Preparation of Nano-Hydroxyapatite Coated Carbon Nanotube Reinforced Hydroxyapatite Composites
Abstract
:1. Introduction
2. Materials and Methods
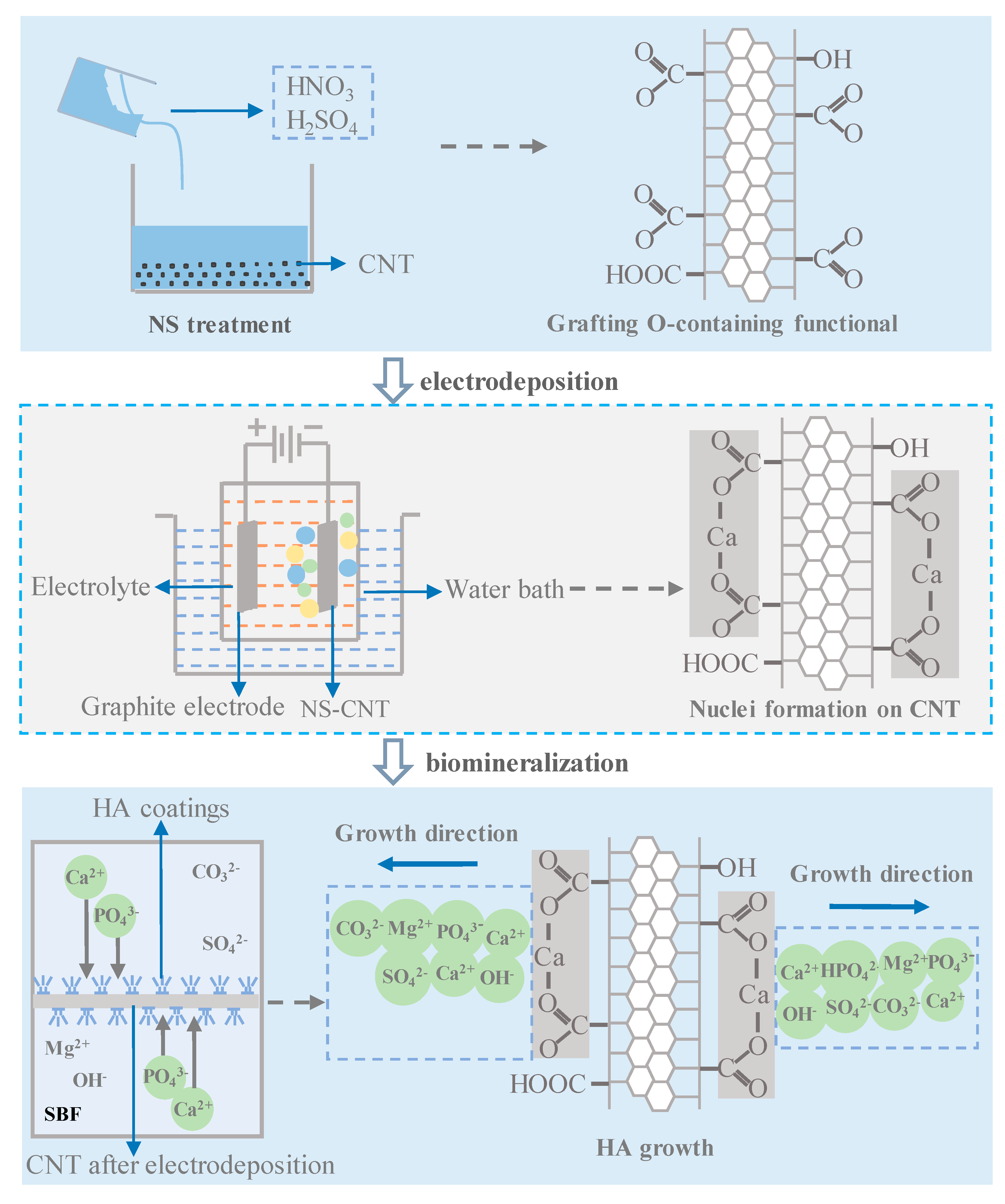
2.1. Electrodeposition and Biomineralization of nHA Coating
2.2. Preparation of CNTs/HA and nHA–CNTs/HA Composites
2.3. Characterization and Mechanical Properties
3. Results and Discussion
3.1. Carbon Nanotube Treatment
3.2. Electrodeposition and Biomineralization of HA Coating
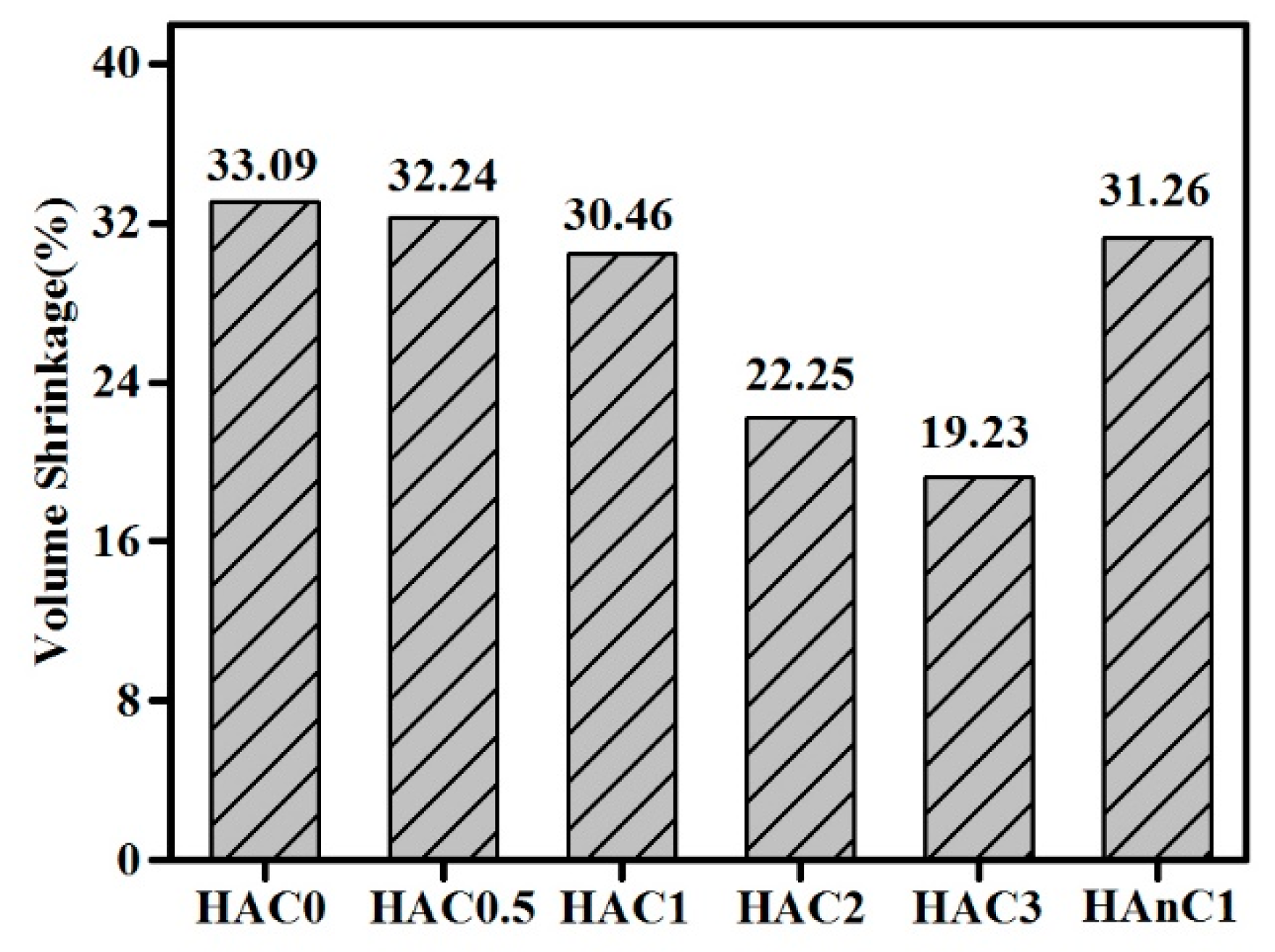
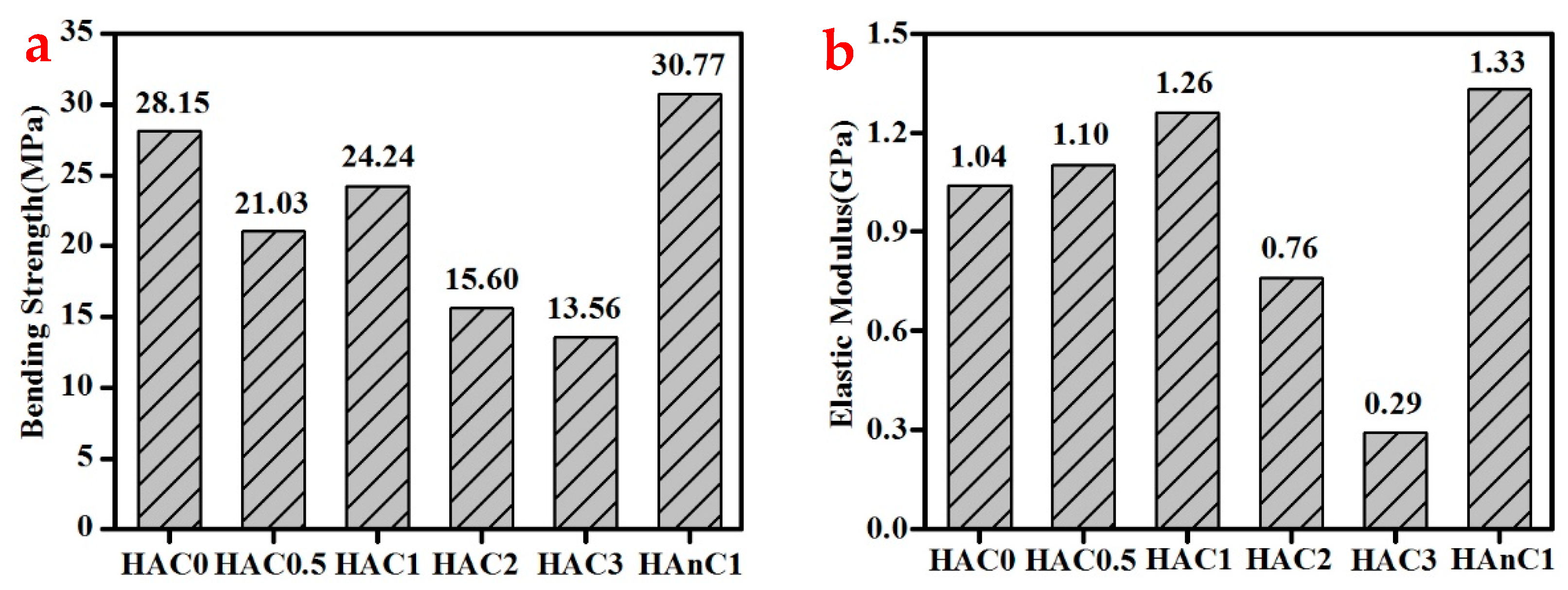
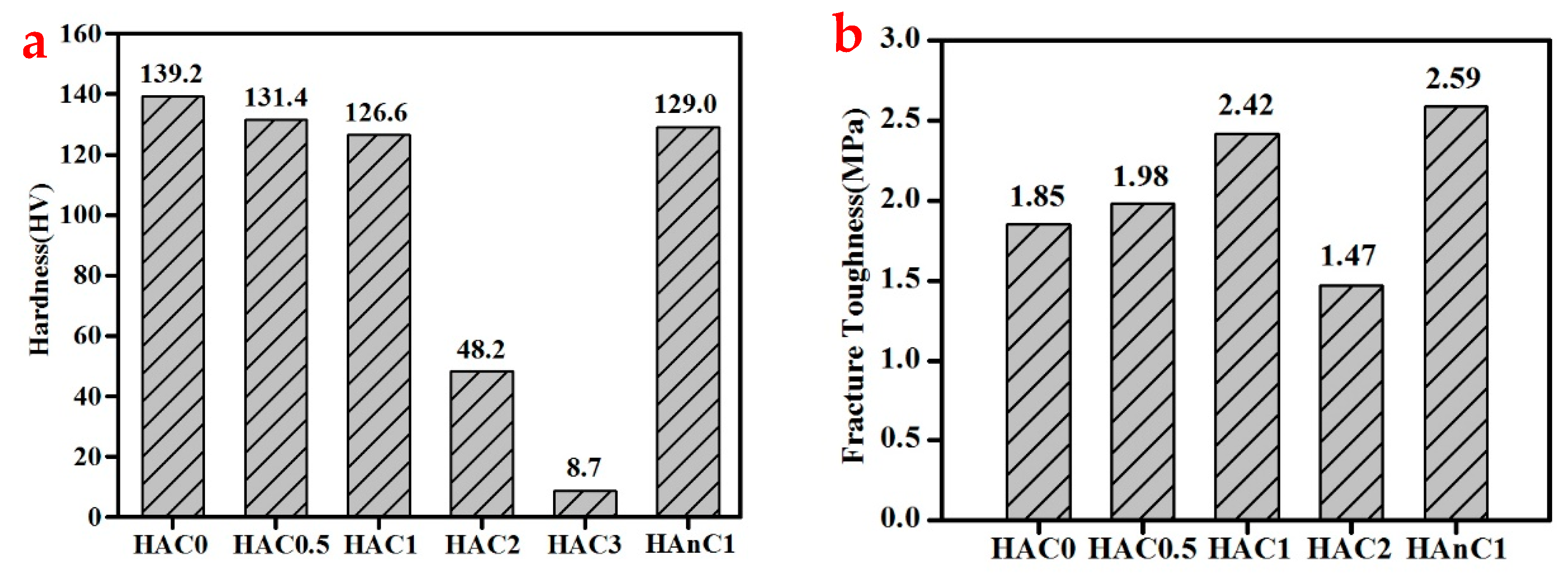
3.3. The Mechanical Properties of the Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Murray, M.G.S.; Wang, J.; Ponton, C.B.; Marquis, P.M. An improvement in processing of hydroxyapatite ceramics. J. Mater. Sci. 1995, 30, 3061–3074. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Zhang, T.; Gan, C.; Zheng, C.; Yu, G. Carbon nanotube reinforced hydroxyapatite composite coatings produced through laser surface alloying. Carbon 2006, 44, 37–45. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kawai, W. Development of carbon nanofiber reinforced hydroxyapatite with enhanced mechanical properties. Compos. Part A Appl. S Manuf. 2007, 38, 114–123. [Google Scholar] [CrossRef]
- Adolfsson, E.; Alberius-Henning, P.; Hermansson, L. Phase analysis and thermal stability of hot isostatically pressed zirconia–hydroxyapatite composites. J. Am. Ceram. Soc. 2000, 83, 2798–2802. [Google Scholar] [CrossRef]
- Dorner-Reisel, A.; Berroth, K.; Neubauer, R.; Nestler, K.; Marx, G.; Scislo, M.; Muller, E.; Slosarcyk, A. Unreinforced and carbon fibre reinforced hydroxyapatite: Resistance against microabrasion. J. Eur. Ceram. Soc. 2004, 24, 2131–2139. [Google Scholar] [CrossRef]
