Development of Silane-Based Coatings with Zirconia Nanoparticles Combining Wetting, Tribological, and Aesthetical Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Preparation of Coatings
2.2. Silane Solution Characterization
2.2.1. Surface Tension
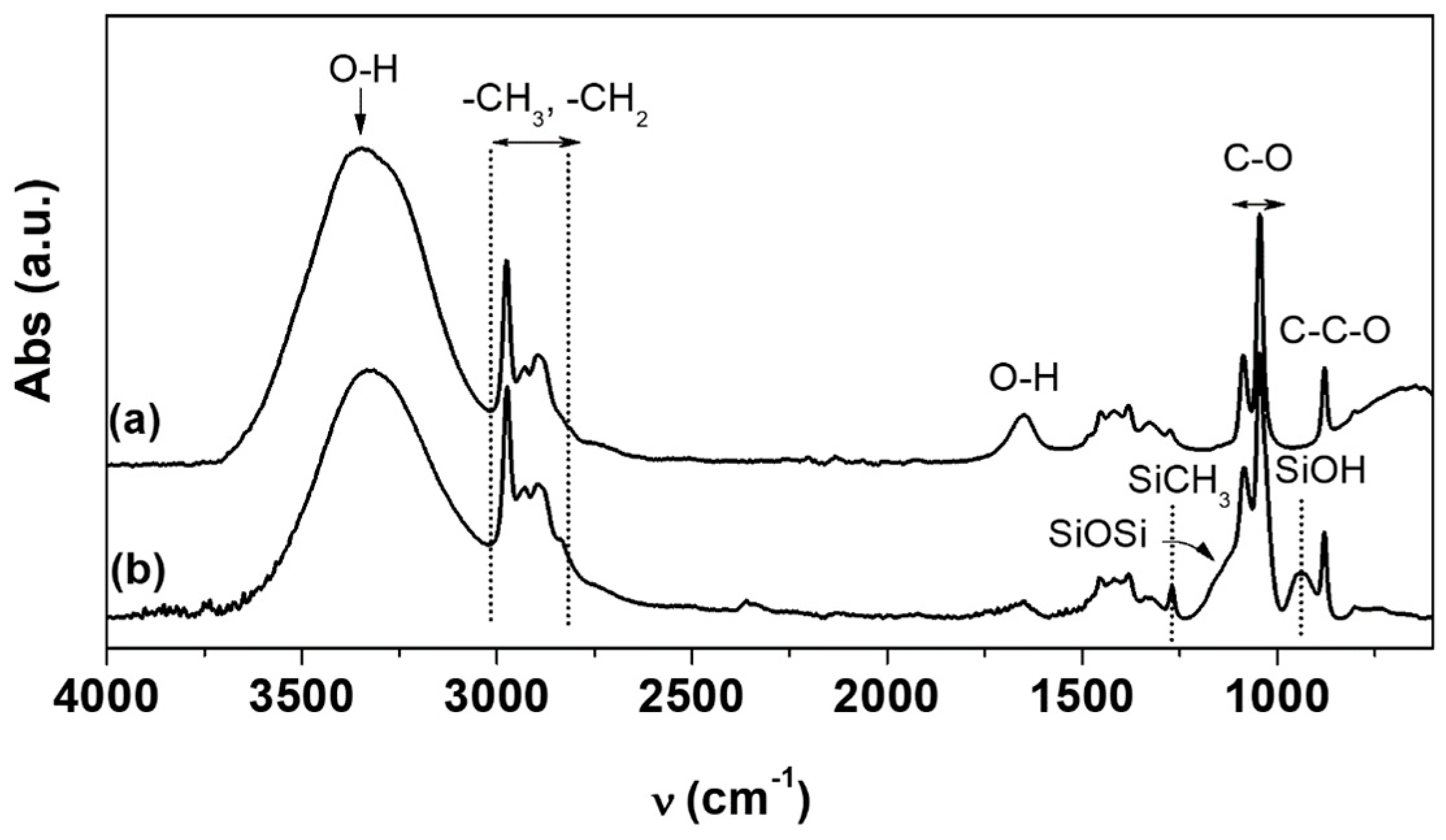
2.2.2. Fourier Transform Infrared Spectroscopy
2.2.3. Dynamic Light Scattering
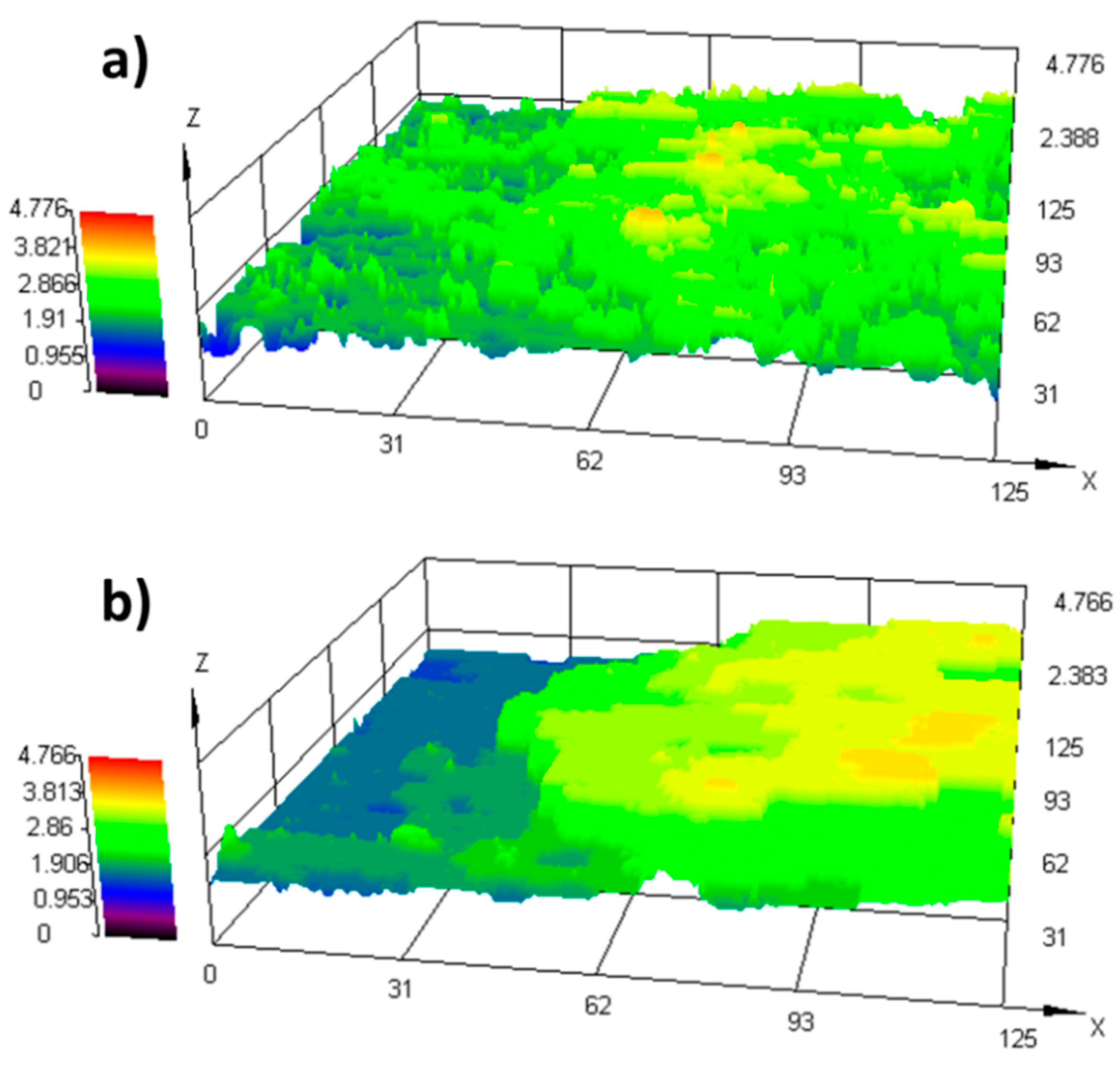
2.3. Surface Characterization
2.3.1. X-ray Fluorescence
2.3.2. Coating Thickness
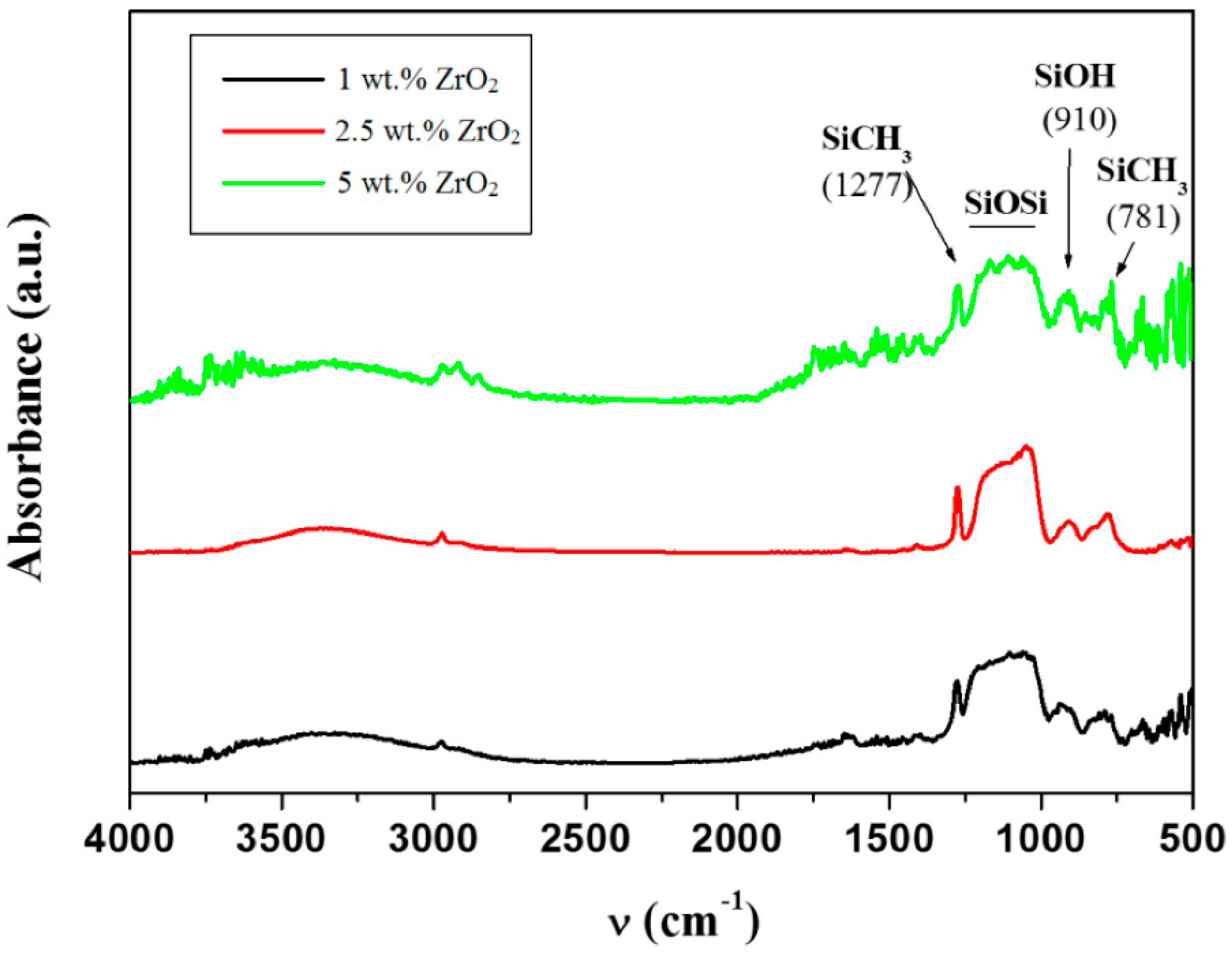
2.3.3. Fourier Transform Infrared Spectroscopy
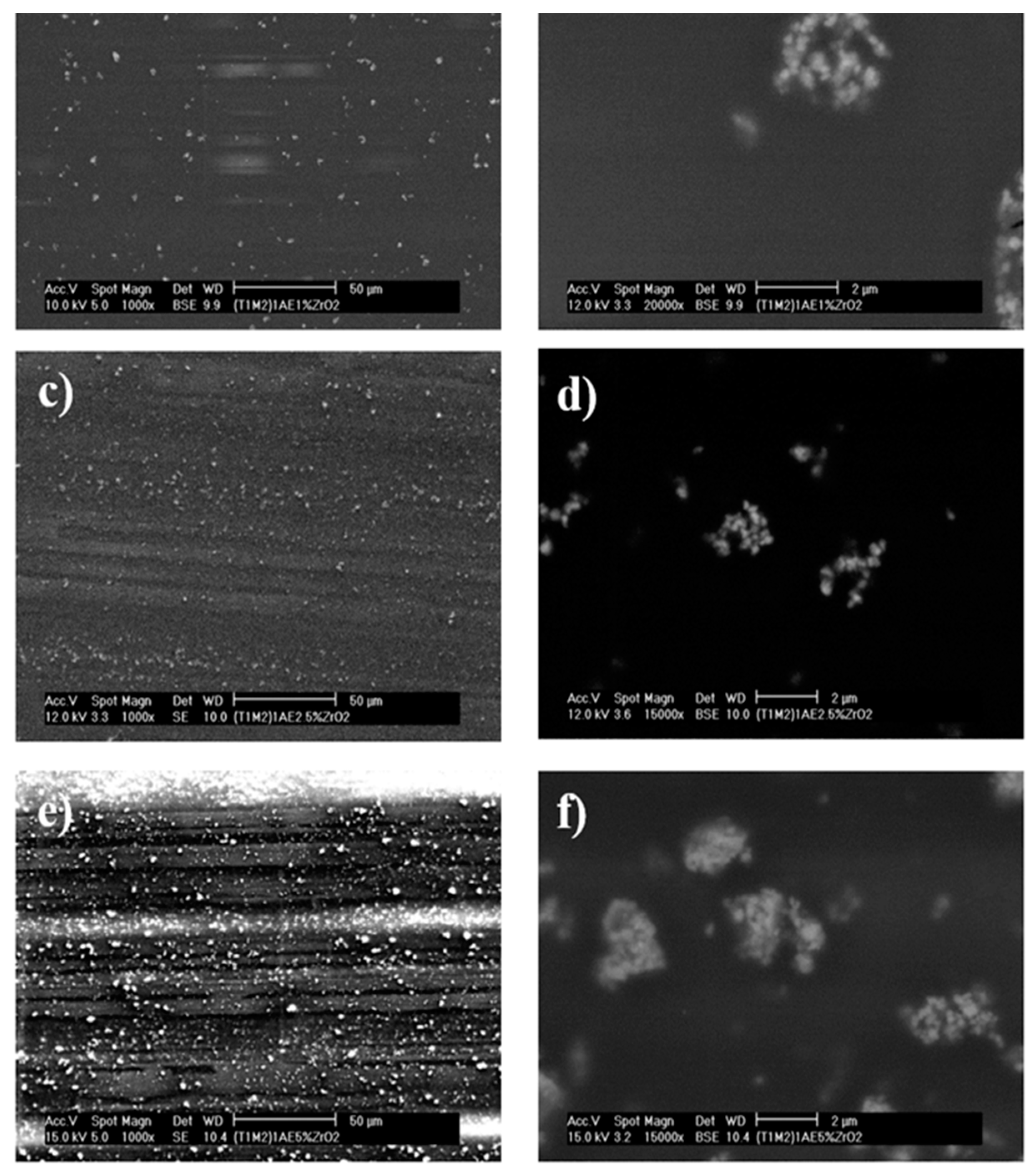
2.3.4. Scanning Electron Microscopy
2.3.5. Color and Gloss
2.3.6. Contact Angle
2.3.7. Wear Resistance
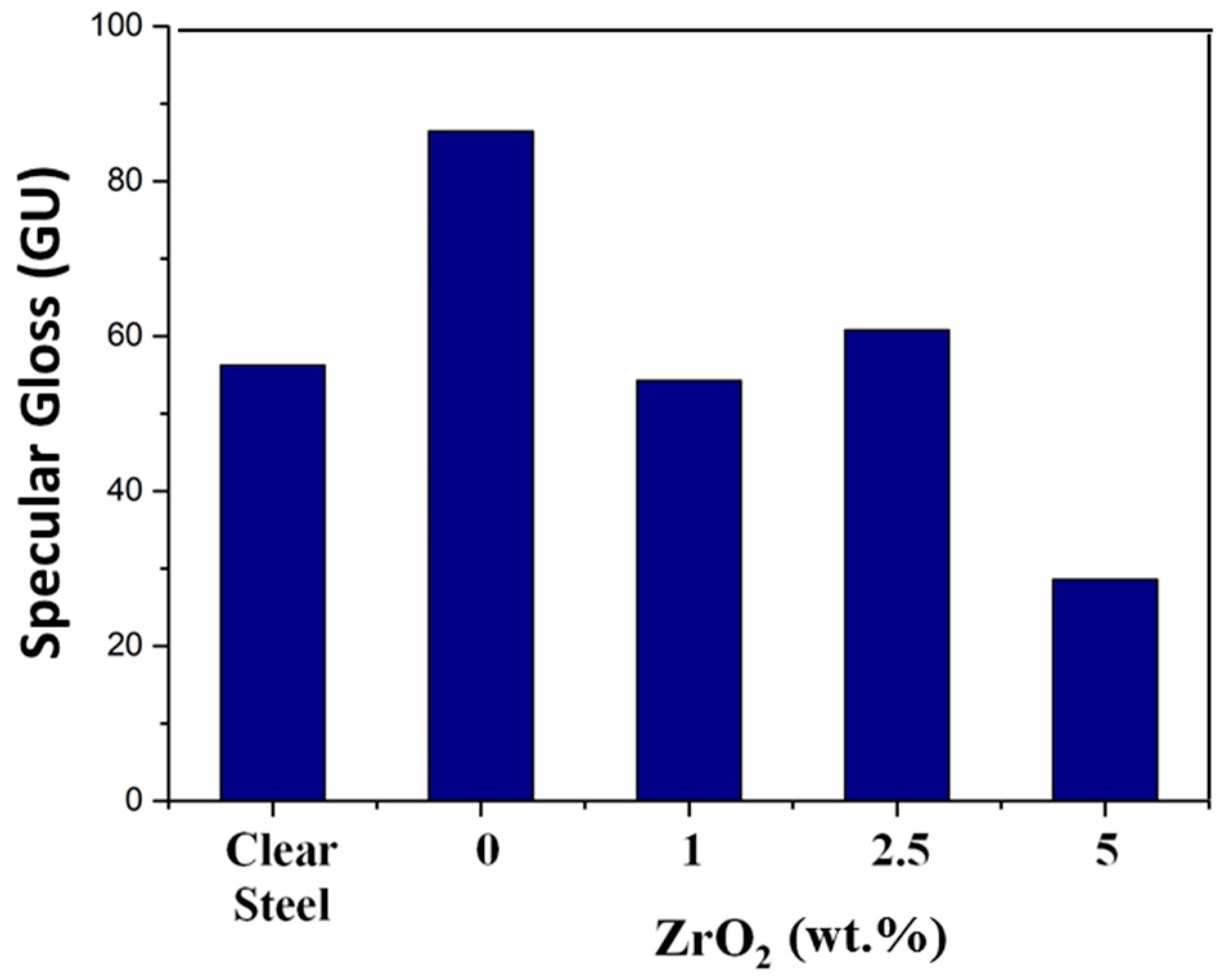
3. Results
3.1. Characterization of Solutions
3.2. Characterization of Coatings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pathak, S.S.; Khanna, A.S. Sol-gel nanocoatings for corrosion protection. In Corrosion Protection and Control Using Nanomaterials, 1st ed.; Saji, V.S., Cook, R., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 304–329. [Google Scholar]
- Montoya, P.; Martins, C.R.; de Melo, H.G.; Aoki, I.V.; Jaramillo, F.; Calderón, J.A. Synthesis of polypyrrole-magnetite/silane coatings on steel and assessment of anticorrosive properties. Electrochim. Acta 2014, 124, 100–108. [Google Scholar] [CrossRef]
- Córdoba, L.C.; Montemor, M.F.; Coradin, T. Silane/TiO2 coating to control the corrosion rate of magnesium alloys in simulated body fluid. Corros. Sci. 2016, 104, 152–161. [Google Scholar] [CrossRef]
