Tribo-Mechanical Properties and Corrosion Behavior Investigation of Anodized Ti–V Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.2.1. Preparation of the Substrates
2.2.2. Fabrication of Self-Organized TiO2 Nanotubular Arrays
2.2.3. TiO2 Nanotube Crystallization
2.3. TiO2 Nanotube Array Characterization
2.3.1. Phase Analysis and Microstructural Characterization
2.3.2. Surface Roughness (AFM)
2.3.3. Wear Test
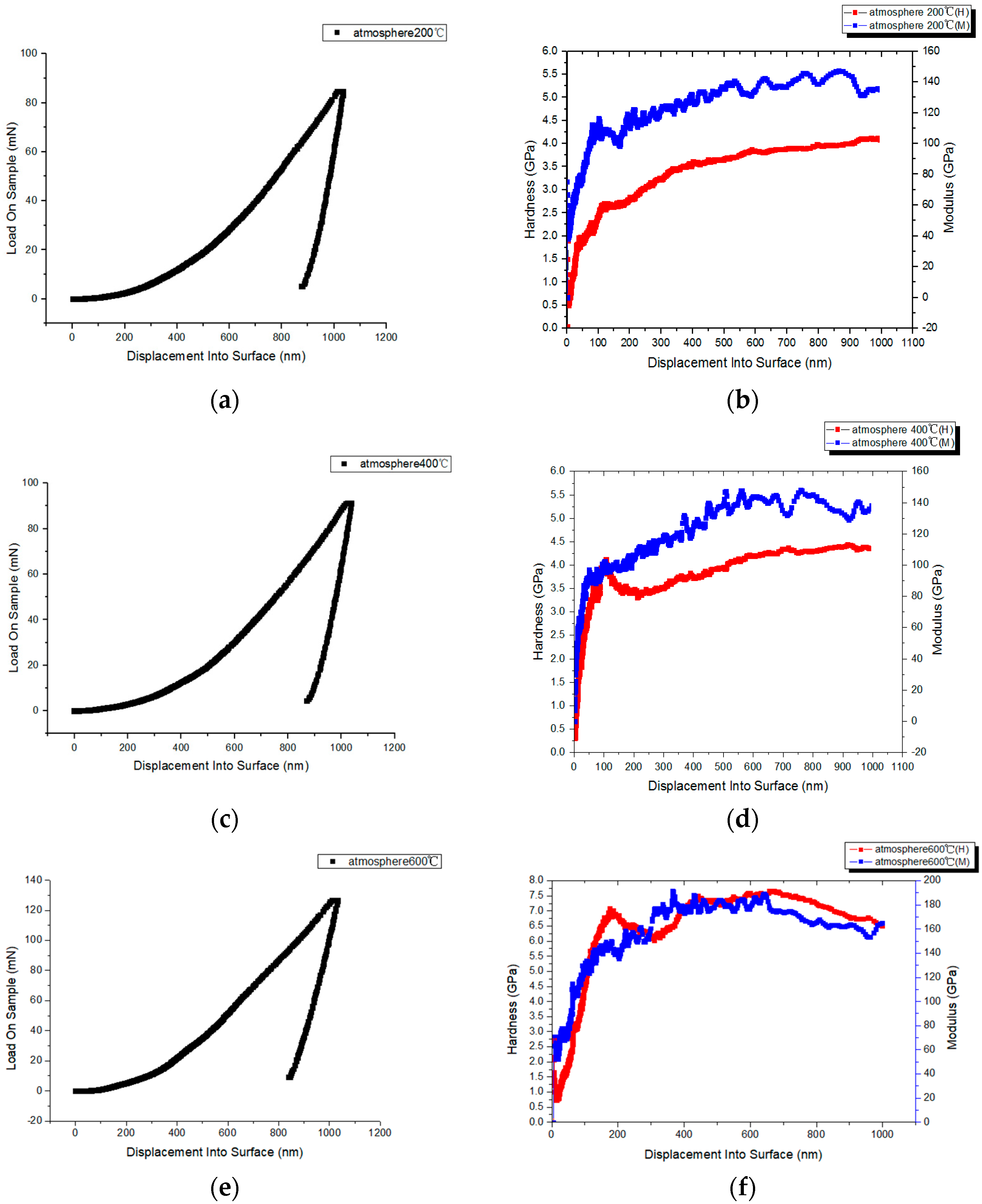
2.3.4. Evaluation of Nanohardness
2.3.5. Corrosion Test
3. Results and Discussion
3.1. TiO2 Nanotube Array Formation Mechanism
- For titanium metal oxide under the action of an electric field, the anode current density decreases exponentially until it reaches a steady state. Due to the creation of a compact oxide film, the current drops rapidly [24];
- Due to the creation of the oxide film, the electric field strength at both ends of the film increases gradually. Due to the corrosion reaction of F− with the oxide film under the influence of an electric field, the oxide film forms dissolved pits, resulting in an increase in current. The rate of decreasing current slows but continues;
- As the corrosion rate and oxidation rate reach equilibrium, ion mobility becomes a major factor in current formation. The hole bottom electric field is much larger than that of the hole wall, and fluoride ions are more concentrated in the bottom of the hole, further deepening the hole. With the current reduction, oxidation rate, and nozzle dissolution rate, the tube bottom corrosion rate reaches dynamic equilibrium. The length of the nanotube array no longer increases.
