Influence of C2H2 Flows on Microstructure and Corrosion Resistance of TiCN Films Doped with Carbon Atoms
Abstract
:1. Introduction
2. Experimental
2.1. Film Preparation
- Base pressure of chamber was 2 × 10−3 Pa;
- Substrate bias was −45 V;
- Thickness of TiN was 600 nm, with an average deposition rate of 24 nm min−1; TiCN was 800 nm, with an average deposition rate of 20 nm min−1;
- DC target current was 0.4 A;
- Total working pressure was 0.5 Pa;
- Ar flow was fixed at 20 sccm; N2 flow was fixed at 5 sccm; C2H2 flows varied from 2 to 5 sccm; and the films fabricated with 2, 3, 4, and 5 sccm were presented as samples C2, C3, C4, and C5, respectively.
2.2. Characterization of Microstructure
2.3. Corrosion Behavior
3. Results and Discussion
3.1. Microstructure
3.2. Morphologies and EDS Analysis
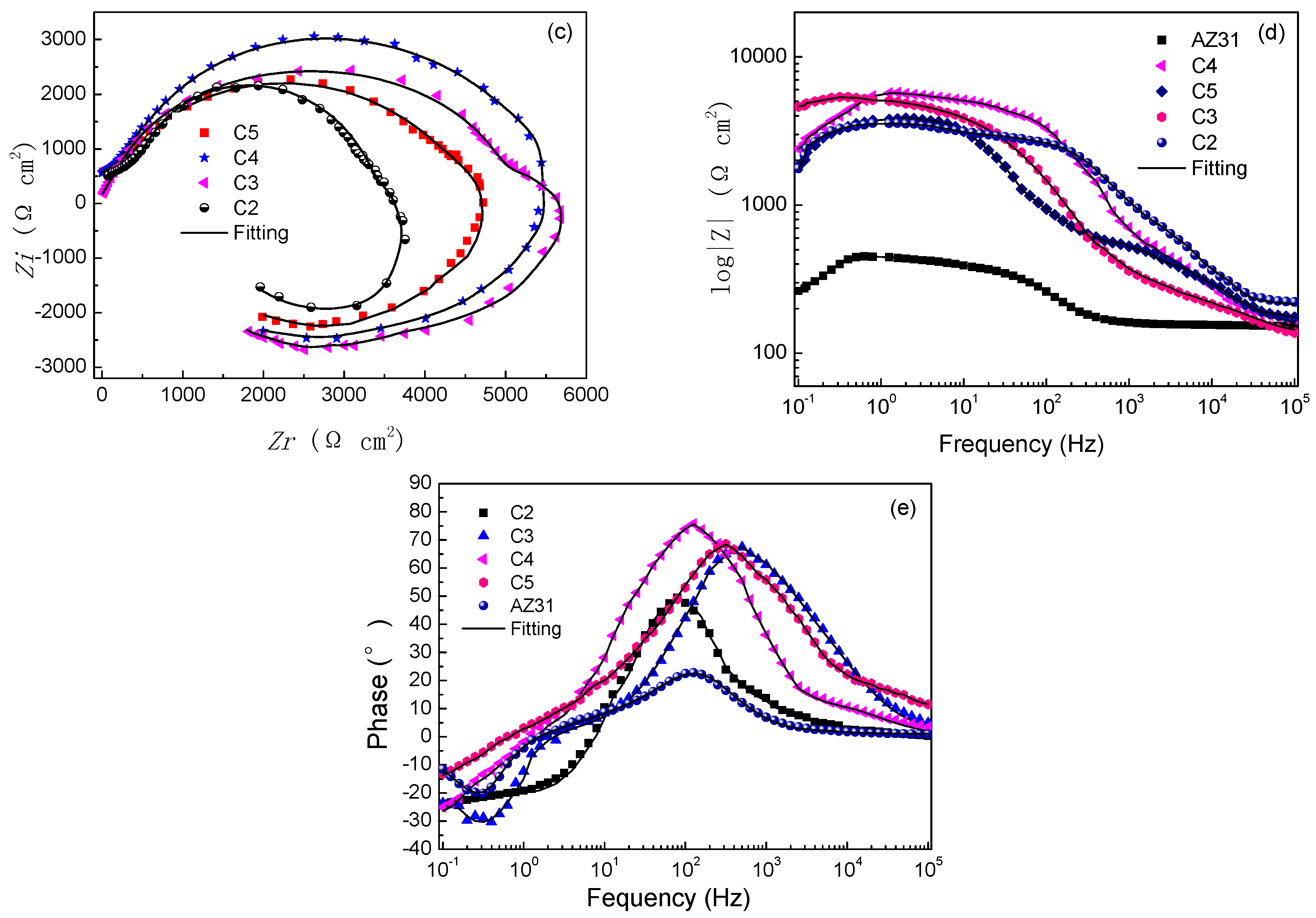
3.3. Corrosion Behavior
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoche, H.; Blawert, C.; Broszeit, E.; Berger, C. Galvanic corrosion properties of differently PVD-treated magnesium die cast alloy AZ91. Surf. Coat. Technol. 2005, 193, 223–229. [Google Scholar] [CrossRef]
- Paraskevas, D.; Dadbakhsh, S.; Vleugels, J.; Vanmeensel, K.; Dewulf, W.; Duflou, J.R. Solid state recycling of pure Mg and AZ31 Mg machining chips via spark plasma sintering. Mater. Des. 2016, 109, 520–529. [Google Scholar] [CrossRef]
- Li, H.; Wang, Q.; Zhuang, M.; Wu, J. Characterization and residual stress analysis of TiN/TiCN films on AZ31 magnesium alloy by PVD. Vacuum 2015, 112, 66–69. [Google Scholar] [CrossRef]
- Rashid, R.A.R.; Sun, S.; Wang, G.; Dargusch, M.S. Experimental investigation of laser assisted machining of AZ91 magnesium alloy. Int. J. Precis. Eng. Manuf. 2013, 14, 1263–1265. [Google Scholar] [CrossRef]