- Ramezani, M.; Vaezi, M.R.; Kazemzadeh, A. Preparation of silane-functionalized silica films via two-step dip coating sol-gel and evaluation of their superhydrophobic properties. Appl. Surf. Sci. 2014, 317, 147–153. [Google Scholar] [CrossRef]
- Ma, M.; Hill, R.M. Superhydrophobic surfaces. Curr. Opin. Colloid Interface Sci. 2006, 11, 193–202. [Google Scholar] [CrossRef]
- Pantoja, M.; Abenojar, J.; Martínez, M.A.; Velasco, F. Silane pretreatment of electrogalvanized steels: Effect on adhesive properties. Int. J. Adhes. Adhes. 2016, 65, 54–62. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Adhesion aspects of hydrophobic silane zeolite coatings for corrosion protection of aluminium substrate. Prog. Org. Coat. 2014, 77, 1341–1350. [Google Scholar] [CrossRef]
- Cheng, X.W.; Liang, C.X.; Guan, J.P.; Yang, X.H.; Tang, R.C. Flame retardant and hydrophobic properties of novel sol-gel derived phytic acid/silica hybrid organic–inorganic coatings for silk fabric. Appl. Surf. Sci. 2018, 427, 69–80. [Google Scholar] [CrossRef]
- Suegama, P.H.; Recco, A.A.C.; Tschiptschin, A.P.; Aoki, I.V. Influence of silica nanoparticles added to an organosilane film on carbon steel electrochemical and tribological behavior. Prog. Org. Coat. 2007, 60, 90–98. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.; Wang, C. Characterization and tribological investigation of sol-gel Al2O3 and doped Al2O3 films. J. Eur. Ceram. Soc. 2002, 22, 2869–2876. [Google Scholar] [CrossRef]
- Piwonski, I.; Soliwoda, K. The effect of ceramic nanoparticles on tribological properties of alumina sol-gel thin coatings. Ceram. Int. 2010, 36, 47–54. [Google Scholar] [CrossRef]