- Wei, W.; Zhu, Y.; Watari, F.; Liao, S.; Yokoyama, A.; Omori, M.; Ai, H.; Cui, F. Carbon nanotubes/hydroxyapatite nanocomposites fabricated by spark plasma sintering for bonegraft applications. Appl. Surf. Sci. 2012, 262, 194–199. [Google Scholar]
- Jong, K.D.; Geus, J. Carbon nanofibers: Catalytic synthesis and applications. J. Catal Rev. 2000, 42, 481–510. [Google Scholar] [CrossRef]
- Dai, H. Carbon nanotubes: Opportunities and challenges. Surf. Sci. 2002, 500, 218–241. [Google Scholar] [CrossRef]
- Hayashida, T.; Pan, L.; Nakayama, Y. Mechanical and electrical properties of carbon tubule nanocoils. Phys. B Condens. Matter 2002, 323, 352–353. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Youn, M.H.; Oh, I.H.; Lee, B.T. Fabrication of CNT-reinforced HAp composites by spark plasma sintering. Mater. Sci. Forum 2007, 534–536, 893–896. [Google Scholar] [CrossRef]
- Xu, J.; Khor, K.; Sui, J.; Chen, W. Preparation and characterization of a novel hydroxyapatite/carbon nanotubes composite and its interaction with osteoblast-like cells. Mater. Sci. Eng. C 2009, 29, 44–49. [Google Scholar] [CrossRef]
- De Heer, W.A.; Chatelain, A.; Ugarte, D. A carbon nanotube field-emission electron source. Science 1995, 270, 1179–1180. [Google Scholar] [CrossRef]
- Di, Z.-C.; Ding, J.; Peng, X.-J.; Li, Y.-H.; Luan, Z.-K.; Liang, J. Chromium adsorption by aligned carbon nanotubes supported ceria nanoparticles. Chemosphere 2006, 62, 861–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Zhu, Y.; Gong, Q. Preparation and properties of the powder SBR composites filled with CNTs by spray drying process. Mater. Lett. 2006, 60, 3769–3775. [Google Scholar] [CrossRef]
