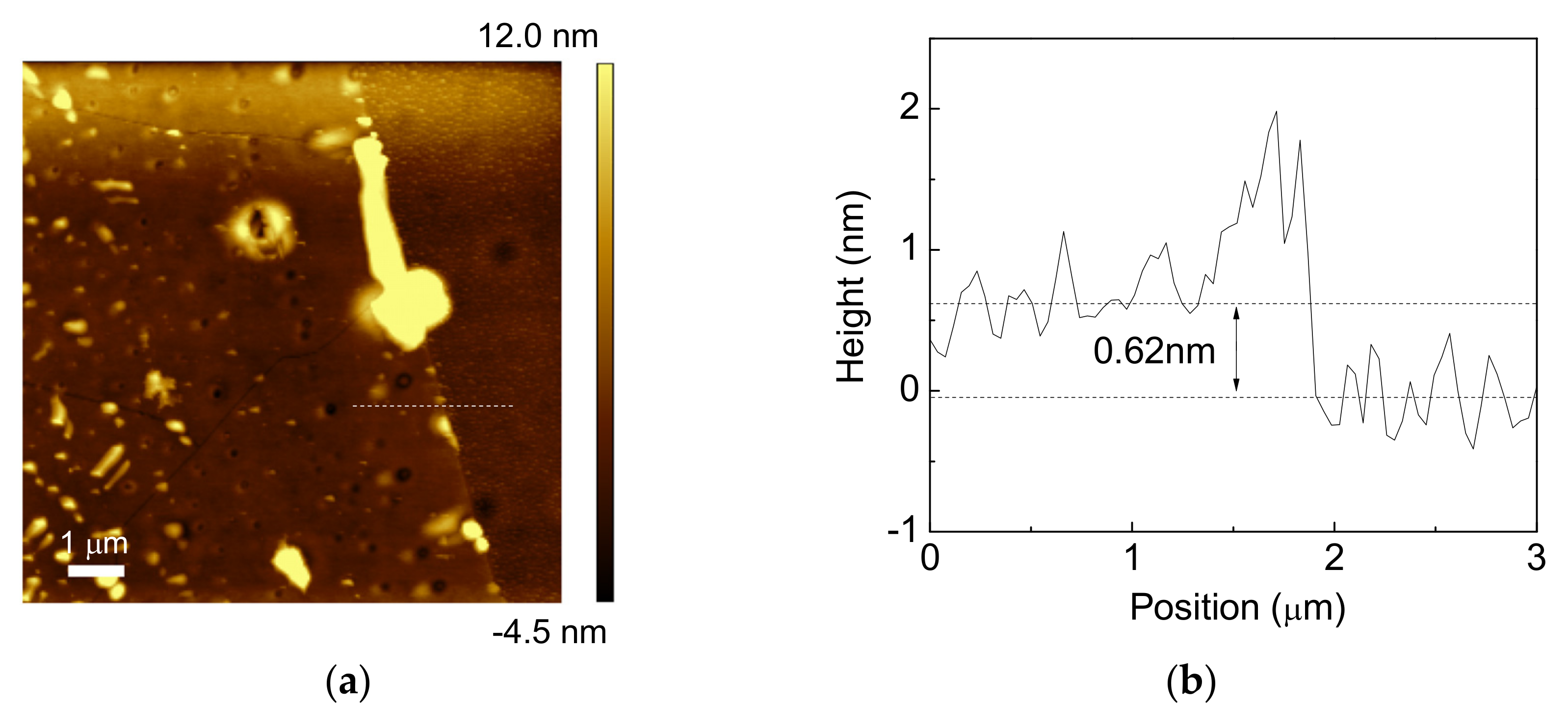
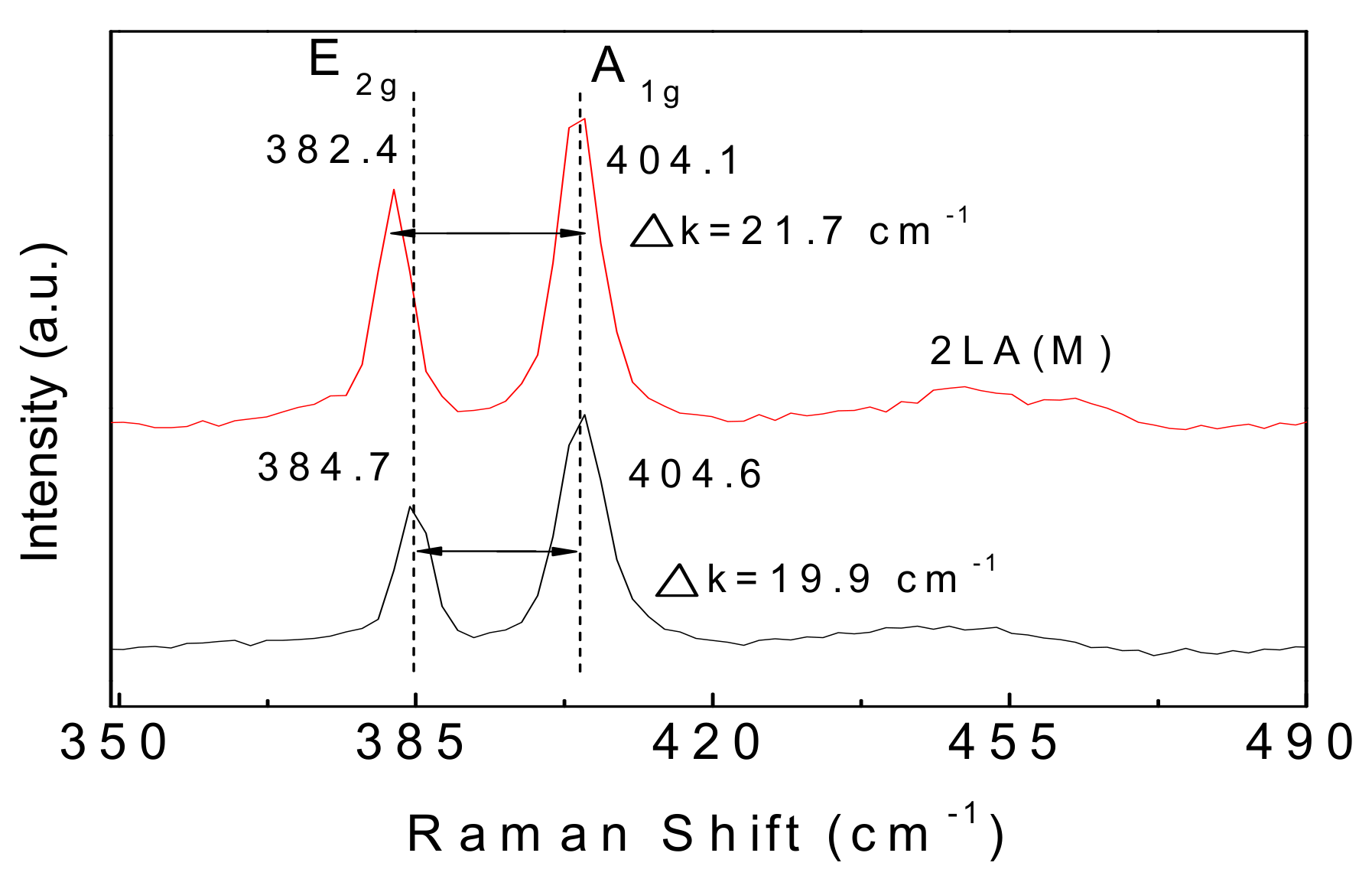
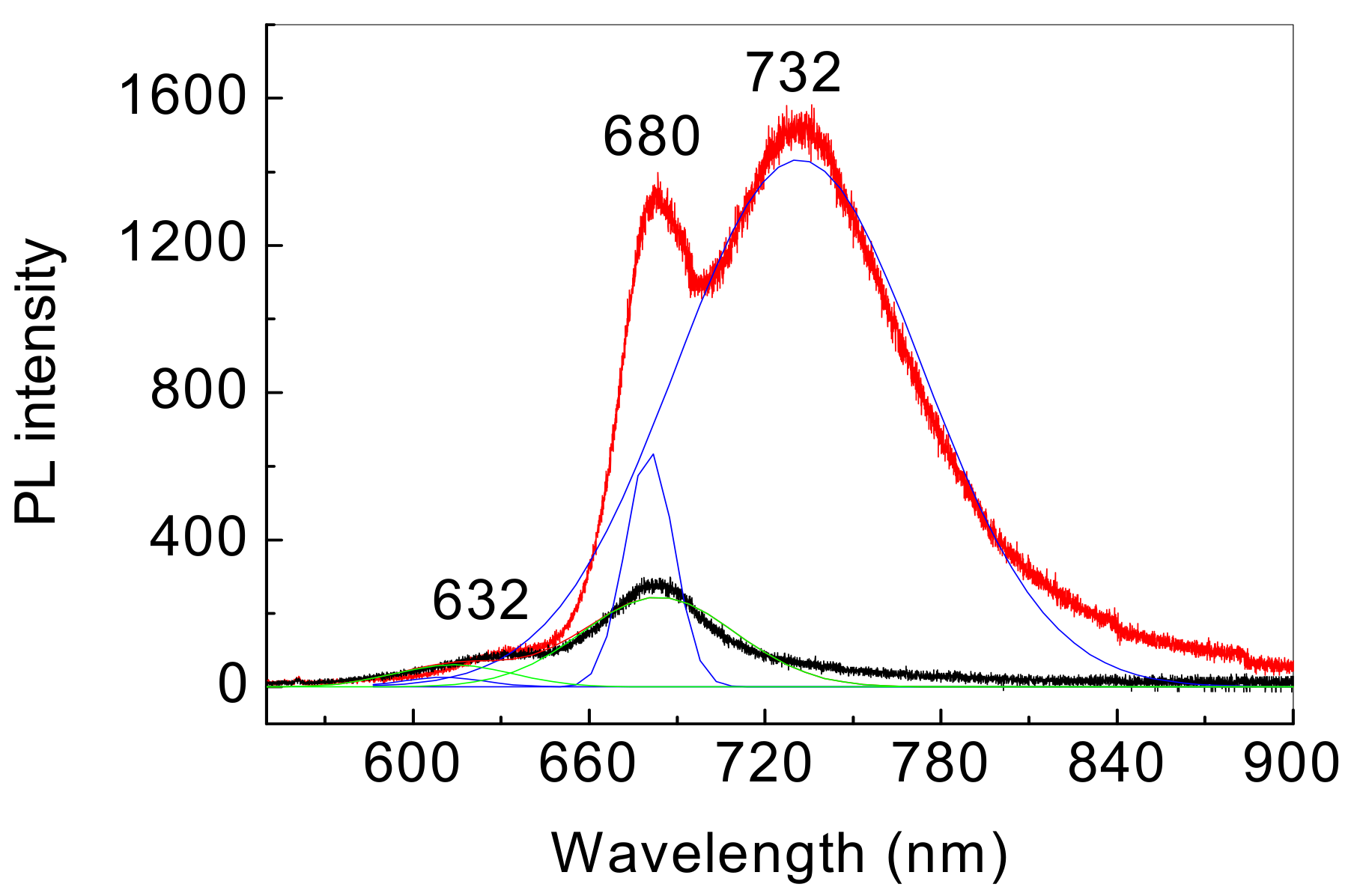
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically Thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805:1–136805:4. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Emerging device applications for semiconducting two-dimensional transition metal dichalcogenides. ACS Nano 2014, 8, 1102–1120. [Google Scholar] [CrossRef] [PubMed]
- Hayamizu, Y.; So, C.R.; Dag, S.; Page, T.S.; Starkebaum, D.; Sarikaya, M. Bioelectronic interfaces by spontaneously organized peptides on 2D atomic single layer materials. Sci. Rep. 2016, 6, 33778:1–33778:9. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.R.; Zhao, H.M.; Quan, X. Two-dimensional MoS2: A promising building block for biosensors. Biosens. Bioelectron. 2017, 89, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.F.; Shan, J. Photonics and optoelectronics of 2D semiconductor transition metal dichalcogenides. Nat. Photonics 2016, 10, 216–226. [Google Scholar] [CrossRef]
- Ma, S.; Xie, J.; Wen, J.Q.; He, K.L.; Li, X.; Liu, W.; Zhang, X.C. Constructing 2D layered hybrid CdS nanosheets/MoS2 heterojunctions for enhanced visible-light photocatalytic H2 generation. Appl. Surf. Sci. 2017, 391, 580–591. [Google Scholar] [CrossRef]
