TiO2 Nanocrystal Based Coatings for the Protection of Architectural Stone: The Effect of Solvents in the Spray-Coating Application for a Self-Cleaning Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2 NRs
2.3. Characterization of TiO2 NRs
2.3.1. Transmission Electron Microscopy
2.3.2. XRD Analysis
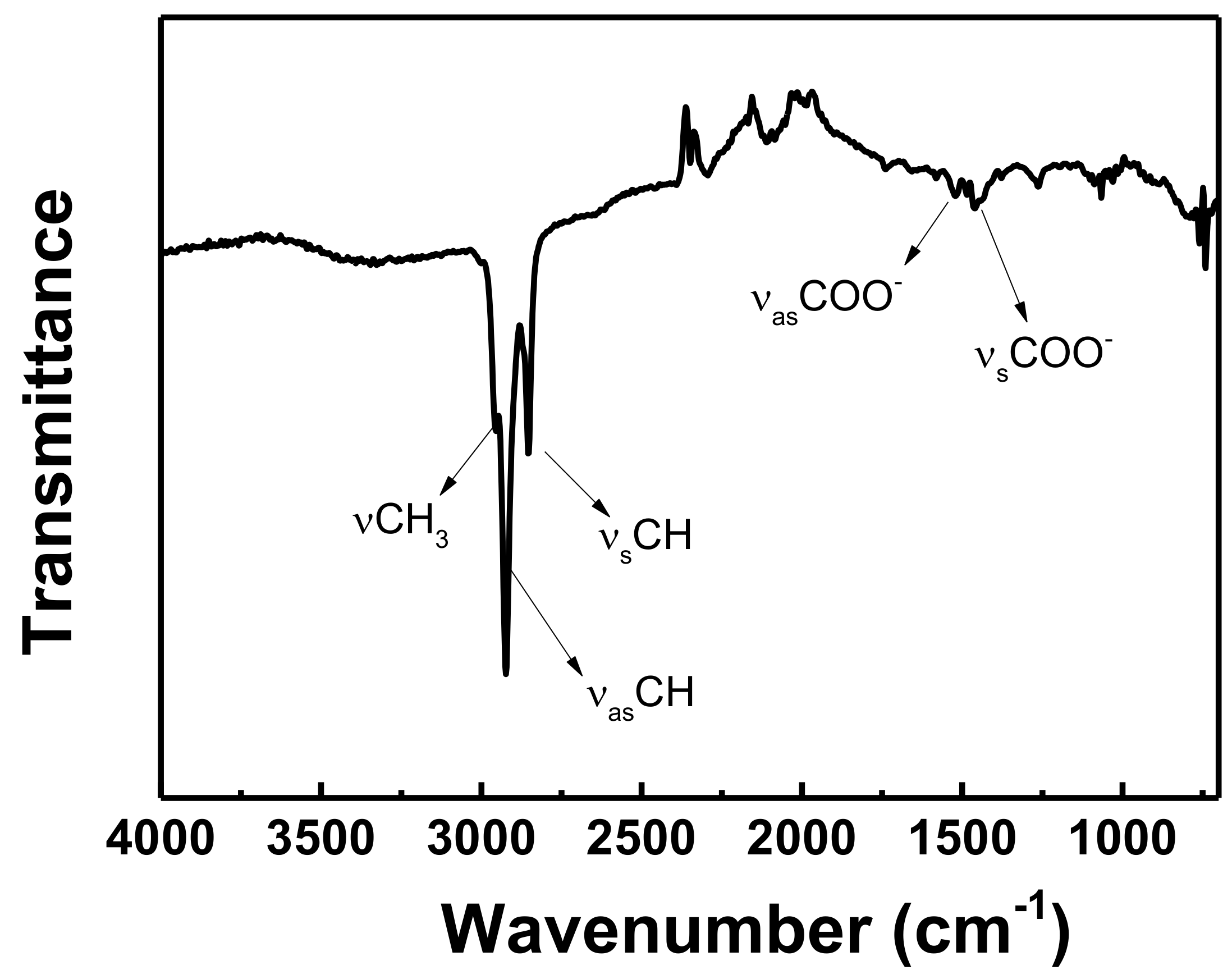
2.3.3. ATR-FTIR Spectroscopy
2.4. Spray-Coating Application Of TiO2 Nanorods onto the Model Substrate
2.5. Characterization of Films of TiO2 NRs on Silicon
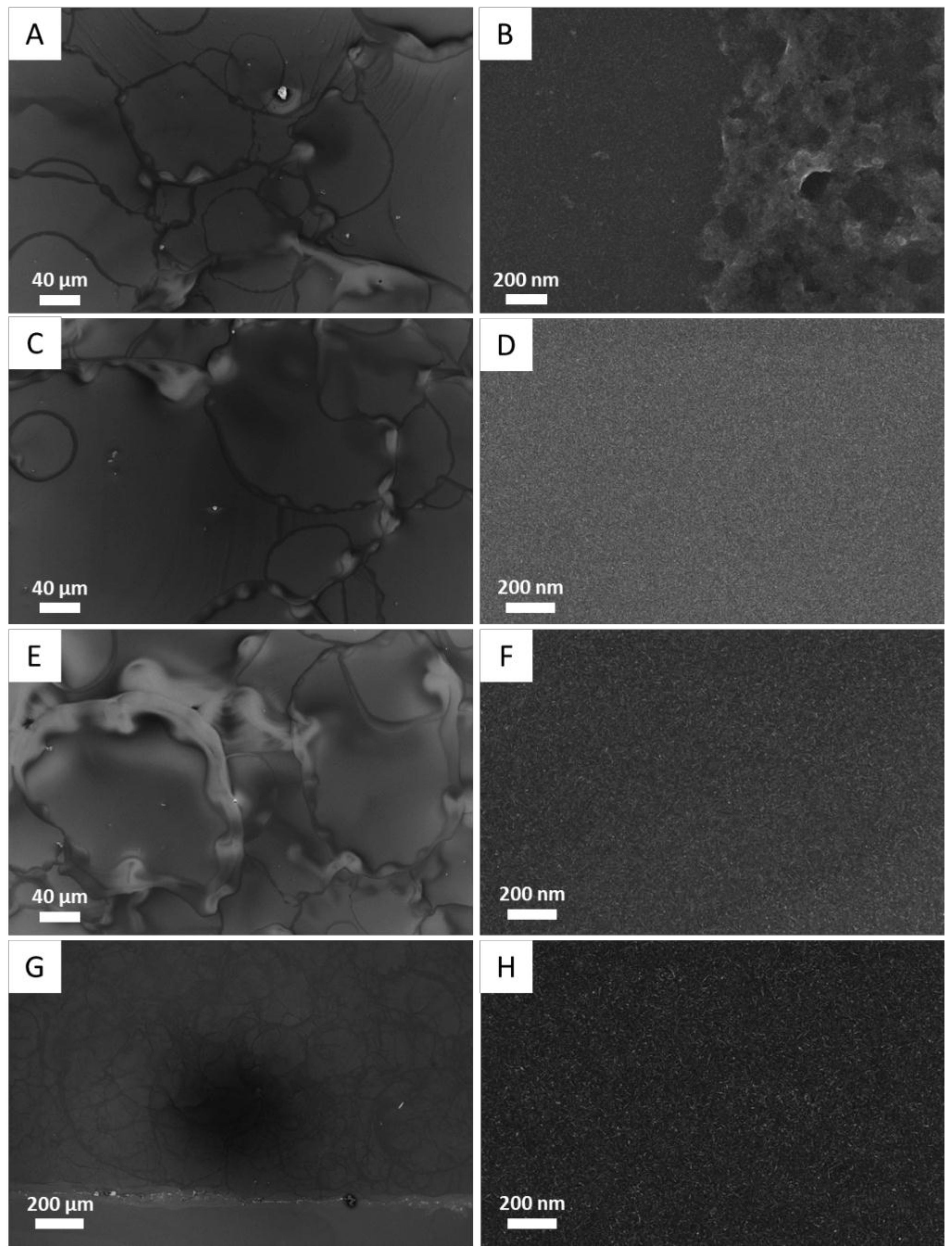
2.5.1. Scanning Electron Microscopy
2.5.2. Atomic Force Microscopy Analysis
2.6. Spray-Coating Application of TiO2 NRs on Stone
2.7. Characterization of TiO2 NRs Coating
2.7.1. Diffuse Reflectance Spectroscopy
2.7.2. Static Contact Angle Measurements
2.7.3. Colour Measurement
2.7.4. Water Absorption Test
2.7.5. Water Vapour Permeability Test
2.8. Photocatalytic Activity of Ti
3. Results and Discussion
3.1. Photocatalyst Characterization
3.2. Optimization of the Deposition Conditions of TiO2NRs onto a Model Substrate
3.3. Deposition of TiO2 NRs on Lecce Stone
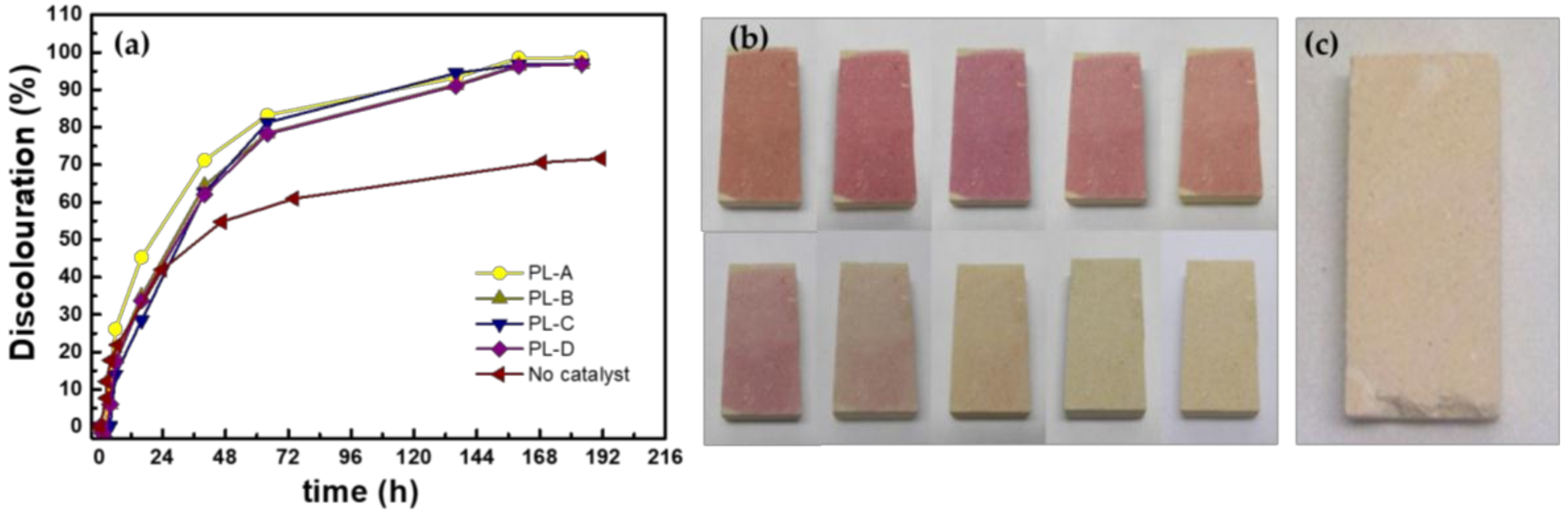
3.4. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sang, L.; Zhao, Y.; Burda, C. TiO2 nanoparticles as functional building blocks. Chem. Rev. 2014, 114, 9283–9318. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Petronella, F.; Truppi, A.; Ingrosso, C.; Placido, T.; Striccoli, M.; Curri, M.L.; Agostiano, A.; Comparelli, R. Nanocomposite materials for photocatalytic degradation of pollutants. Catal. Today 2017, 281, 85–100. [Google Scholar] [CrossRef]
- Lai, Y.; Huang, J.; Cui, Z.; Ge, M.; Zhang, K.-Q.; Chen, Z.; Chi, L. Recent advances in TiO2-based nanostructured surfaces with controllable wettability and adhesion. Small 2016, 12, 2203–2224. [Google Scholar] [CrossRef] [PubMed]