- Li, C.; Tang, Y.; Yao, K.; Zhou, F.; Ma, Q.; Lin, H.; Tao, M.; Liang, J. Decoration of multiwall nanotubes with cadmium sulfide nanoparticles. Carbon 2006, 44, 2021–2026. [Google Scholar] [CrossRef]
- Kwon, Y.K.; Berber, S.; Tománek, D. Thermal contraction of carbon fullerenes and nanotubes. Phys. Rev. Lett. 2004, 92, 015901. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, X.; Li, Z.; Wang, W.; Jing, Z.; He, F.; Yang, J. Design and fabrication of carbon fibers with needle-like nano-HA coating to reinforce granular nano-HA composites. Mater. Sci. Eng. C 2017, 77, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhu, Y.; Wang, F.; Chen, W. Effects of plasma and acid treatment on the dispersion of carbon nanotubes in liquids. Plasma Chem. Plasma Proc. 2011, 31, 441–448. [Google Scholar] [CrossRef]
- Okpalugo, T.I.T.; Papakonstantinou, P.; Murphy, H.; Mclaughlin, J.; Brown, N.M.D. High resolution XPS characterization of chemical functionalised MWCNTs and SWCNTs. Carbon 2005, 43, 153–161. [Google Scholar] [CrossRef]
- Zimmermann, K.A.; Leblanc, J.M.; Sheets, K.T.; Fox, R.W.; Gatenholm, P. Biomimetic design of a bacterial cellulose/hydroxyapatite nanocomposite for bone healing applications. Mater. Sci. Eng. C 2011, 31, 43–49. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, W.; Zheng, Y.; Liu, A.; Yao, J.; Li, C. The structural and biological properties of hydroxyapatite-modified titanate nanowire scaffolds. Biomaterials 2011, 32, 5837–5846. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, Q.; Liu, X.; Liu, H. Biomimetic synthesis and characterization of carbon nanofiber/hydroxyapatite composite scaffolds. Carbon 2013, 51, 335–345. [Google Scholar] [CrossRef]