- Wu, G.; Zeng, X.; Ding, W.; Guo, X.; Yao, S. Characterization of ceramic PVD thin films on AZ31 magnesium alloys. Appl. Surf. Sci. 2006, 252, 7422–7429. [Google Scholar] [CrossRef]
- Ji, P.; Long, R.; Hou, L.; Wu, R.; Zhang, J.; Zhang, M. Study on hydrophobicity and wettability transition of Ni-Cu-SiC coating on Mg-Li alloy. Surf. Coat. Technol. 2018, 350, 428–435. [Google Scholar] [CrossRef]
- Apelfeld, A.; Krit, B.; Ludin, V.; Morozova, N.; Vladimirov, B.; Wu, R. The characterization of plasma electrolytic oxidation coatings on AZ41 magnesium alloy. Surf. Coat. Technol. 2017, 322, 127–133. [Google Scholar] [CrossRef]
- Li, H.; Rong, S.; Sun, P.; Wang, Q. Microstructure, Residual Stress, Corrosion and Wear Resistance of Vacuum Annealed TiCN/TiN/Ti Films Deposited on AZ31. Metals 2016, 7, 5. [Google Scholar] [CrossRef]
- Wu, G.; Zeng, X.; Yuan, G. Growth and corrosion of aluminum PVD-coating on AZ31 magnesium alloy. Mater. Lett. 2008, 62, 4325–4327. [Google Scholar]
- Altun, H.; Sen, S. The effect of DC magnetron sputtering AlN coatings on the corrosion behaviour of magnesium alloys. Surf. Coat. Technol. 2005, 197, 193–200. [Google Scholar] [CrossRef]
- Hollstein, F.; Wiedemann, R.; Scholz, J. Characteristics of PVD-coating on AZ31hp magnesium alloys. Surf. Coat. Technol. 2003, 162, 261–268. [Google Scholar] [CrossRef]
- Huo, H.; Li, Y.; Wang, F. Corrosion of AZ91D magnesium alloy with a chemical conversion coating and electroless nickel layer. Corros. Sci. 2004, 46, 1467–1477. [Google Scholar] [CrossRef]