- Varshney, G.; Kanel, S.R.; Kempisty, D.M.; Varshney, V.; Agrawal, A.; Sahle-Demessie, E.; Varma, R.S.; Nadagouda, M.N. Nanoscale TiO2 films and their application in remediation of organic pollutants. Coord. Chem. Rev. 2016, 306, 43–64. [Google Scholar] [CrossRef]
- Rtimi, S.; Giannakis, S.; Pulgarin, C. Self-sterilizing sputtered films for applications in hospital facilities. Molecules 2017, 22, 1074. [Google Scholar] [CrossRef] [PubMed]
- Salthammer, T.; Fuhrmann, F. Photocatalytic surface reactions on indoor wall paint. Environ. Sci. Technol. 2007, 41, 6573–6578. [Google Scholar] [CrossRef] [PubMed]
- Hernández Rodríguez, M.J.; Pulido Melián, E.; González Díaz, O.; Araña, J.; Macías, M.; González Orive, A.; Doña Rodríguez, J.M. Comparison of supported TiO2 catalysts in the photocatalytic degradation of NOx. J. Mol. Catal. A Chem. 2016, 413, 56–66. [Google Scholar] [CrossRef]
- Munafò, P.; Goffredo, G.B.; Quagliarini, E. TiO2-based nanocoatings for preserving architectural stone surfaces: An overview. Constr. Build. Mater. 2015, 84, 201–218. [Google Scholar] [CrossRef]
- Baglioni, P.; Carretti, E.; Chelazzi, D. Nanomaterials in art conservation. Nat. Nanotechnol. 2015, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Antizar-Ladislao, B.; Galil, N.I. Biofilm and colloidal biomass dynamics in a shallow sandy contaminated aquifer under in-situ remediation conditions. Int. Biodeterior. Biodegrad. 2010, 64, 331–338. [Google Scholar] [CrossRef]
- Kapridaki, C.; Maravelaki-Kalaitzaki, P. TiO2–SiO2–PDMS nano-composite hydrophobic coating with self-cleaning properties for marble protection. Prog. Org. Coat. 2013, 76, 400–410. [Google Scholar] [CrossRef]
- Cappelletti, G.; Fermo, P.; Camiloni, M. Smart hybrid coatings for natural stones conservation. Prog. Org. Coat. 2015, 78, 511–516. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Kolinkeová, B.; Panayiotou, C. Superhydrophobic films for the protection of outdoor cultural heritage assets. Appl. Phys. A 2009, 97, 351–360. [Google Scholar] [CrossRef]