- Li, H.; Zhao, X.; Cao, S.; Li, K.; Chen, M.; Xu, Z.; Lu, J.; Zhang, L. Na-doped hydroxyapatite coating on carbon/carbon composites: Preparation, in vitro bioactivity and biocompatibility. Appl. Surf. Sci. 2012, 263, 163–173. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Malafaya, P.B.; Reis, R.L. Sodium silicate gel as a precursor for the in vitro nucleation and growth of a bone-like apatite coating in compact and porous polymeric structures. Biomaterials 2003, 24, 2575–2584. [Google Scholar] [CrossRef] [Green Version]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Matsushita, T.; Kokubo, T. A bioactive Ti metal with a Ca-enriched surface layer releases Mg ions. RSC Adv. 2013, 3, 11274–11282. [Google Scholar] [CrossRef]
- Fu, S.; Yang, L.; Fan, J.; Wen, Q.; Lin, S.; Wang, B. In vitro mineralization of hydroxyapatite on electrospun poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) fibrous scaffolds for tissue engineering application. Colloids Surf. B Biointerface 2013, 107, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Cordell, J.M.; Vogl, M.L.; Johnson, A.J.W. The influence of micropore size on the mechanical properties of bulk hydroxyapatite and hydroxyapatite scaffolds. J. Mech. Behav. Biomed. Mater. 2009, 2, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Ebbesen, T.W.; Ajayan, P.M. Large-scale synthesis of carbon nanotubes. Nature 1992, 358, 220–222. [Google Scholar] [CrossRef]
- Zhou, W.; Sasaki, S.; Kawasaki, A. Effective control of nanodefects in multiwalled carbon nanotubes by acid treatment. Carbon 2014, 78, 121–129. [Google Scholar] [CrossRef]
- Hiura, H.; Ebbesen, T.W.; Tanigaki, K.; Takahashi, H. Raman studies of carbon nanotubes. Chem. Phys. Lett. 1993, 202, 509–512. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Estili, M.; Kawasaki, A.; Sakamoto, H.; Mekuchi, Y.; Kuno, M.; Tsukada, T. The homogeneous dispersion of surfactantless, slightly disordered, crystalline, multiwalled carbon nanotubes in α-alumina ceramics for structural reinforcement. Acta Mater. 2008, 56, 4070–4079. [Google Scholar] [CrossRef]