- Van Der Zande, A.M.; Huang, P.Y.; Chenet, D.A.; Berkelbach, T.C.; You, Y.M.; Lee, G.H.; Heinz, T.F.; Reichman, D.R.; Muller, D.A.; Hone, J.C. Grains and grain boundaries in highly crystalline monolayer molybdenum disulphide. Nat. Mater. 2013, 12, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, Z.; Zhan, Y.J.; Zhang, P.; Wu, R.X.; Jiang, T.; Wu, S.W.; Wang, H.; Zhao, Y.; Nan, T.; et al. Controllable growth of monolayer MoS2 by chemical vapor deposition via close MoO2 precursor for electrical and optical applications. Nanotechnology 2017, 28, 084001:1–084001:11. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.F.; Huang, C.M.; Aivazian, G.; Ross, J.S.; Cobden, D.H.; Xu, X.D. Vapor-solid growth of high optical quality MoS2 monolayers with near-unity valley polarization. ACS Nano 2013, 7, 2768–2772. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.W.; Qi, X.; Ren, L.; Hao, G.L.; Fan, Y.P.; Liu, Y.D.; Han, W.J.; Zang, C.; Li, J.; Zhong, J.X. Photoresponse properties of large-area MoS2 atomic layer synthesized by vapor phase deposition. J. Appl. Phys. 2014, 116, 164304:1–164304:6. [Google Scholar] [CrossRef]
- Kim, Y.; Bark, H.; Ryu, G.H.; Lee, Z.; Lee, C. Wafer-scale monolayer MoS2 grown by chemical vapor deposition using a reaction of MoO3 and H2S. J. Phys. Condens. Matter 2016, 28, 184002:1–184002:6. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Zhang, X.Q.; Zhang, W.J.; Chang, M.T.; Lin, C.T.; Chang, K.D.; Yu, Y.C.; Wang, J.T.W.; Chang, C.S.; Li, L.J.; et al. Synthesis of large-area MoS2 atomic layers with chemical vapor deposition. Adv. Mater. 