- La Russa, M.F.; Ruffolo, S.A.; Rovella, N.; Belfiore, C.M.; Palermo, A.M.; Guzzi, M.T.; Crisci, G.M. Multifunctional TiO2 coatings for cultural heritage. Prog. Org. Coat. 2012, 74, 186–191. [Google Scholar] [CrossRef]
- Kapridaki, C.; Pinho, L.; Mosquera, M.J.; Maravelaki-Kalaitzaki, P. Producing photoactive, transparent and hydrophobic SiO2-crystalline TiO2 nanocomposites at ambient conditions with application as self-cleaning coatings. Appl. Catal. B Environ. 2014, 156–157, 416–427. [Google Scholar] [CrossRef]
- Arturi, K.R.; Jepsen, H.; Callsen, J.N.; Søgaard, E.G.; Simonsen, M.E. Superhydrophilicity and durability of fluoropolymer-TiO2 coatings. Prog. Org. Coat. 2016, 90, 132–138. [Google Scholar] [CrossRef]
- Ingrosso, C.; Esposito Corcione, C.; Striani, R.; Comparelli, R.; Striccoli, M.; Agostiano, A.; Curri, M.L.; Frigione, M. UV-curable nanocomposite based on methacrylic-siloxane resin and surface-modified TiO2 nanocrystals. ACS Appl. Mater. Interfaces 2015, 7, 15494–15505. [Google Scholar] [CrossRef] [PubMed]
- Esposito Corcione, C.; Ingrosso, C.; Petronella, F.; Comparelli, R.; Striccoli, M.; Agostiano, A.; Frigione, M.; Curri, M.L. A designed UV–vis light curable coating nanocomposite based on colloidal TiO2 nrs in a hybrid resin for stone protection. Prog. Org. Coat. 2018, 122, 290–301. [Google Scholar] [CrossRef]
- Franzoni, E.; Fregni, A.; Gabrielli, R.; Graziani, G.; Sassoni, E. Compatibility of photocatalytic TiO2-based finishing for renders in architectural restoration: A preliminary study. Build. Environ. 2014, 80, 125–135. [Google Scholar] [CrossRef]