- Cho, J.; Boccaccini, A.R.; Shaffer, M.S.P. The influence of reagent stoichiometry on the yield and aspect ratio of acid-oxidised injection CVD-grown multi-walled carbon nanotubes. Carbon 2012, 50, 3967–3976. [Google Scholar] [CrossRef]
- Osswald, S.; Havel, M.; Gogotsi, Y. Monitoring oxidation of multiwalled carbon nanotubes by Raman spectroscopy. J. Raman Spectrosc. 2010, 38, 728–736. [Google Scholar] [CrossRef]
- Li, P.; Ohtsuki, C.; Kokubo, T.; Nakanishi, K.; Soga, N.; Groot, K.D. The role of hydrated silica, titania, and alumina in inducing apatite on implants. J. Biomed. Mater. Res. 1994, 28, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Hu, H.; And, S.K.M.; Haddon, R.C. A bone mimic based on the self-assembly of hydroxyapatite on chemically functionalized single-walled carbon nanotubes. Chem. Mater. 2005, 17, 3235–3241. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Wei, J.; De, G.K. Development of biomimetic nano-hydroxyapatite/poly(hexamethylene adipamide) composites. Biomaterials 2002, 23, 4787–4791. [Google Scholar] [CrossRef]
- Mukherjee, S.; Nandi, S.K.; Kundu, B.; Chanda, A.; Sen, S.; Das, P.K. Enhanced bone regeneration with carbon nanotube reinforced hydroxyapatite in animal model. J. Mech. Behav. Biomed. Mater. 2016, 60, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Saheb, N.; Khalil, A.; Hakeem, A.S.; Laoui, T.; Al-Aqeeli, N.; Al-Qutub, A.M. Age hardening behavior of carbon nanotube reinforced aluminum nanocomposites. J. Nano Res. 2012, 21, 29–35. [Google Scholar] [CrossRef]