2012, 24, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Lee, Y.H.; Lin, Y.X.; Fang, W.J.; Yu, L.L.; Dresselhaus, M.S.; Kong, J. Role of the seeding promoter in MoS2 growth by chemical vapor deposition. Nano Lett. 2014, 14, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Loh, T.A.J.; Chua, D.H.C. Growth mechanism of pulsed laser fabricated few-layer MoS2 on metal substrates. ACS Appl. Mater. Interface 2014, 6, 15966–15971. [Google Scholar] [CrossRef] [PubMed]
- Loh, T.A.J.; Tanemura, M.; Chua, D.H.C. Ultrathin MoS2 and WS2 layers on silver nano-tips as electron emitters. Appl. Phys. Lett. 2016, 109, 133102. [Google Scholar] [CrossRef]
- Zhang, K.H.; Feng, S.M.; Wang, J.J.; Azcatl, A.L.; Lu, N.; Addou, R.; Wang, N.; Zhou, C.J.; Lerach, J.; Bojan, V.; et al. Manganese doping of Monolayer MoS2: The substrate is critical. Nano Lett. 2015, 15, 6586–6591. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Rong, Y.M.; Fan, Y.; Pacios, M.; Bhaskaran, H.; He, K.; Warner, J.H. Shape evolution of monolayer MoS2 crystals grown by chemical vapor deposition. Chem. Mater. 2014, 26, 6371–6379. [Google Scholar] [CrossRef]
- Amani, M.; Chin, M.L.; Birdwell, A.G.; O’Regan, T.P.; Najmaei, S.; Liu, Z.; Ajayan, P.M.; Lou, J.; Dubey, M. Electrical performance of monolayer MoS2 field-effect transistors prepared by chemical vapor deposition. Appl. Phys. Lett. 2013, 102, 193107:1–193107:4. [Google Scholar] [CrossRef]