- Zhi, Y.; Sun, X.; Li, N.; Yuan, S.; Wang, Z.; Jin, L.; Hang, J.; Shi, L. UV curable organic–inorganic hybrid coatings on microporous polyethylene separator for enhancing mechanical and electrochemical performance. Appl. Surf. Sci. 2018, 743, 756–762. [Google Scholar] [CrossRef]
- Banerjee, D.A.; Kessman, A.J.; Cairns, D.R.; Sierros, K.A. Tribology of silica nanoparticle-reinforced, hydrophobic sol-gel composite coatings. Surf. Coat. Technol. 2014, 260, 214–219. [Google Scholar] [CrossRef]
- Behzadnasab, M.; Mirabedini, S.M.; Kabiri, K.; Jamali, S. Corrosion performance of epoxy coatings containing silane treated ZrO2 nanoparticles on mild steel in 3.5% NaCl solution. Corros. Sci. 2011, 53, 89–98. [Google Scholar] [CrossRef]
- Montemor, M.F.; Trabelsi, W.; Lamaka, S.V.; Yasakau, K.A.; Zheludkevich, M.L.; Bastos, A.C.; Ferreira, M.G.S. The synergistic combination of bis-silane and CeO2·ZrO2 nanoparticles on the electrochemical behaviour of galvanised steel in NaCl solutions. Electrochim. Acta 2008, 53, 5913–5922. [Google Scholar] [CrossRef]
- Gusmano, G.; Montesperelli, G.; Rapone, M.; Padeletti, G.; Cusmà, A.; Kaciulis, S.; Mezzi, A.; Di Maggio, R. Zirconia primers for corrosion resistant coatings. Surf. Coat. Technol. 2007, 201, 5822–5828. [Google Scholar] [CrossRef]
- Zhu, R.; Zhang, J.; Chang, C.; Gao, S.; Ni, N. Effect of silane and zirconia on the thermal property of cathodic electrophoretic coating on AZ31 magnesium alloy. J. Magnes. Alloy. 2013, 1, 235–241. [Google Scholar] [CrossRef]
- Mirabedini, S.M.; Behzadnasab, M.; Kabiri, K. Effect of various combinations of zirconia and organoclay nanoparticles on mechanical and thermal properties of an epoxy nanocomposite coating. Compos. Part A Appl. Sci. Manuf. 2012, 43, 2095–2106. