Improvement of Corrosion Protection of Coating System via Inhibitor Response Order
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Nanocontainers Preparation and Inhibitor Loading
2.3. Coating System Preparation
2.4. Characterization
3. Results and Discussion
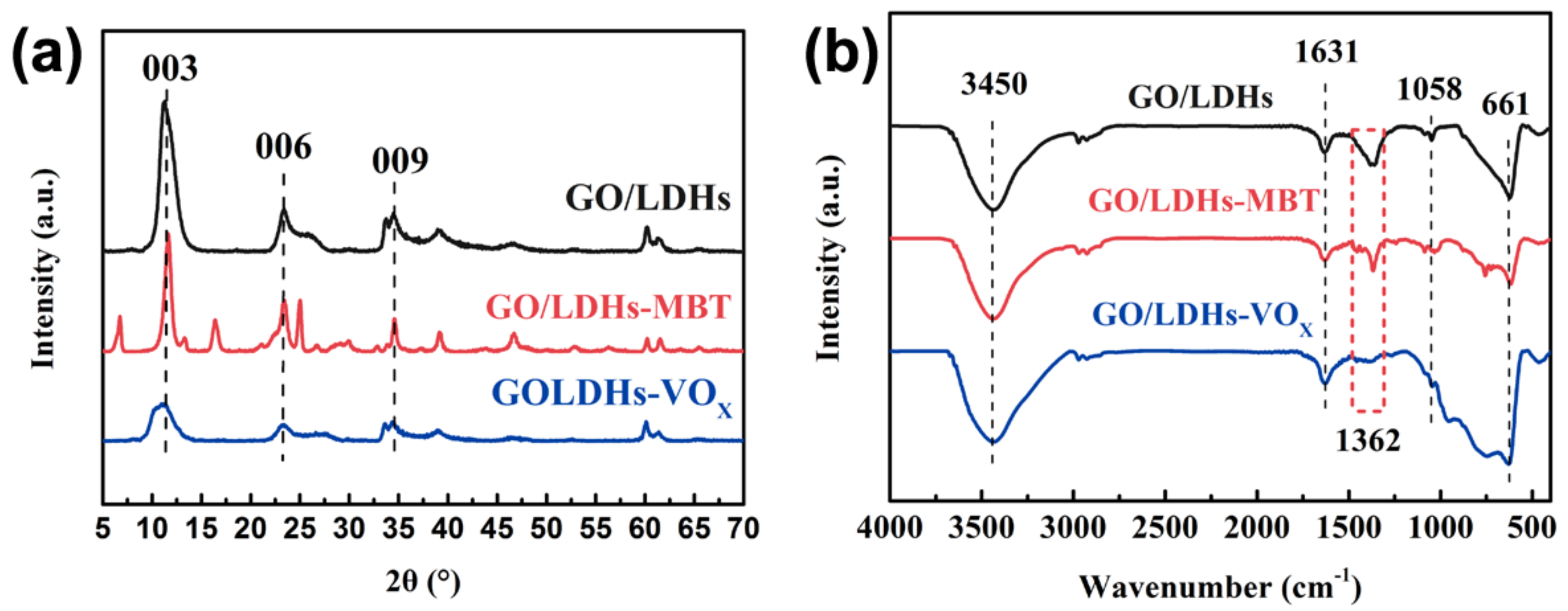
3.1. Characterization of Nanofillers
3.2. Corrosion Inhibition Properties of Inhibitor Mixture
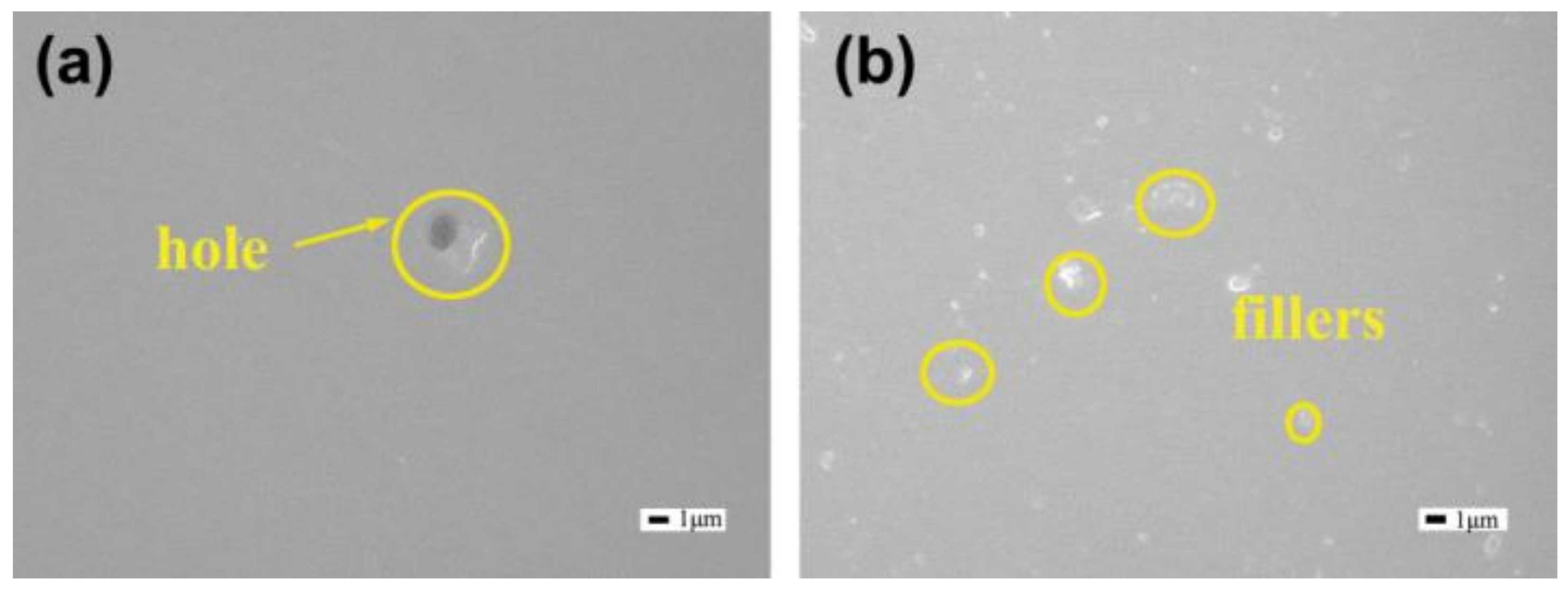
3.3. Preparation and Morphology of Coating Systems
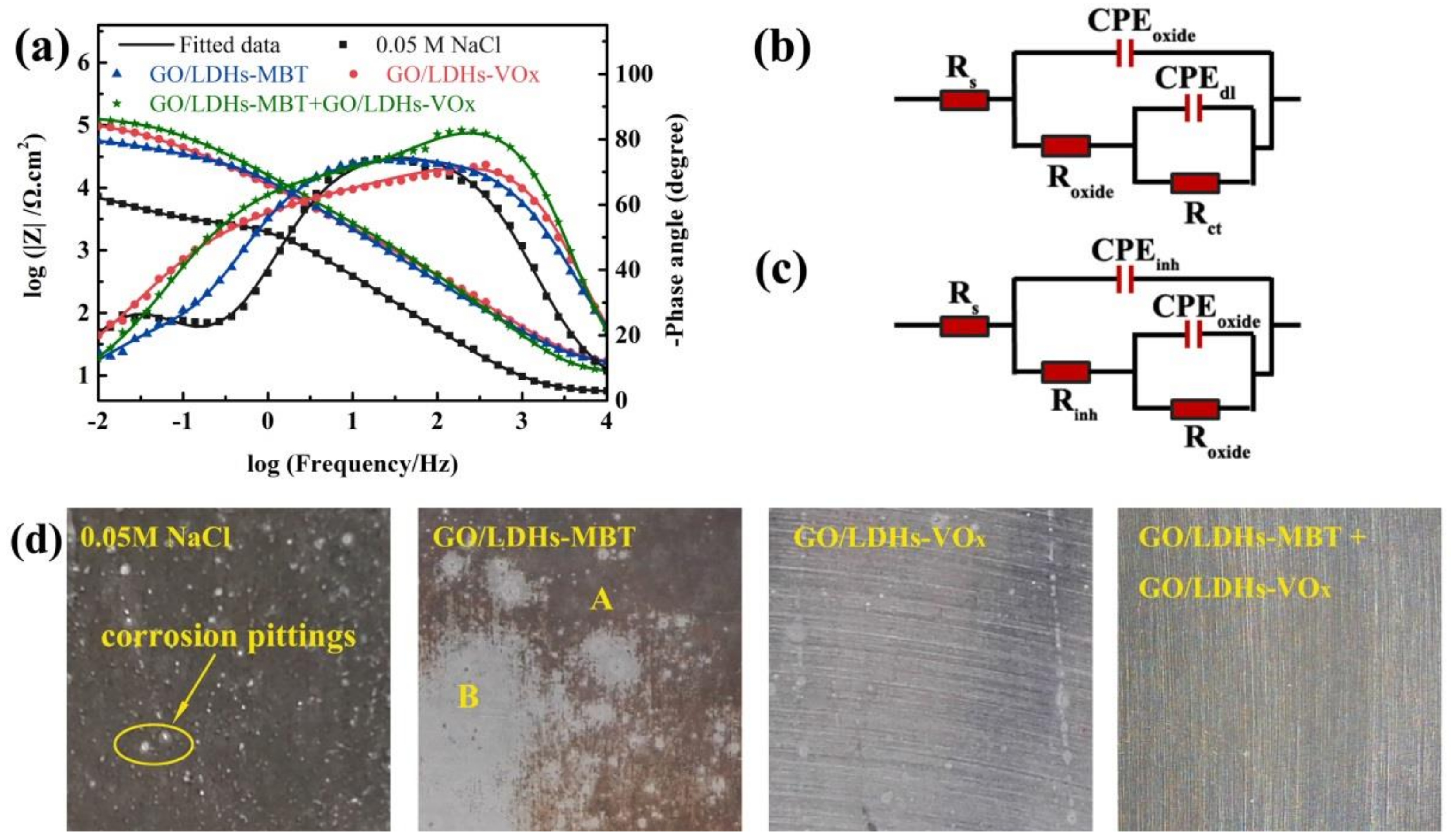
3.4. EIS Measurement of Coating Systems
3.5. Salt Spray Test
3.6. Active Corrosion Protection of Coating Systems
3.7. Corrosion Protection Mechanism of the M + V Coating System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sathiyanarayanan, S.; Azim, S.S.; Venkatachari, G. A new corrosion protection coating with polyaniline–TiO2 composite for steel. Electrochim. Acta 2007, 52, 2068–2074. [Google Scholar] [CrossRef]
- García, S.J.; Fischer, H.R.; van der Zwaag, S. A critical appraisal of the potential of self healing polymeric coatings. Prog. Org. Coat. 2011, 72, 211–221. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.V.; Cardoso, M.M.; Condeço, J.A.D.; Ferreira, H.E.C.S.; de Fatima Montemor, M. pH-sensitive polymeric particles with increased inhibitor-loading capacity as smart additives for corrosion protective coatings for AA2024. Electrochim. Acta 2014, 145, 123–131. [Google Scholar] [CrossRef]