3.2. Phase Evolution and Structural Features (XRD Analysis)
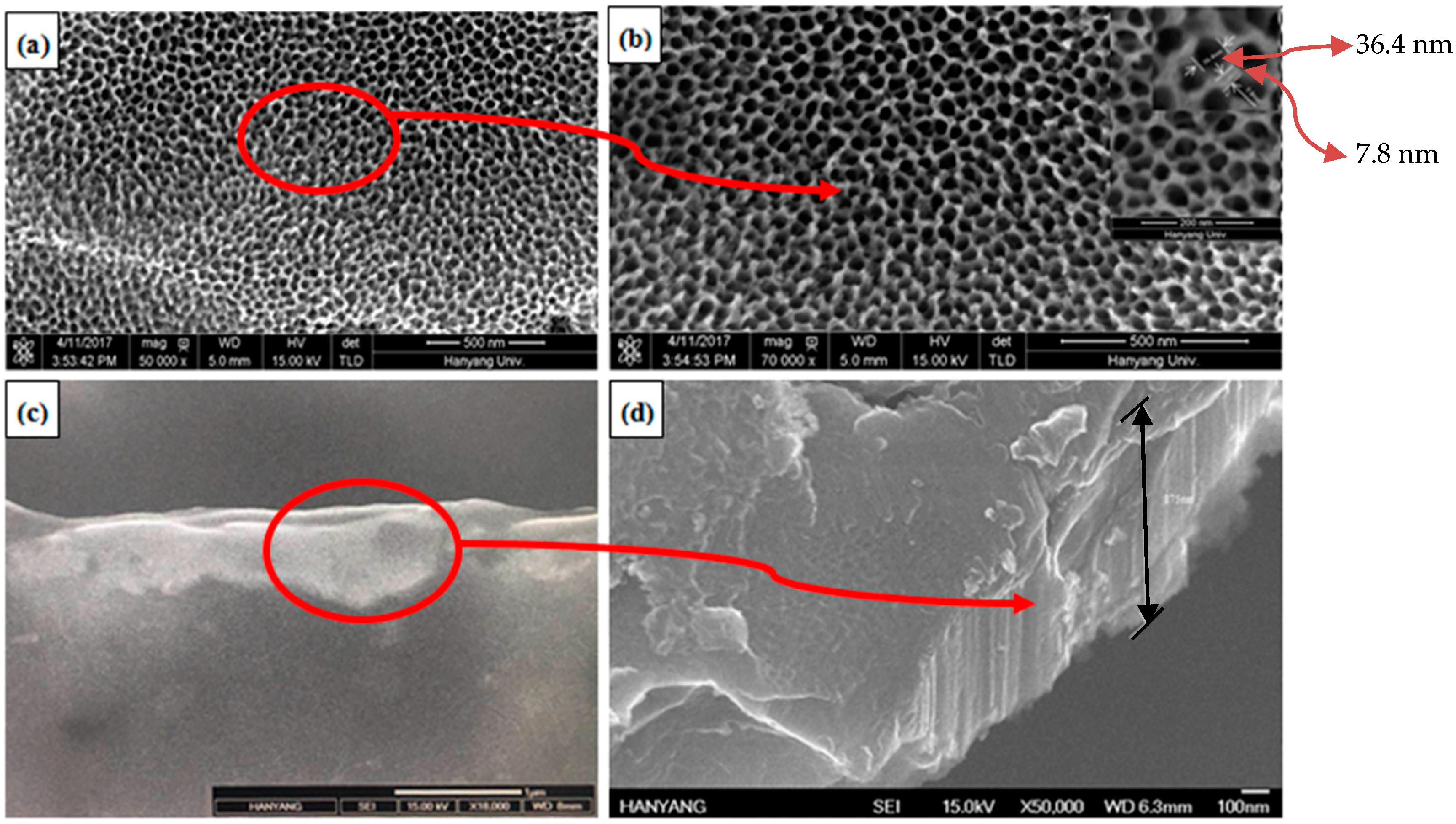
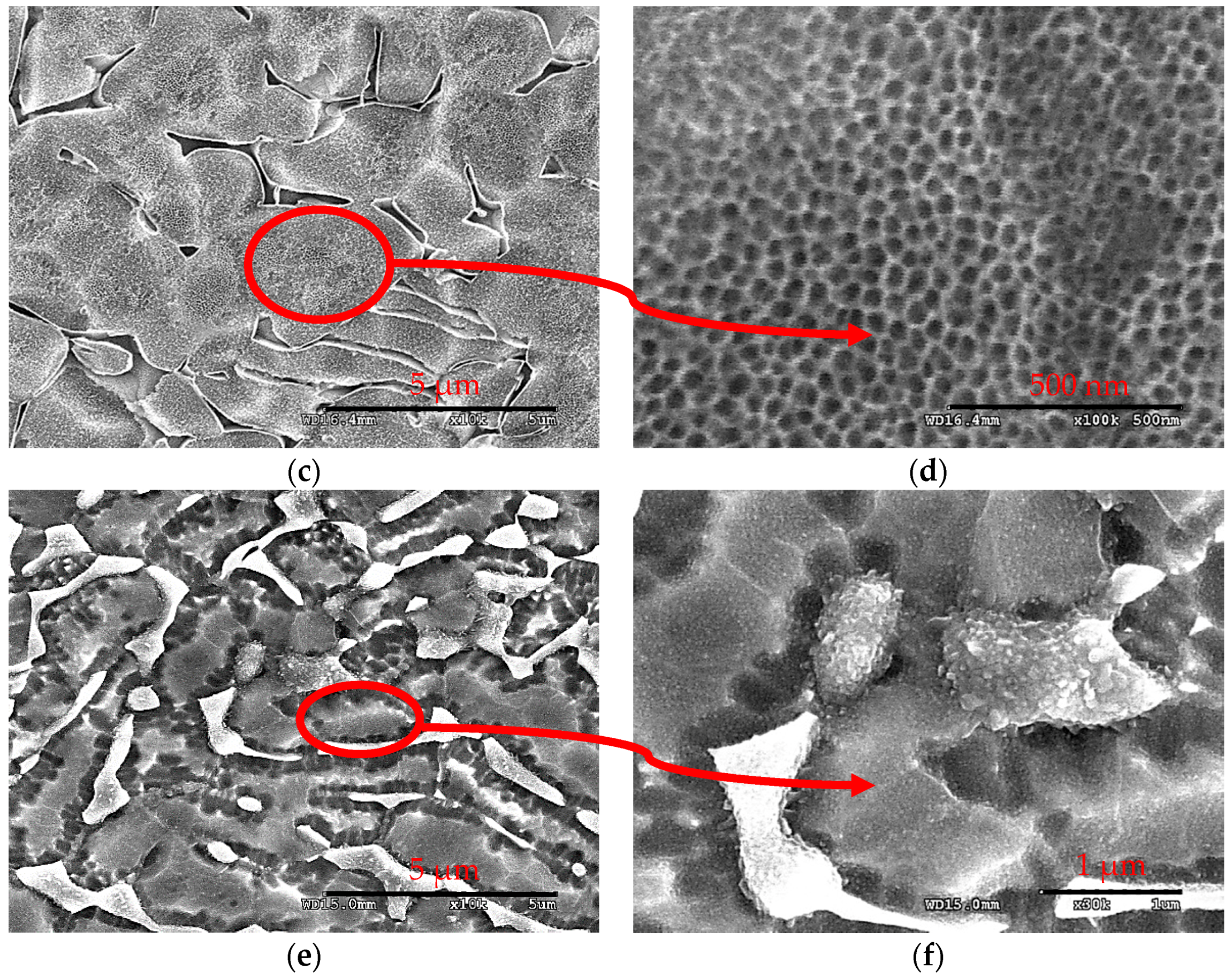
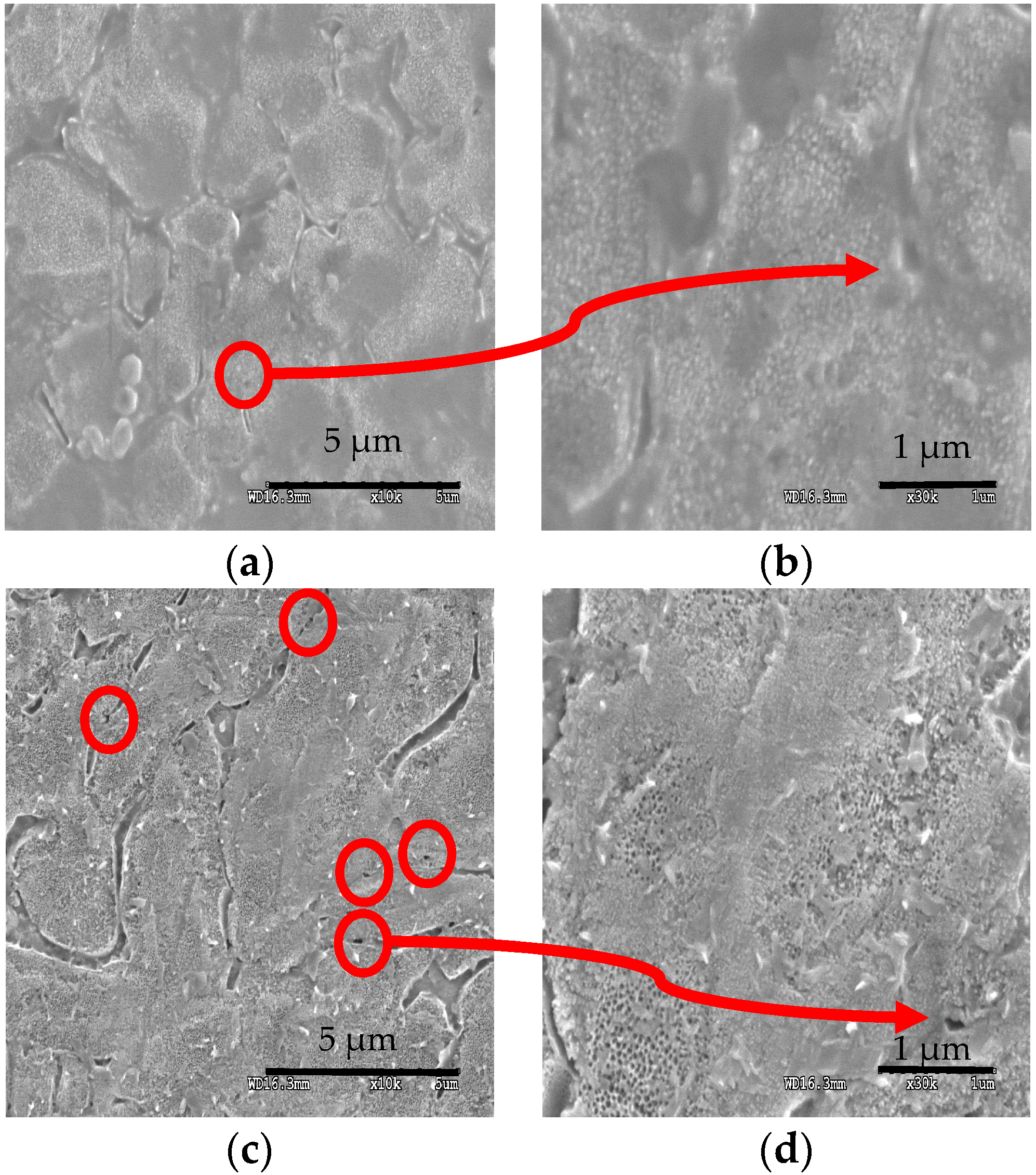
3.3. Microstructural Characterization
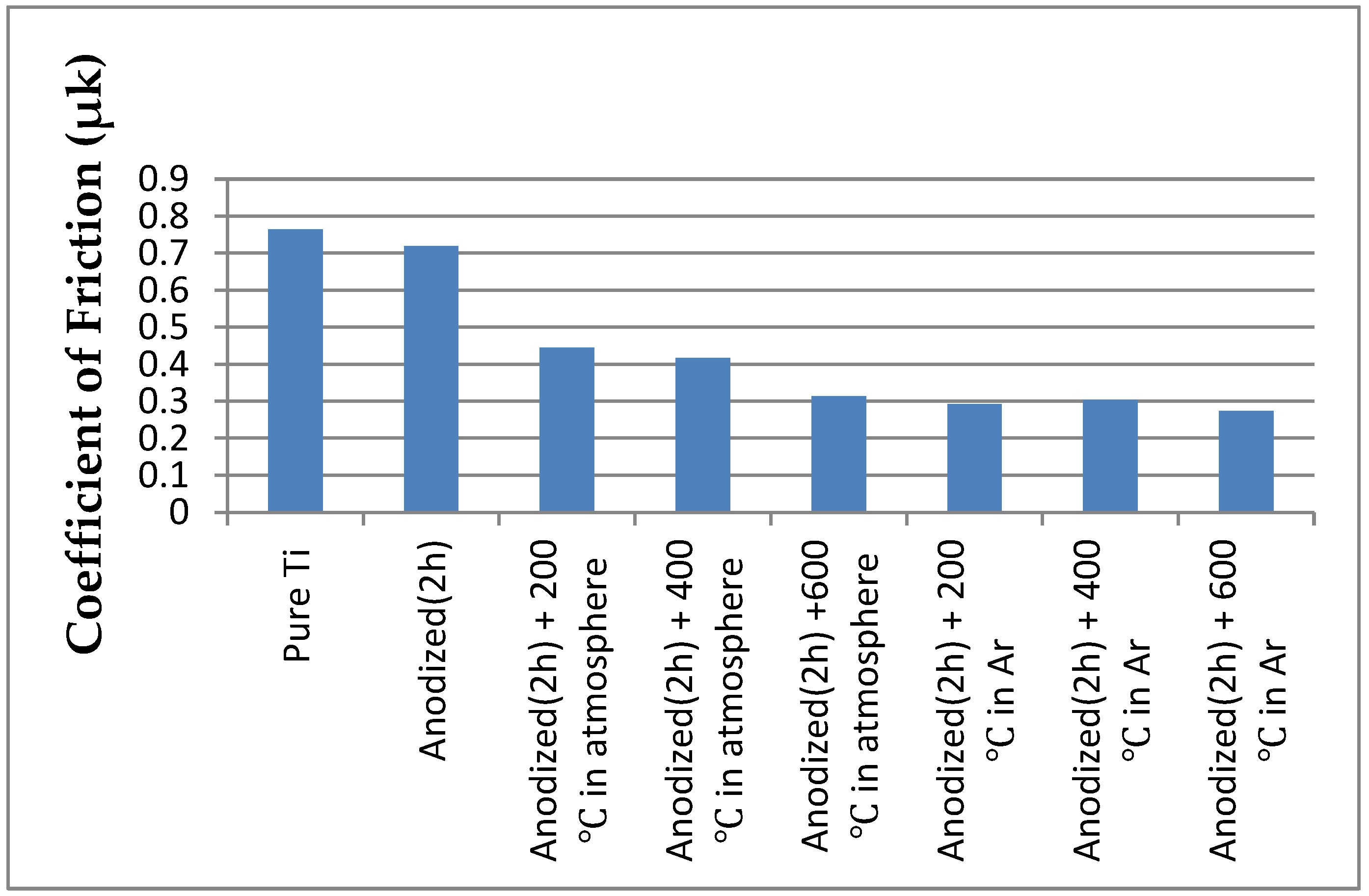
3.4. Friction and Wear Behaviors
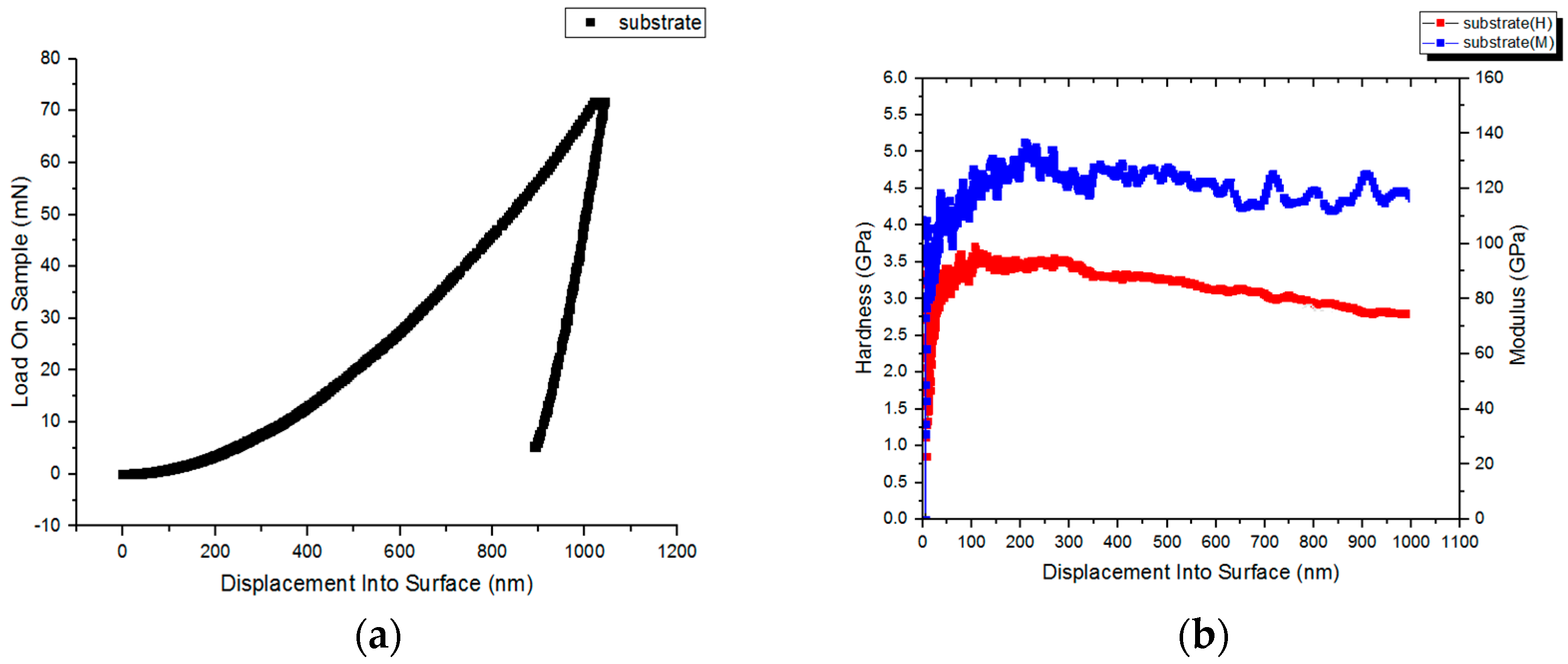
3.5. Nanoindentation
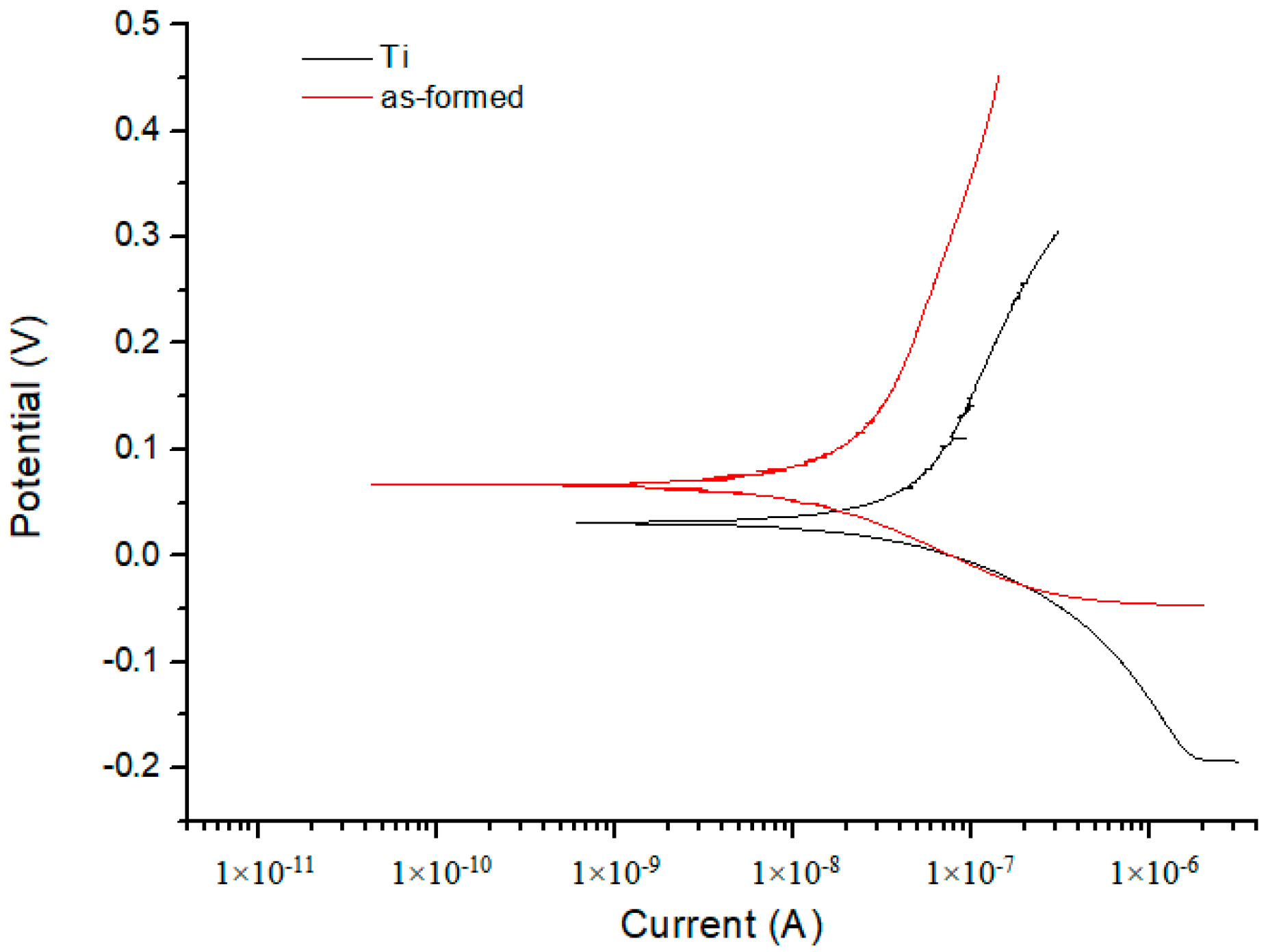
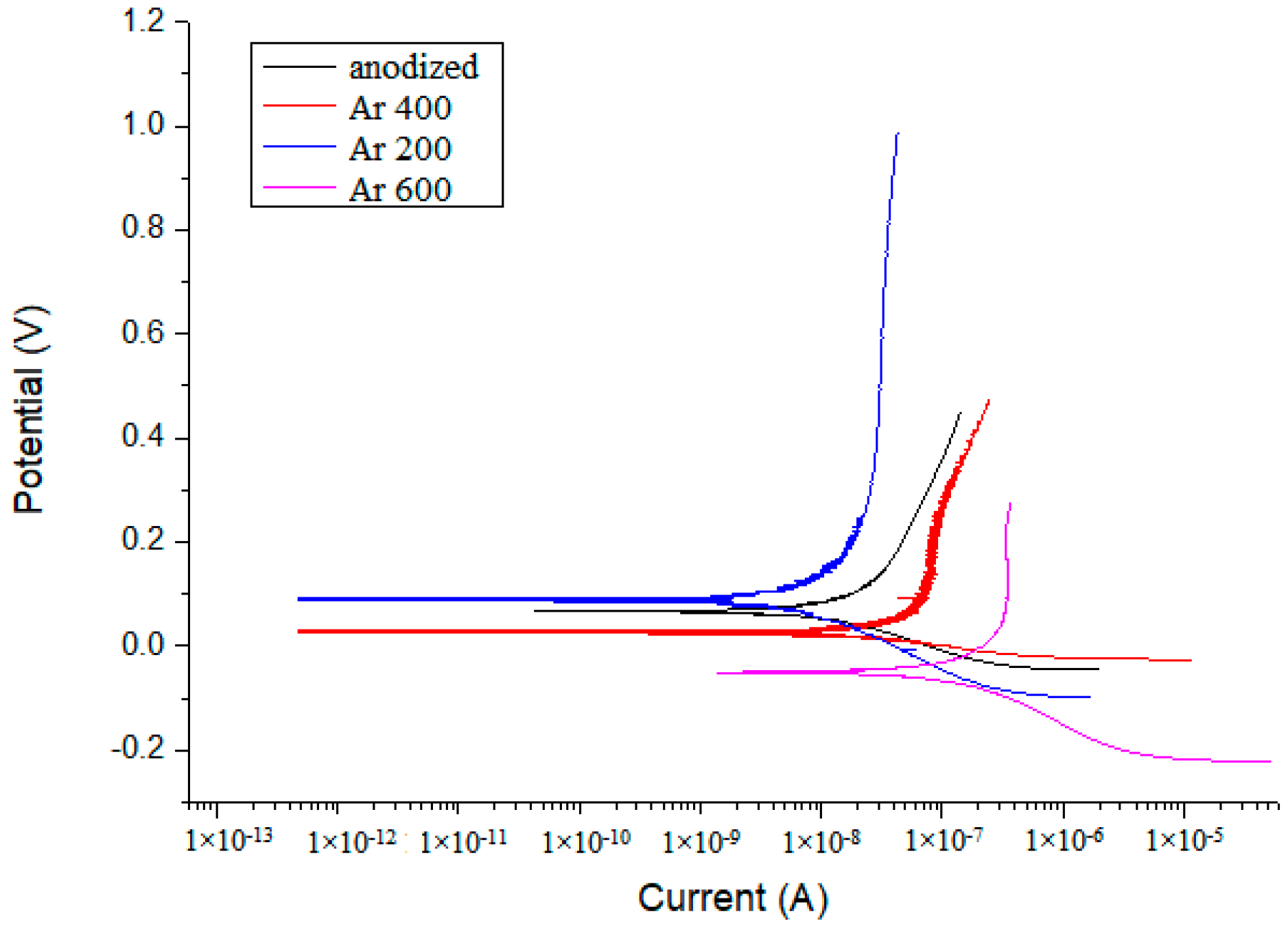
3.6. Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti-based biomaterials, the ultimate choice for orthopedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Narayanan, R.; Seshadri, S.K. Phosphoric acid anodization of Ti–6Al–4V—Structural and corrosion aspects. Corros. Sci. 2007, 49, 542–558. [Google Scholar] [CrossRef]
- Hedzelek, W.; Sikorska, B.; Domka, L. Evaluation of selected mechanical and chemical methods of modifications of titanium. Physicochem. Probl. Miner. Process. 2005, 39, 149–154. [Google Scholar]
- Variola, F.; Yi, J.-H.; Richert, L.; Wuest, J.D.; Rosei, F.; Nanci, A. Tailoring the surface properties of Ti6Al4V by controlled chemical oxidation. Biomaterials 2008, 29, 1285–1298. [Google Scholar] [CrossRef]