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q.; Li, Z.; Yuan, W.; Zhen, Y.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Wu, S. A combined coating strategy based on atomic layer deposition for enhancement of corrosion resistance of AZ31 magnesium alloy. Appl. Surf. Sci. 2018, 434, 1101–1111. [Google Scholar] [CrossRef]
- Kumar, P.; Oka, M.; Ikenchi, K.; Shimizu, K.; Yamamuro, T.; Okumura, H.; Kotoura, Y. Low wear rate of UHMWPE against zirconia ceramic (Y-PSZ) in comparison to alumina ceramic and SUS 316L alloy. J. Biomed. Mater. Res. 1991, 25, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Kurahatti, R.V.; Surendranathan, A.O.; Srivastava, S.; Singh, N.; Ramesh Kumar, A.V.; Suresha, B. Role of zirconia filler on friction and dry sliding wear behaviour of bismaleimide nanocomposites. Mater. Des. 2011, 32, 2644–2649. [Google Scholar] [CrossRef]
- Yen, S.K.; Guo, M.J.; Zan, H.Z. Characterization of electrolytic ZrO2 coating on CoCrMo implant alloys of hip prosthesis. Biomaterials 2001, 22, 125–133. [Google Scholar] [CrossRef]
- Pantoja, M.; Abenojar, J.; Martinez, M.A. Influence of the type of solvent on the development of superhydrophobicity from silane-based solution containing nanoparticles. Appl. Surf. Sci. 2017, 397, 87–94. [Google Scholar] [CrossRef]
- Gonnet, J. Colour effects of co-pigmentation of anthocyanins revisited—2. A colorimetric look at the solucions of cyanin co-pigmented by rutin using the CIELAB scale. Food Chem. 1999, 66, 387–394. [Google Scholar] [CrossRef]
- Franceschi, E.; Letardi, P.; Luciano, G. Colour measurements on patinas and coatings system for outdoor bronze monuments. J. Cult. Herit. 2006, 7, 166–170. [Google Scholar] [CrossRef]