- Plawecka, M.; Snihirova, D.; Martins, B.; Szczepanowicz, K.; Warszynski, P.; Montemor, M.F. Self healing ability of inhibitor-containing nanocapsules loaded in epoxy coatings applied on aluminium 5083 and galvanneal substrates. Electrochim. Acta 2014, 140, 282–293. [Google Scholar] [CrossRef]
- Markley, T.A.; Forsyth, M.; Hughes, A.E. Corrosion Protection of AA2024-T3 using rare earth diphenyl phosphates. Electrochim. Acta 2007, 52, 4024–4031. [Google Scholar] [CrossRef]
- Montemor, M.F.; Snihirova, D.V.; Taryba, M.G.; Lamaka, S.V.; Kartsonakis, I.A.; Balaskas, A.C.; Kordas, G.; Tedim, J.; Kuznetsova, A.; Zheludkevich, M.L.; et al. Evaluation of self-healing ability in protective coatings modified with combinations of layered double hydroxides and cerium molibdate nanocontainers filled with corrosion inhibitors. Electrochim. Acta 2012, 60, 31–40. [Google Scholar] [CrossRef]
- Kallip, S.; Bastos, A.C.; Yasakau, K.A.; Zheludkevich, M.L.; Ferreira, M.G.S. Synergistic corrosion inhibition on galvanically coupled metallic materials. Electrochem. Commun. 2012, 20, 101–104. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, G. The synergistic inhibition effect of oleic-based imidazoline and sodium benzoate on mild steel corrosion in a CO2-saturated brine solution. Electrochim. Acta 2012, 69, 247–255. [Google Scholar] [CrossRef]