- Jonášová, L.; Müller, F.A.; Helebrant, A.; Strnad, J.; Greil, P. Biomimetic apatite formation on chemically treated titanium. Biomaterials 2004, 25, 1187–1194. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Viswanathan, B.; Varadaraju, U.V. Fabrication and characterization of uniform TiO2 nanotube arrays by sol-gel template method. Bull. Mater. Sci. 2006, 29, 705–708. [Google Scholar]
- Raja, K.S.; Misra, M.; Paramguru, K. Deposition of calcium phosphate coating on nanotubular anodized titanium. Mater. Lett. 2005, 59, 2137–2141. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Wlodarski, W.; Kandasamy, S.; Kalantar-zadeh, K. Fabrication of nanostructured TiO2 by anodization: A comparison between electrolytes and substrates. Sensor. Actuator B Chem. 2008, 130, 25–31. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Taveira, L.; Ghicov, A.; Schmuki, P. Self-organized nanotubular oxide layers on Ti–6Al–7Nb and Ti–6Al–4V formed by anodization in NH4F solutions. J. Biomed. Mater. Res. 2005, 75, 928–933. [Google Scholar] [CrossRef]
- Kim, H.M.; Kaneko, H.; Masakazu, K.; Kokubo, T.; Nakamura, T. Mechanism of apatite formation on anodically oxidized titanium metal in simulated body fluid. Key Eng. Mater. 2004, 254–265, 741–744. [Google Scholar] [CrossRef]
- Zwilling, V.; Darque-Ceretti, E.; Boutry-Forveille, A.; David, D.; Perrin, M.Y.; Aucouturier, M. Structure and physicochemistry of anodic oxide films on titanium and TA6V alloy. Surf. Interface Anal. 1999, 27, 629–637. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Taveira, L.; Aldabergerova, S.; Schmuki, P. Smooth anodic TiO2 nanotubes. Angew. Chem. Int. Ed. 2005, 44, 7463–7465. [Google Scholar] [CrossRef]