- Berry, J.D.; Neeson, M.J.; Dagastine, R.R.; Chan, D.Y.C.; Tabor, R.F. Measurement of surface and interfacial tension using pendant drop tensiometry. J. Colloid Interface Sci. 2015, 454, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A. Colloidal processing of ceramics. J. Am. Ceram. Soc. 2000, 83, 2341–2359. [Google Scholar] [CrossRef]
- Tang, F.; Huang, X.; Zhang, Y.Z.; Guo, J. Effect of dispersants on surface chemical properties of nano-zirconia supensions. Ceram. Int. 2000, 26, 93–97. [Google Scholar] [CrossRef]
- Dos Santos, V.; da Silveira, N.P.; Bergmann, C.P. In-situ evaluation of particle size distribution of ZrO2-nanoparticles obtained by sol-gel. Powder Technol. 2014, 267, 392–397. [Google Scholar] [CrossRef]
- Puomi, P.; Fagerholm, H.M. Charaterization of hot-dip galvanized (HDG) steel treated with UPS, VS and tetrasulfide. J. Adhes. Sci. Technol. 2001, 15, 509–533. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Zhang, Q.; Wang, H.Z.; Xu, Z.C.; Zhang, L.; Liu, D.D.; Zhang, L. Wettability of a PTFE surface by aqueous solutions of zwitterionic surfactants: Effect of molecular structure. Colloids Surf. A 2016, 489, 370–377. [Google Scholar] [CrossRef]
- Anderson, D.R. Infra-red, Raman and ultraviolet spectroscopy. In Analysis of Silicones; Smith, A.L., Ed.; Wiley Interscience: New York, NY, USA, 1974; pp. 247–286. [Google Scholar]
- Pantoja, M.; Encinas, N.; Abenojar, J.; Martínez, M.A. Effect of tetraethoxysilane coating on the improvement of plasma treated polypropylene adhesion. Appl. Surf. Sci. 2013, 280, 850–857. [Google Scholar] [CrossRef]