- Fonseca, A.J.; Pina, F.; Macedo, M.F.; Leal, N.; Romanowska-Deskins, A.; Laiz, L.; Gómez-Bolea, A.; Saiz-Jimenez, C. Anatase as an alternative application for preventing biodeterioration of mortars: Evaluation and comparison with other biocides. Int. Biodeterior. Biodegrad. 2010, 64, 388–396. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Ortíz, N.; De la Rosa-García, S.; González-Gómez, W.; Soria-Castro, M.; Quintana, P.; Oskam, G.; Ortega-Morales, B. Antifungal coatings based on Ca(OH)2 mixed with ZnO/TiO2 nanomaterials for protection of limestone monuments. ACS Appl. Mater. Interfaces 2013, 5, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.; Chan, C.k.; Tytgat, T.; Craeye, B.; Costarramone, N.; Lacombe, S.; Lenaerts, S. Photocatalytic degradation of soot deposition: Self-cleaning effect on titanium dioxide coated cementitious materials. Chem. Eng. J. 2013, 222, 411–418. [Google Scholar] [CrossRef]
- Bergamonti, L.; Alfieri, I.; Lorenzi, A.; Predieri, G.; Barone, G.; Gemelli, G.; Mazzoleni, P.; Raneri, S.; Bersani, D.; Lottici, P.P. Nanocrystalline TiO2 coatings by sol–gel: Photocatalytic activity on Pietra di Noto biocalcarenite. J. Sol-Gel Sci. Technol. 2015, 75, 141–151. [Google Scholar] [CrossRef]
- Calia, A.; Lettieri, M.; Masieri, M.; Pal, S.; Licciulli, A.; Arima, V. Limestones coated with photocatalytic TiO2 to enhance building surface with self-cleaning and depolluting abilities. J. Clean. Product. 2017, 165, 1036–1047. [Google Scholar] [CrossRef]
- Quagliarini, E.; Bondioli, F.; Goffredo, G.B.; Licciulli, A.; Munafò, P. Self-cleaning materials on architectural heritage: Compatibility of photo-induced hydrophilicity of TiO2 coatings on stone surfaces. J. Cult. Herit. 2013, 14, 1–7. [Google Scholar] [CrossRef]
- Gherardi, F.; Colombo, A.; D'Arienza, M.; Di Credico, B.; Goidanich, S.; Morazzoni, F.; Simonutti, R.; Toniolo, L. Efficient self-cleaning treatments for built heritage based on highly photo-active and well-dispersible TiO2 nanocrystals. Microchem. J. 2016, 126, 54–62. [Google Scholar] [CrossRef]