- Macak, J.M.; Taveira, L.V.; Tsuchiya, H.; Sirotna, K.; Macak, J.; Schmuki, P. Influence of different fluoride containing electrolytes on the formation of self-organized titania nanotubes by Ti anodization. J. Electroceram. 2006, 16, 29–34. [Google Scholar] [CrossRef]
- Ghicov, A.; Macak, J.M.; Tsuchiya, H.; Kunze, J.; Haeublein, V.; Frey, L.; Schmuki, P. Ion implantation and annealing for an efficient N-doping of TiO2 nanotubes. Nano Lett. 2006, 6, 1080–1082. [Google Scholar] [CrossRef]
- Yu, W.; Qiu, J.; Xu, L.; Zhang, F. Corrosion behaviors of TiO2 nanotube layers on titanium in Hank’s solution. Biomed. Mater. 2009, 4, 065012. [Google Scholar] [CrossRef]
- Kunze, J.; Müller, L.; Macak, J.M.; Greil, P.; Schmuki, P.; Müller, F.A. Time-dependent growth of biomimetic apatite on anodic TiO2 nanotubes. Electrochim. Acta 2008, 53, 6995–7003. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G.; Li, Y.; Yu, Z.; Xi, Z. Fabrication of TiO2 nanotube arrays on biologic titanium alloy and properties. Trans. Nonferr. Met. Soc. Chin. 2007, 17, 692–695. [Google Scholar]
- Petukhov, D.I.; Eliseeva, A.A.; Kolesnik, I.V.; Napolskii, K.S.; Lukashin, A.V.; Tretyakov, Y.D.; Grigoriev, S.V.; Grigorieva, N.A.; Eckerlebe, H. Formation mechanism and packing options in tubular anodic titania films. Microporous Microporous Mater. 2008, 114, 440–447. [Google Scholar] [CrossRef]