- Nadargi, D.Y.; Latthe, S.S.; Venkateswara, A. Effect of post-treatment (gel aging) on the properties of methyltrimethoxysilane based silica aerogels prepared by two-step sol-gel process. J. Sol-Gel Sci. Technol. 2009, 49, 53–59. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; John Willey & Sons: Chichester, UK, 2001. [Google Scholar]
- Department of Chemistry and Biochemistry, University of Colorado at Boulder. Organic Chemistry al CU Boulder. Available online: http://orgchem.colorado.edu/Spectroscopy/irtutor/alcoholsir.html (accessed on 5 April 2016).
- Pantoja, M.; Velasco, F.; Broekema, D.; Abenojar, J.; del Real, J.C. The influence of pH on the hydrolysis process of γ-Methacryloxypropyltrimethoxysilane, analyzed by FT-IR, and the silanization of electrogalvanized steel. J. Adhes. Sci. Technol. 2010, 24, 1131–1143. [Google Scholar] [CrossRef]
- Onishi, T.; Abe, H.; Maruya, K.; Domen, K. I.r. spectra of hydrogen absorbed on ZrO2. J. Chem. Soc. Chem. Commun. 1985, 617–618. [Google Scholar] [CrossRef]
- Onishi, T. IR spectroscopic studies on surface reactions. In Dynamic Processes on Solid Surfaces, 1st ed.; Tamaru, K., Ed.; Springer: New York, NY, USA, 1993; pp. 237–257. [Google Scholar]
- Phanasgaonkar, A.; Raja, V.S. Influence of curing temperatura, silica nanoparticles- and cerium on surface morphology and corrosion behavior of hybrid silane coatings on mild steel. Surf. Coat. Technol. 2009, 203, 2260–2271. [Google Scholar] [CrossRef]
- Kapoor, P.; Mhaske, S.T.; Joshi, K. Modification and characterisation of pre-hydrolysed silanes by acrylate utilizing sol–gel process. Prog. Org. Coat. 2016, 94, 124–130. [Google Scholar] [CrossRef]