- Garcia, S.J.; Markley, T.A.; Mol, J.M.C.; Hughes, A.E. Unravelling the corrosion inhibition mechanisms of bi-functional inhibitors by EIS and SEM-EDS. Corros. Sci. 2013, 69, 346–358. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Gao, L.; Zhang, D. Synergism between cerium nitrate and sodium dodecylbenzenesulfonate on corrosion of AA5052 aluminium alloy in 3 wt.% NaCl solution. Appl. Surf. Sci. 2016, 389, 369–377. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.V.; Taheri, P.; Mol, J.M.C.; Montemor, M.F. Comparison of the synergistic effects of inhibitor mixtures tailored for enhanced corrosion protection of bare and coated AA2024-T3. Surf. Coat. Technol. 2016, 303, 342–351. [Google Scholar] [CrossRef]
- Abdolah Zadeh, M.; Tedim, J.; Zheludkevich, M.; van der Zwaag, S.; Garcia, S.J. Synergetic active corrosion protection of AA2024-T3 by 2D-anionic and 3D-cationic nanocontainers loaded with Ce and mercaptobenzothiazole. Corros. Sci. 2018, 135, 35–45. [Google Scholar] [CrossRef]
- Saei, E.; Ramezanzadeh, B.; Amini, R.; Kalajahi, M.S. Effects of combined organic and inorganic corrosion inhibitors on the nanostructure cerium based conversion coating performance on AZ31 magnesium alloy: Morphological and corrosion studies. Corros. Sci. 2017, 127, 186–200. [Google Scholar] [CrossRef]