- Yang, B.; Uchida, M.; Kim, H.-M.; Zhang, X.; Kokubo, T. Preparation of bioactive titanium metal via anodic oxidation treatment. Biomaterials 2004, 25, 1003–1010. [Google Scholar] [CrossRef]
- Boehlert, C.J.; Cowen, C.J.; Quast, J.P.; Akahori, T.; Niinomi, M. Fatigue and wear evaluation of Ti–Al–Nb alloys for biomedical applications. Mater. Sci. Eng. C 2008, 28, 323–330. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639. [Google Scholar] [CrossRef]
- Chang, W.-Y.; Fang, T.-H.; Chiu, Z.-W.; Hsiao, Y.-J.; Ji, L.-W. Nanomechanical properties of array TiO2 nanotubes. Microporous Microporous Mater. 2011, 145, 87–92. [Google Scholar] [CrossRef]
- Prida, V.M.; Manova, E.; Vega, V.; Hernandez-Velez, M.; Aranda, P.; Pirota, K.R.; Vázquez, M.; Ruiz-Hitzky, E. Temperature influence on the anodic growth of self-aligned Titanium dioxide nanotube arrays. J. Magn. Magn. Mater. 2007, 316, 110–113. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Santo, V.D. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef]
- Sarraf, M.; Zalnezhad, E.; Bushroa, A.R.; Hamouda, A.M.S.; Baradaran, S.; Nasiri-Tabrizi, B.; Rafieerad, A.R. Structural and mechanical characterization of Al/Al2O3 nanotube thin film on TiV alloy. Appl. Surf. Sci. 2014, 321, 511–519. [Google Scholar] [CrossRef]