- Fei, T.; Chen, H.; Lin, J. Transparent superhydrophobic films possessing high thermal stability and improved moisture resistance from the deposition of MTMS-based aerogels. Colloids Surf. A 2014, 443, 255–264. [Google Scholar] [CrossRef]
- Múgica, R.; Alba, F.; Sainz, E.; Pantoja, M. Hydrophobicity attainment and wear resistance enhancement on glass substrates by atmospheric plasma-polymerization of mixtures of an aminosilane and a fluorocarbon. Appl. Surf. Sci. 2015, 347, 325–335. [Google Scholar] [CrossRef]
- Masuko, M.; Miyamoto, H.; Suzuki, A. Tribological characteristics of self-assembled monolayer with siloxane bonding to Si surface. Tribol. Int. 2007, 40, 1587–1596. [Google Scholar] [CrossRef]
- Kahn, H.; Ernst, F.; Heuer, A.H. Increasing the coefficient of sliding friction of NiCr at low loads by interstitial surface hardening. Wear 2016, 346–347, 1–5. [Google Scholar] [CrossRef]
- Ou, Y.X.; Lin, J.; Tong, S.; Che, H.L.; Sproul, W.D.; Lei, M.K. Wear and corrosion resistance of CrN/TiN superlattice coatings deposited by a combined deep oscillation magnetron sputtering and pulsed dc magnetron sputtering. Appl. Surf. Sci. 2015, 351, 332–343. [Google Scholar] [CrossRef]






| Solution | σT (mN/m) | σP (mN/m) | σD (mN/m) |
|---|---|---|---|
| EW | 27.7 ± 0.3 | 9.9 ± 0.1 (36%) | 17.8 ± 0.4 (64%) |
| Silane–EW | 24.1 ± 2.9 | 19.0 ± 3 (79%) | 5.1 ± 1.6 (21%) |
| Silane–EW + 1 wt.% ZrO2 | 32.1 ± 0.9 | 6.5 ± 1.4 (20%) | 25.6 ± 1 (80%) |
| Silane–EW + 2.5 wt.% ZrO2 | 32.8 ± 0.8 | 12.9 ± 1.7 (39%) | 19.9 ± 1.5 (61%) |
| Silane–EW + 5 wt.% ZrO2 | 32.3 ± 0.2 | 7.4 ± 0.3 (23%) | 24.9 ± 0.3 (77%) |
| Coating | % Si | % Zr | Coating Thickness (µm) (Scratch) | Coating Thickness (µm) (Opto-Digital Microscope) |
|---|---|---|---|---|
| Pristine steel | 0.33 ± 0.02 | <0.0001 | – | – |