- Kowalczyk, K.; Kugler, S.; Spychaj, T. Antistatic polyurethane coats with hybrid carbon nanofillers. Polimery 2014, 59, 650–655. [Google Scholar] [CrossRef]
- Okafor, P.A.; Singh-Beemat, J.; Iroh, J.O. Thermomechanical and corrosion inhibition properties of graphene/epoxy ester–siloxane–urea hybrid polymer nanocomposites. Prog. Org. Coat. 2015, 88, 237–244. [Google Scholar] [CrossRef]
- Jena, K.K.; Narayan, R.; Alhassan, S.M. Highly branched graphene siloxane−polyurethane-urea (Pu-urea) hybrid coatings. Prog. Org. Coat. 2017, 111, 343–353. [Google Scholar] [CrossRef]
- Xue, B.; Yu, M.; Liu, J.; Li, S.; Xiong, L.; Kong, X. Synthesis of inhibitor nanocontainers with two-dimensional structure and their anticorrosion action in sol-gel coating on Aa2024-T3 aluminum alloy. J. Electrochem. Soc. 2017, 164, C641–C652. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Serra, R.; Montemor, M.F.; Yasakau, K.A.; Salvado, I.M.M.; Ferreira, M.G.S. Nanostructured sol-gel coatings doped with cerium nitrate as pre-treatments for AA2024-T3: Corrosion protection performance. Electrochim. Acta 2005, 51, 208–217. [Google Scholar] [CrossRef]
- ASTM B117–18 Standard Practice for Operating Salt Spray (Fog) Apparatus; ASTM International: West Conshohocken, PA, USA, 2018.