- Petronella, F.; Pagliarulo, A.; Striccoli, M.; Calia, A.; Lettieri, M.; Colangiuli, D.; Curri, M.; Comparelli, R. Colloidal nanocrystalline semiconductor materials as photocatalysts for environmental protection of architectural stone. Crystals 2017, 7, 30. [Google Scholar] [CrossRef]
- Calia, A.; Lettieri, M.; Masieri, M. Durability assessment of nanostructured TiO2 coatings applied on limestones to enhance building surface with self-cleaning ability. Build. Environ. 2016, 110, 1–10. [Google Scholar] [CrossRef]
- Lorenzo Graziani, E.Q.; D’Orazio, M. Superfici autopulenti e biocide nel restauro archeologico di pietre e laterizi. Restauro Archeologico 2016, 25, 28–43. [Google Scholar]
- Esposito Corcione, C.; De Simone, N.; Santarelli, M.L.; Frigione, M. Protective properties and durability characteristics of experimental and commercial organic coatings for the preservation of porous stone. Prog. Org. Coat. 2017, 103, 193–203. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Carbajo, J.; Bahamonde, A.; Crippa, M.; Polizzi, S.; Scotti, R.; Wahba, L.; Morazzoni, F. Photogenerated defects in shape-controlled TiO2 anatase nanocrystals: A probe to evaluate the role of crystal facets in photocatalytic processes. J. Am. Chem. Soc. 2011, 133, 17652–17661. [Google Scholar] [CrossRef] [PubMed]
- Fittipaldi, M.; Curri, M.L.; Comparelli, R.; Striccoli, M.; Agostiano, A.; Grassi, N.; Sangregorio, C.; Gatteschi, D. A multifrequency EPR study on organic-capped anatase TiO2 nanocrystals. J. Phys. Chem. C 2009, 113, 6221–6226. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Cozzoli, P.D.; Kornowski, A.; Weller, H. Low-temperature synthesis of soluble and processable organic-capped anatase TiO2 nanorods. J. Am. Chem. Soc. 2003, 125, 14539–14548. [Google Scholar] [CrossRef] [PubMed]
- EN 15802 Conservation of Cultural Property—Test Methods—Determination of Static Contact Angle; CEN (European Committee for Standardization): Brussels, Belgium, 2010.