| 0 wt.% ZrO2 | 14.89 ± 3.08 | <0.0001 | – | – |
| 1 wt.% ZrO2 | 21.54 ± 1.26 | 0.03 ± 0.00 | 0.8 ± 0.10 | 1.0 ± 0.10 |
| 2.5 wt.% ZrO2 | 18.48 ± 1.17 | 0.14 ± 0.02 | 1.2 ± 0.53 | 1.2 ± 0.10 |
| 5 wt.% ZrO2 | 18.86 ± 1.57 | 0.24 ± 0.04 | 2.4 ± 0.06 | 2.3 ± 0.17 |
| Coating | L* | a* | b* | ΔE |
|---|---|---|---|---|
| Clear steel | 68.66 | 0.37 | 4.6 | – |
| Silane | 67.44 | 0.40 | 4.33 | 1.24 |
| Silane + 1 wt.% ZrO2 | 65.41 | 0.31 | 3.97 | 3.31 |
| Silane + 2.5 wt.% ZrO2 | 64.61 | 0.40 | 4.13 | 4.08 |
| Silane + 5 wt.% ZrO2 | 65.78 | 0.51 | 4.26 | 2.90 |
| Coating | Initial Friction Coefficient | No. Cycles to Wear Down |
|---|---|---|
| Silane | 0.38 | <25 (5 N) |
| Silane + 1 wt.% ZrO2 | 0.35 | 35 (5 N) |
| Silane + 2.5 wt.% ZrO2 | 0.25 | 1610 (10 N) |
| Silane + 5 wt.% ZrO2 | 0.23 | 3340 (10 N) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez de Armentia, S.; Pantoja, M.; Abenojar, J.; Martinez, M.A. Development of Silane-Based Coatings with Zirconia Nanoparticles Combining Wetting, Tribological, and Aesthetical Properties. Coatings 2018, 8, 368. https://doi.org/10.3390/coatings8100368
Lopez de Armentia S, Pantoja M, Abenojar J, Martinez MA. Development of Silane-Based Coatings with Zirconia Nanoparticles Combining Wetting, Tribological, and Aesthetical Properties. Coatings. 2018; 8(10):368. https://doi.org/10.3390/coatings8100368
Chicago/Turabian StyleLopez de Armentia, Sara, Mariola Pantoja, Juana Abenojar, and Miguel Angel Martinez. 2018. "Development of Silane-Based Coatings with Zirconia Nanoparticles Combining Wetting, Tribological, and Aesthetical Properties" Coatings 8, no. 10: 368. https://doi.org/10.3390/coatings8100368