- Jen, Y.M.; Huang, C.Y. Combined temperature and moisture effect on the strength of carbon nanotube reinforced epoxy materials. Transac. Can. Soc. Mech. Eng. 2013, 37, 755–763. [Google Scholar] [CrossRef]
- Jagannatham, M.; Sankaran, S.; Haridoss, P. Microstructure and mechanical behavior of copper coated multiwall carbon nanotubes reinforced aluminum composites. Mater. Sci. Eng. A 2015, 638, 197–207. [Google Scholar] [CrossRef]
- Snyders, R.; Music, D.; Sigumonrong, D.; Schelnberger, B. Experimental and ab initio study of the mechanical properties of hydroxyapatite. Appl. Phys. Lett. 2007, 90, 19390. [Google Scholar] [CrossRef]
- Lahiri, D.; Singh, V.; Keshri, A.K.; Seal, S.; Agarwal, A. Carbon nanotube toughened hydroxyapatite by spark plasma sintering: Microstructural evolution and multiscale tribological properties. Carbon 2010, 48, 3103–3120. [Google Scholar] [CrossRef]
- Yan, J. Elastic-Plastic Fracture Mechanics of Compact Bone. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, January 2005. [Google Scholar]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Chen, X.; Zhang, L.; Liu, Q.; Wang, Y.; Zhang, W.; Zheng, J. Preparation of Nano-Hydroxyapatite Coated Carbon Nanotube Reinforced Hydroxyapatite Composites. Coatings 2018, 8, 357. https://doi.org/10.3390/coatings8100357
Zhao X, Chen X, Zhang L, Liu Q, Wang Y, Zhang W, Zheng J. Preparation of Nano-Hydroxyapatite Coated Carbon Nanotube Reinforced Hydroxyapatite Composites. Coatings. 2018; 8(10):357. https://doi.org/10.3390/coatings8100357
Chicago/Turabian StyleZhao, Xueni, Xueyan Chen, Li Zhang, Qingyao Liu, Yao Wang, Weigang Zhang, and Jiamei Zheng. 2018. "Preparation of Nano-Hydroxyapatite Coated Carbon Nanotube Reinforced Hydroxyapatite Composites" Coatings 8, no. 10: 357. https://doi.org/10.3390/coatings8100357