- Saharudin, K.A.; Sreekantan, S.; Aziz, S.N.; Hazan, R.; Lai, C.W.; Mydin, R.B.; Mat, I. Surface modification and bioactivity of anodic Ti6Al4V alloy. J. Nanosci. Nanotechnol. 2013, 13, 1696–1705. [Google Scholar] [CrossRef]
- Tian, T.; Xiao, X.; Liu, R.; She, H.; Hu, X. Study on titania nanotube arrays prepared by titanium anodization in NH4F/H2SO4 solution. J. Mater. Sci. 2007, 42, 5539–5543. [Google Scholar] [CrossRef]
- Kummer, K.M.; Taylor, E.; Webster, T.J. Biological applications of anodized TiO2 nanostructures: A review from orthopedic to stent applications. Nanosci. Nanotechnol. Lett. 2012, 4, 483–493. [Google Scholar] [CrossRef]
- Baradaran, S.; Basirun, W.J.; Zalnezhad, E.; Hamdi, M.; Sarhan, A.A.; Alias, Y. Fabrication and deformation behaviour of multilayer Al2O3/Ti/TiO2 nanotube arrays. J. Mech. Behav. Biomed. Mater. 2013, 20, 272–282. [Google Scholar] [CrossRef]
- Zalnezhad, E.; Baradaran, S.; Bushroa, A.R.; Sarhan, A.A.D. Mechanical property enhancement of Ti-6Al-4V by multilayer thin solid film Ti/TiO2 nanotubular array coating for biomedical application. Metall. Mater. Trans. A 2014, 45, 785–797. [Google Scholar] [CrossRef]
- Yaghoubi, H.; Taghavinia, N.; Alamdari, E.K.; Volinsky, A.A. Nanomechanical properties of TiO2 granular thin films. ACS Appl. Mater. Interfaces 2010, 2, 2629–2636. [Google Scholar] [CrossRef]