- Mignuzzi, S.; Pollard, A.J.; Bonini, N.; Brennan, B.; Gilmore, I.S.; Pimenta, M.A.; Richards, D.; Roy, D. Effect of disorder on Raman scattering of single-layer MoS2. Phys. Rev. B 2015, 91, 195411:1–195411:7. [Google Scholar] [CrossRef]
- Splendiani, A.; Sun, L.; Zhang, Y.B.; Li, T.S.; Kim, J.; Chim, C.Y.; Galli, G.; Wang, F. Emerging photoluminescence in monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Tongay, S.; Suh, J.; Ataca, C.; Fan, W.; Luce, A.; Kang, J.S.; Liu, J.; Ko, C.; Raghunathanan, R.; Zhou, J.; et al. Defects activated photoluminescence in two-dimensional semiconductors: Interplay between bound, charged, and free excitons. Sci. Rep. 2013, 3, 2657:1–2657:5. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.; Zhao, S.; Weng, J.; Lv, Y. Mn-Promoted Growth and Photoluminescence of Molybdenum Disulphide Monolayer. Coatings 2017, 7, 78. https://doi.org/10.3390/coatings7060078
Jin S, Zhao S, Weng J, Lv Y. Mn-Promoted Growth and Photoluminescence of Molybdenum Disulphide Monolayer. Coatings. 2017; 7(6):78. https://doi.org/10.3390/coatings7060078
Chicago/Turabian StyleJin, Shengzhong, Shichao Zhao, Jiaxin Weng, and Yanfei Lv. 2017. "Mn-Promoted Growth and Photoluminescence of Molybdenum Disulphide Monolayer" Coatings 7, no. 6: 78. https://doi.org/10.3390/coatings7060078