- Wu, G.; Wang, X.; Ding, K.; Zhou, Y.; Zeng, X. Corrosion behavior of Ti-Al-N/Ti-Al duplex coating on AZ31 magnesium alloy in NaCl aqueous solution. Mater. Charact. 2009, 60, 803–807. [Google Scholar] [CrossRef]
- Altun, H.; Sen, S. The effect of PVD coatings on the wear behaviour of magnesium alloys. Mater. Charact. 2007, 58, 917–921. [Google Scholar] [CrossRef]
- Antunes, R.; Rodas, A.C.D.; Lima, N.; Higa, O.Z.; Costa, I. Costa, Study of the corrosion resistance and in vitro biocompatibility of PVD TiCN-coated AISI 316 L austenitic stainless steel for orthopedic applications. Surf. Coat. Technol. 2010, 205, 2074–2081. [Google Scholar] [CrossRef]
- Karyaoui, M.; Mhamdi, A.; Kaouach, H.; Labidi, A.; Boukhachem, A.; Boubaker, K.; Amlouk, M.; Chtourou, R. Some physical investigations on silver-doped ZnO sprayed thin films. Mater. Sci. Semicond. Proc. 2015, 30, 255–262. [Google Scholar] [CrossRef]
- Xu, L.; Xiao, S.; Zhang, C.; Zheng, G.; Su, J.; Zhao, L.; Wang, J. Optical and structural properties of Sr-doped ZnO thin films. Mater. Chem. Phys. 2014, 148, 720–726. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, T.; Zhang, W.; Li, J.; Chen, Z.; Chang, F.; Zhang, H.; Zhou, H. Effect of one-step laser processed biomimetic coupling units’ degrees on rolling contact fatigue wear resistance of train track alloy steel. Surf. Coat. Technol. 2015, 277, 181–187. [Google Scholar] [CrossRef]
- Verdian, M.M.; Raeissi, K.; Salehi, M.; Sabooni, S. Characterization and corrosion behavior of NiTi-Ti2Ni-Ni3Ti multiphase intermetallics produced by vacuum sintering. Vacuum 2011, 86, 91–95. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.M.; Bakhsheshi-Rad, H.R.; Yusof, N.M.; Izman, S.; Hamzah, E.; Kadir, M.R.A. Corrosion resistance investigation of nanostructured Si-and Si/TiO2-coated Mg alloy in 3.5% NaCl solution. Vacuum 2014, 108, 61–65. [Google Scholar] [CrossRef]