- Sarraf, M.; Zalnezhad, E.; Bushroa, A.R.; Hamouda, A.M.S.; Rafieerad, A.R.; Nasiri-Tabrizi, B. Effect of microstructural evolution on wettability and tribological behavior of TiO2 nanotubular arrays coated on Ti–6Al–4V. Ceram. Int. 2015, 41, 7952–7962. [Google Scholar] [CrossRef]












| Element | wt % |
|---|---|
| Al | 5.84 |
| V | 4.12 |
| Cr | 0.12 |
| C | 0.35 |
| Fe | 0.13 |
| Si | 0.02 |
| Ti | Balance |
| Chemical Composition | Concentration (g/dm3) |
|---|---|
| CaCl2 | 1 |
| Na2HPO4 | 22.98 |
| NaH2PO4 | 13.78 |
| Sample | Wear Loss (g) |
|---|---|
| Bare sample | 0.0007 |
| Anodized sample | 0.0005 |
| Anodized + 200 °C in Ar | 0.0004 |
| Anodized + 400 °C in Ar | 0.0004 |
| Anodized + 600 °C in Ar | 0.0002 |
| Anodized + 200 °C in air | 0.0004 |
| Anodized + 400 °C in air | 0.0001 |
| Anodized + 600 °C in air | 0.0001 |
| Sample | Hardness (GPa) | Elastic Modulus (GPa) |
|---|---|---|
| Bare substrate | 3.38 | 115.51 |
| Anodized sample | 3.66 | 135.55 |
| Anodized + 200 °C in air | 3.71 | 140.34 |
| Anodized + 400 °C in air | 4.11 | 147.46 |
| Anodized + 600 °C in air | 6.15 | 160.87 |
| Anodized + 200 °C in Ar | 3.28 | 140.45 |
| Anodized + 400 °C in Ar | 3.57 | 148.35 |
| Anodized + 600 °C in Ar | 4.36 | 156.64 |
| Sample | Ecorr (V) | βc (V) | βa (V) | Icorr (A/cm2) |
|---|---|---|---|---|