- EN 15886 Conservation of Cultural Property—Test Methods—Colour Measurement of Surfaces; CEN (European Committee for Standardization): Brussels, Belgium, 2010.
- UNI 11432 Cultural Heritage—Natural and Artificial Stone—Determination of the Water Absorption by Contact Sponge; Ente Italiano di Normazione: Milan, Italy, 2011. (In Italian)
- NORMAL Rec. 21/85 Permeabilità al vapor d’acqua Italy; C.I.R.: Rome, Italy, 1985. (In Italian) [Google Scholar]
- Chen, Y.; Kang, K.S.; Yoo, K.H.; Jyoti, N.; Kim, J. Cause of slow phase transformation of TiO2 nanorods. J. Phys. Chem. C 2009, 113, 19753–19755. [Google Scholar] [CrossRef]
- Kim, D.-O.; Pack, M.; Hu, H.; Kim, H.; Sun, Y. Deposition of colloidal drops containing ellipsoidal particles: Competition between capillary and hydrodynamic forces. Langmuir 2016, 32, 11899–11906. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, I.M. Handbook of Organic Solvent Properties; Butterworth-Heinemann: Oxford, UK, 1996. [Google Scholar]
- Mackison, F.W.; Stricoff, R.S.; Partridge, L.J., Jr. Occupational Health Guidelines for Chemical Hazards; DHHS (NIOSH) Publication Number 81-123; The National Institute for Occupational Safety and Health (NIOSH): Atlanta, GA, USA, 1981. [Google Scholar]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Vandevoorde, D.; Pamplona, M.; Schalm, O.; Vanhellemont, Y.; Cnudde, V.; Verhaeven, E. Contact sponge method: Performance of a promising tool for measuring the initial water absorption. J. Cult. Herit. 2009, 10, 41–47. [Google Scholar] [CrossRef]
- Kronlund, D.; Bergbreiter, A.; Meierjohann, A.; Kronberg, L.; Lindén, M.; Grosso, D.; Smått, J.-H. Hydrophobization of marble pore surfaces using a total immersion treatment method—Product selection and optimization of concentration and treatment time. Prog. Org. Coat. 2015, 85, 159–167. [Google Scholar] [CrossRef]
- Dumée, L.; Germain, V.; Sears, K.; Schütz, J.; Finn, N.; Duke, M.; Cerneaux, S.; Cornu, D.; Gray, S. Enhanced durability and hydrophobicity of carbon nanotube bucky paper membranes in membrane distillation. J. Membr. Sci. 2011, 376, 241–246. [Google Scholar] [CrossRef]
- Licciulli, A.; Calia, A.; Lettieri, M.; Diso, D.; Masieri, M.; Franza, S.; Amadelli, R.; Casarano, G. Photocatalytic TiO2 coatings on limestone. J. Sol-Gel Sci. Technol. 2011, 60, 437–444. [Google Scholar] [CrossRef]
- Petronella, F.; Fanizza, E.; Mascolo, G.; Locaputo, V.; Bertinetti, L.; Martra, G.; Coluccia, S.; Agostiano, A.; Curri, M.L.; Comparelli, R. Photocatalytic activity of nanocomposite catalyst films based on nanocrystalline metal/semiconductors. J. Phys. Chem. C 2011, 115, 12033–12040. [Google Scholar] [CrossRef]