- Luo, X.H.; Yuan, S.; Pan, X.Y.; Zhang, C.X.; Du, S.; Liu, Y.L. Synthesis and enhanced corrosion protection performance of reduced graphene oxide nanosheet/ZnAl layered double hydroxide composite films by hydrothermal continuous flow method. ACS Appl. Mater. Interfaces 2017, 9, 18263–18275. [Google Scholar] [CrossRef] [PubMed]
- Tedim, J.; Poznyak, S.K.; Kuznetsova, A.; Raps, D.; Hack, T.; Zheludkevich, M.L.; Ferreira, M.G. Enhancement of active corrosion protection via combination of inhibitor-loaded nanocontainers. ACS Appl. Mater. Interfaces 2010, 2, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, G.; Liu, Z.-H.; Yang, Z.; Wang, Z. Fabrication of a hybrid graphene/layered double hydroxide material. Carbon 2010, 48, 4391–4396. [Google Scholar] [CrossRef]
- Fang, J.; Li, M.; Li, Q.; Zhang, W.; Shou, Q.; Liu, F.; Zhang, X.; Cheng, J. Microwave-assisted synthesis of coal-layered double hydroxide/graphene oxide composite and its application in supercapacitors. Electrochim. Acta 2012, 85, 248–255. [Google Scholar] [CrossRef]
- Beattie, D.A.; Kempson, I.M.; Fan, L.-J.; Skinner, W.M. Synchrotron XPS studies of collector adsorption and co-adsorption on gold and gold: Silver alloy surfaces. Int. J. Miner. Process. 2009, 92, 162–168. [Google Scholar] [CrossRef]
- Kazansky, L.P.; Selyaninov, I.A.; Kuznetsov, Y.I. Adsorption of 2-mercaptobenzothiazole on copper surface from phosphate solutions. Appl. Surf. Sci. 2012, 258, 6807–6813. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Lin, Z.; Whiddon, R.; Qiu, K.; Kuang, M.; Cen, K. Transformation of nitrogen and sulphur impurities during hydrothermal upgrading of low quality coals. Fuel 2016, 164, 254–261. [Google Scholar] [CrossRef]
- Motola, M.; Satrapinskyy, L.; Čaplovicová, M.; Roch, T.; Gregor, M.; Grančič, B.; Greguš, J.; Čaplovič, L’.; Plesch, G. Enhanced photocatalytic activity of hydrogenated and vanadium doped TiO2 nanotube arrays grown by anodization of sputtered Ti layers. Appl. Surf. Sci. 2018, 434, 1257–1265. [Google Scholar] [CrossRef]
- Luo, H.; Dong, C.F.; Xiao, K.; Li, X.G. Characterization of passive film on 2205 duplex stainless steel in sodium thiosulphate solution. Appl. Surf. Sci. 2011, 258, 631–639. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Q.; Yu, M.; Li, S.; Zhao, K.; Xue, B.; Zu, H. Silane modification of titanium dioxide-decorated graphene oxide nanocomposite for enhancing anticorrosion performance of epoxy coatings on AA-2024. J. Alloys Compd. 2018, 744, 728–739. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Ahmadi, A.; Mahdavian, M. Enhancement of the corrosion protection performance and cathodic delamination resistance of epoxy coating through treatment of steel substrate by a novel nanometric sol-gel based silane composite film filled with functionalized graphene oxide nanosheets. Corros. Sci. 2016, 109, 182–205. [Google Scholar] [CrossRef]
- Borisova, D.; Mohwald, H.; Shchukin, D.G. Influence of embedded nanocontainers on the efficiency of active anticorrosive coatings for aluminum alloys part I: Influence of nanocontainer position. ACS Appl. Mater. Interfaces 2013, 5, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Maia, F.; Tedim, J.; Lisenkov, A.D.; Salak, A.N.; Zheludkevich, M.L.; Ferreira, M.G. Silica nanocontainers for active corrosion protection. Nanoscale 2012, 4, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- McMurray, H.N.; Williams, G.; O’Driscoll, S. Chromate inhibition of filiform corrosion on organic coated AA2024-T3 studied using the scanning kelvin probe. J. Electrochem. Soc. 2004, 151, B406–B414. [Google Scholar] [CrossRef]