| Substrate | 2.32 × 10−1 | 1.72 × 10−1 | 8.13 × 10−1 | 1.04 × 10−7 |
| Anodized | 2.55 × 10−1 | 7.53 × 10−2 | 7.01 × 10−1 | 2.33 × 10−8 |
| Anodized + 200 °C in air | 2.82 × 10−1 | 7.4 × 10−2 | 1.04 | 3.53 × 10−9 |
| Anodized + 400 °C in air | 2.33 × 10−1 | 8.28 × 10−2 | 6.91 × 10−2 | 6.35 × 10−9 |
| Anodized + 600 °C in air | 2.64 × 10−1 | 1.18 × 10−2 | 1.09 | 1.87 × 10−8 |
| Anodized + 200 °C in Ar | 2.18 × 10−1 | 4.58 × 10−2 | 8.08 × 10−1 | 1.86 × 10−8 |
| Anodized + 400 °C in Ar | 2.17 × 10−1 | 4.16 × 10−2 | 6.4 × 10−1 | 4.54 × 10−8 |
| Anodized + 600 °C in Ar | −5.19 × 10−2 | 1.76 × 10−2 | 1.41 × 10−1 | 4.33 × 10−7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, B.; Zal Nezhad, E.; Musharavati, F.; Jaber, F.; Bae, S. Tribo-Mechanical Properties and Corrosion Behavior Investigation of Anodized Ti–V Alloy. Coatings 2018, 8, 459. https://doi.org/10.3390/coatings8120459
Han B, Zal Nezhad E, Musharavati F, Jaber F, Bae S. Tribo-Mechanical Properties and Corrosion Behavior Investigation of Anodized Ti–V Alloy. Coatings. 2018; 8(12):459. https://doi.org/10.3390/coatings8120459
Chicago/Turabian StyleHan, Bingrong, Erfan Zal Nezhad, Farayi Musharavati, Fadi Jaber, and Sungchul Bae. 2018. "Tribo-Mechanical Properties and Corrosion Behavior Investigation of Anodized Ti–V Alloy" Coatings 8, no. 12: 459. https://doi.org/10.3390/coatings8120459