- Chen, R.; Tu, J.; Liu, D.; Mai, Y.; Gu, C. Microstructure, mechanical and tribological properties of TiCN nanocomposite films deposited by DC magnetron sputtering. Surf. Coat. Technol. 2011, 205, 5228–5234. [Google Scholar] [CrossRef]
- Lin, J.; Moore, J.J.; Mishra, B.; Pinkas, M.; Sproul, W.D. The structure and mechanical and tribological properties of TiBCN nanocomposite coatings. Acta Mater. 2010, 58, 1554–1564. [Google Scholar] [CrossRef]
- Korhonen, A.S. Corrosion of thin hard PVD coatings. Vacuum 1994, 45, 1031–1034. [Google Scholar] [CrossRef]
- Panjan, P.; Čekada, M.; Panjan, M.; Kek-Merl, D. Growth defects in PVD hard coatings. Vacuum 2009, 84, 209–214. [Google Scholar] [CrossRef]
- Wu, G.; Dai, W.; Zheng, H.; Wang, A. Improving wear resistance and corrosion resistance of AZ31 magnesium alloy by DLC/AlN/Al coating. Surf. Coat. Technol. 2010, 205, 2067–2073. [Google Scholar] [CrossRef]
- Shi, P.; Niu, B.; Shanshan, E.; Chen, Y.; Li, Q. Preparation and characterization of PLA coating and PLA/MAO composite coatings on AZ31 magnesium alloy for improvement of corrosion resistance. Surf. Coat. Technol. 2015, 262, 26–32. [Google Scholar] [CrossRef]
- Shi, Z.; Atrens, A. An innovative specimen configuration for the study of Mg corrosion. Corros. Sci. 2011, 53, 226–246. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.; Hamzah, E.; Daroonparvar, M.; Saud, S.N.; Abdul-Kadir, M.R. Bi-layer nano-TiO2/FHA composite coatings on Mg-Zn-Ce alloy prepared by combined physical vapour deposition and electrochemical deposition methods. Vacuum 2014, 110, 127–135. [Google Scholar] [CrossRef]