- Williams, G.; McMurray, H.N. Polyaniline inhibition of filiform corrosion on organic coated AA2024-T3. Electrochim. Acta 2009, 54, 4245–4252. [Google Scholar] [CrossRef]
- Ohsawa, M.; Suetaka, W. Spectro-electrochemical studied of the corrosion inhibition of copper by mercaptobenzothiazole. Corros. Sci. 1979, 19, 709–722. [Google Scholar] [CrossRef]
- Iannuzzi, M.; Frankel, G.S. Mechanisms of corrosion inhibition of AA2024-T3 by vanadates. Corros. Sci. 2007, 49, 2371–2391. [Google Scholar] [CrossRef] [Green Version]
- Khramov, A.N.; Voevodin, N.N.; Balbyshev, V.N.; Mantz, R.A. Sol–gel-derived corrosion-protective coatings with controllable release of incorporated organic corrosion inhibitors. Thin Solid Films 2005, 483, 191–196. [Google Scholar] [CrossRef]
- Ralston, K.D.; Young, T.L.; Buchheit, R.G. electrochemical evaluation of constituent intermetallics in aluminum alloy 2024-T3 exposed to aqueous vanadate inhibitors. J. Electrochem. Soc. 2009, 156, C135–C146. [Google Scholar] [CrossRef]







| Coating Label | Description | |
|---|---|---|
| Sol-Gel Layer with Inhibitors | Epoxy Coating with Inhibitors | |
| Blank | – | – |
| M + M | GO/LDHs-MBT | GO/LDHs-MBT |
| V + V | GO/LDHs-VOx | GO/LDHs-VOx |
| S + M/V | – | GO/LDHs-MBT + GO/LDHs-VOx |
| V + M | GO/LDHs-VOx | GO/LDHs-MBT |
| M + V | GO/LDHs-MBT | GO/LDHs-VOx |
| Coating System | Rs (Ω·cm2) | Rcoat (Ω·cm2) | CPEcoat | Rct (Ω·cm2) | CPEdl | Rw (Ω·cm2) | CPEw | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Ycoat | n | Ydl | n | Yw | n | |||||
| Blank | 6.42 | 2.46 × 103 | 3.42 × 10−9 | 0.974 | 4.99 × 104 | 7.41 × 10−6 | 0.647 | 1.01 × 105 | 7.98 × 10−5 | 0.595 |
| S + M/V | 5.37 | 3.43 × 104 | 4.56 × 10−9 | 0.821 | 2.73 × 105 | 1.62 × 10−7 | 0.846 | 1.27 × 105 | 2.56 × 10−5 | 0.918 |
| V + V | 2.56 | 5.14 × 104 | 1.21 × 10−9 | 0.815 | 4.76 × 105 | 1.84 × 10−7 | 0.818 | 6.27 × 106 | 1.01 × 10−5 | 0.572 |
| M + M | 2.63 | 5.71 × 104 | 1.93 × 10−9 | 0.785 | 3.30 × 106 | 6.84 × 10−8 | 0.759 | 4.95 × 106 | 2.00 × 10−6 | 0.717 |
| V + M | 1.87 | 5.92 × 104 | 4.18 × 10−10 | 0.887 | 2.84 × 106 | 8.31 × 10−8 | 0.712 | 8.63 × 106 | 3.67 × 10−7 | 0.796 |
| M + V | 2.07 | 6.04 × 104 | 3.50 × 10−10 | 0.914 | 5.95 × 106 | 2.68 × 10−8 | 0.845 | 2.13 × 107 | 1.76 × 10−7 | 0.934 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Zhao, X.; Xiong, L.; Xue, B.; Kong, X.; Liu, J.; Li, S. Improvement of Corrosion Protection of Coating System via Inhibitor Response Order. Coatings 2018, 8, 365. https://doi.org/10.3390/coatings8100365
Yu M, Zhao X, Xiong L, Xue B, Kong X, Liu J, Li S. Improvement of Corrosion Protection of Coating System via Inhibitor Response Order. Coatings. 2018; 8(10):365. https://doi.org/10.3390/coatings8100365
Chicago/Turabian StyleYu, Mei, Xiangni Zhao, Liangliang Xiong, Bing Xue, Xiangxin Kong, Jianhua Liu, and Songmei Li. 2018. "Improvement of Corrosion Protection of Coating System via Inhibitor Response Order" Coatings 8, no. 10: 365. https://doi.org/10.3390/coatings8100365