- Comparelli, R.; Fanizza, E.; Curri, M.L.; Cozzoli, P.D.; Mascolo, G.; Passino, R.; Agostiano, A. Photocatalytic degradation of azo dyes by organic-capped anatase TiO2 nanocrystals immobilized onto substrates. Appl. Catal. B Environ. 2005, 55, 81–91. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Wu, H.B.; Hng, H.H.; Lou, X.W. Direct synthesis of anatase TiO2 nanowires with enhanced photocatalytic activity. Adv. Mater. 2012, 24, 2567–2571. [Google Scholar] [CrossRef] [PubMed]








| Sample Name | Application Time (min) |
|---|---|
| A-ch | 1.00 |
| B-ch | 1.30 |
| C-ch | 2.00 |
| D-ch | 2.30 |
| A-he | 1.00 |
| B-he | 1.30 |
| C-he | 2.00 |
| D-he | 2.30 |
| Sample Name | Rq |
|---|---|
| A-ch | 1.61 ± 0.72 |
| B-ch | 1.12 ± 0.5 |
| C-ch | 1.44 ± 0.77 |
| D-ch | 1.54 ± 0.8 |
| A-he | 1.97 ± 0.73 |
| B-he | 1.45 ± 0.75 |
| C-he | 1.56 ± 0.54 |
| D-he | 2.73 ± 0.86 |
| Sample | Application Time | ΔE* | α (°) |
|---|---|---|---|
| PL-A | 1 min | 0.18± 0.08 | 130± 10 |
| PL-B | 1 min 30 s | 0.24± 0.18 | 135± 4 |
| PL-C | 2 min | 1.81± 0.15 | 136± 4 |
| PL-D | 2 min 30s | 3.33± 1.28 | 133±3 |
| Sample | Wa (g·min–1·m–2) | ΔWa (%) | WVP (g·m–2/24h) | ΔWVP (%) | ||
|---|---|---|---|---|---|---|
| Before Coating | After Coating | Before Coating | After Coating | |||
| PL-A | 851.1 ± 19.5 | 37.3 ± 24.4 | -95.6 | 244 ± 15 | 199 ± 18 | -19 |
| PL-B | 858.6 ± 41.7 | 16.1 ± 10.8 | -98.1 | 225 ± 13 | 195 ± 12 | -13 |
| PL-C | 736.4 ± 99.2 | 8.8 ± 0.9 | -98.8 | 198 ± 12 | 224±3 | +13 |
| PL-D | 757.2 ± 193.6 | 9.2 ± 1.7 | -98.8 | 329 ± 48 | 253±44 | -23 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petronella, F.; Pagliarulo, A.; Truppi, A.; Lettieri, M.; Masieri, M.; Calia, A.; Curri, M.L.; Comparelli, R. TiO2 Nanocrystal Based Coatings for the Protection of Architectural Stone: The Effect of Solvents in the Spray-Coating Application for a Self-Cleaning Surfaces. Coatings 2018, 8, 356. https://doi.org/10.3390/coatings8100356
Petronella F, Pagliarulo A, Truppi A, Lettieri M, Masieri M, Calia A, Curri ML, Comparelli R. TiO2 Nanocrystal Based Coatings for the Protection of Architectural Stone: The Effect of Solvents in the Spray-Coating Application for a Self-Cleaning Surfaces. Coatings. 2018; 8(10):356. https://doi.org/10.3390/coatings8100356
Chicago/Turabian StylePetronella, Francesca, Antonella Pagliarulo, Alessandra Truppi, Mariateresa Lettieri, Maurizio Masieri, Angela Calia, M. Lucia Curri, and Roberto Comparelli. 2018. "TiO2 Nanocrystal Based Coatings for the Protection of Architectural Stone: The Effect of Solvents in the Spray-Coating Application for a Self-Cleaning Surfaces" Coatings 8, no. 10: 356. https://doi.org/10.3390/coatings8100356