- Gu, C.; Yan, W.; Zhang, J.; Tu, J. Corrosion resistance of AZ31B magnesium alloy with a conversion coating produced from a choline chloride-Urea based deep eutectic solvent. Corros. Sci. 2016, 106, 108–116. [Google Scholar] [CrossRef]

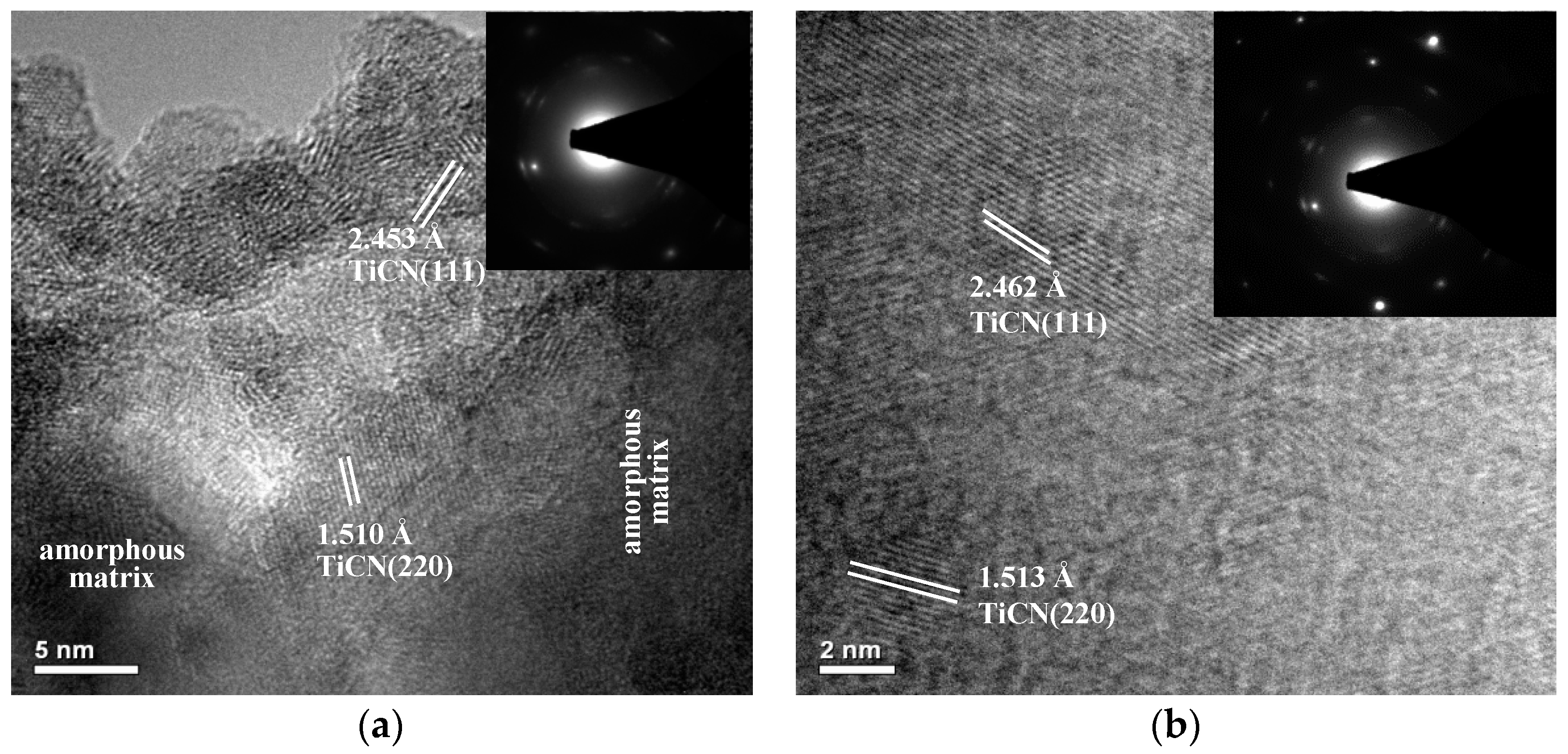

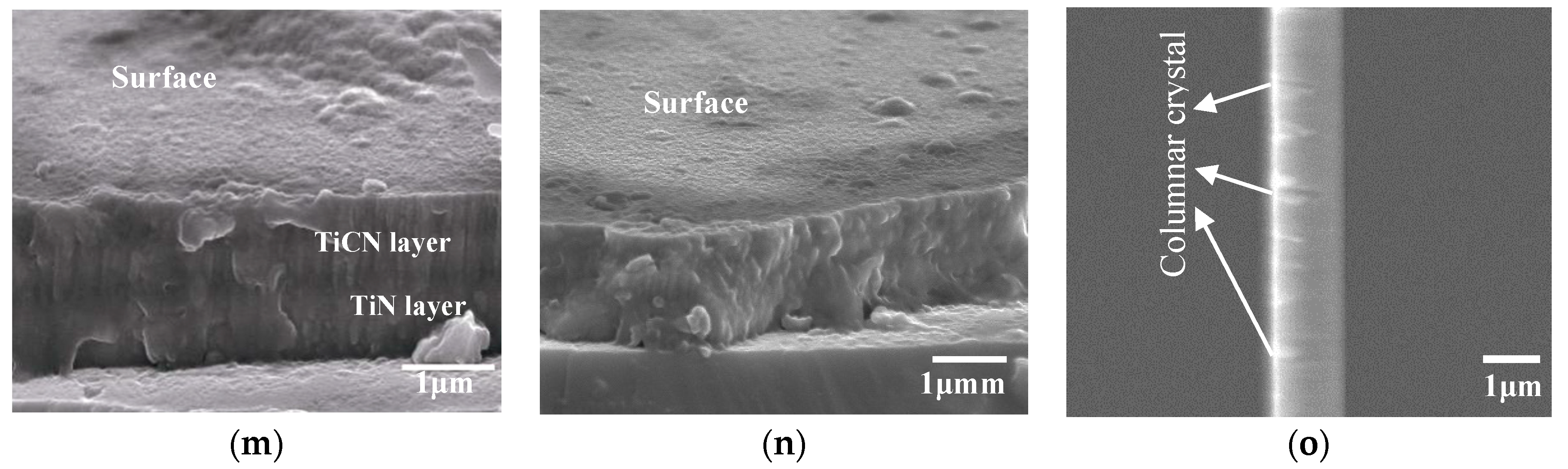

| Coatings | Grain Size (nm) |
|---|---|
| C2 | 33.67 |
| C3 | 30.03 |
| C4 | 25.51 |
| C5 | 25.95 |
| Samples | Ti | C | N |
|---|---|---|---|
| C2 | 49.13 | 18.55 | 32.32 |
| C3 | 46.26 | 22.99 | 30.75 |
| C4 | 44.97 | 25.89 | 29.14 |
| C5 | 42.08 | 28.74 | 29.18 |
| Samples | Corrosion Potential Ecorr (V) | Current Density Icorr (A cm−2) | Corrosion Rate CR (mm/day) |
|---|---|---|---|
| Uncoated AZ31 | −1.562 | 8.15 × 10−4 | 5.1 × 10−2 |
| C2 | −1.188 | 4.78 × 10−6 | 2.99 × 10−4 |
| C3 | −1.151 | 7.65 × 10−7 | 4.79 × 10−5 |
| C4 | −1.129 | 7.07 × 10−7 | 4.43 × 10−5 |
| C5 | −1.173 | 1.05 × 10−6 | 6.57 × 10−5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Sun, P.; Zhu, Y.; Wu, M.; Wang, Q. Influence of C2H2 Flows on Microstructure and Corrosion Resistance of TiCN Films Doped with Carbon Atoms. Coatings 2018, 8, 458. https://doi.org/10.3390/coatings8120458
Li H, Sun P, Zhu Y, Wu M, Wang Q. Influence of C2H2 Flows on Microstructure and Corrosion Resistance of TiCN Films Doped with Carbon Atoms. Coatings. 2018; 8(12):458. https://doi.org/10.3390/coatings8120458
Chicago/Turabian StyleLi, Haitao, Pengfei Sun, Yongchang Zhu, Mingzhong Wu, and Qiang Wang. 2018. "Influence of C2H2 Flows on Microstructure and Corrosion Resistance of TiCN Films Doped with Carbon Atoms" Coatings 8, no. 12: 458. https://doi.org/10.3390/coatings8120458





