Scan-Mode Atmospheric-Pressure Plasma Jet Processed Reduced Graphene Oxides for Quasi-Solid-State Gel-Electrolyte Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. rGO Pastes Preparation
2.2. Supercapacitor Fabrication
2.3. Characterization of rGO-Coated Carboncloth and Supercapacitors
3. Results and Discussion
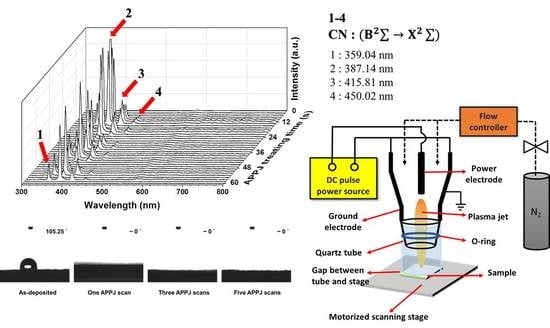
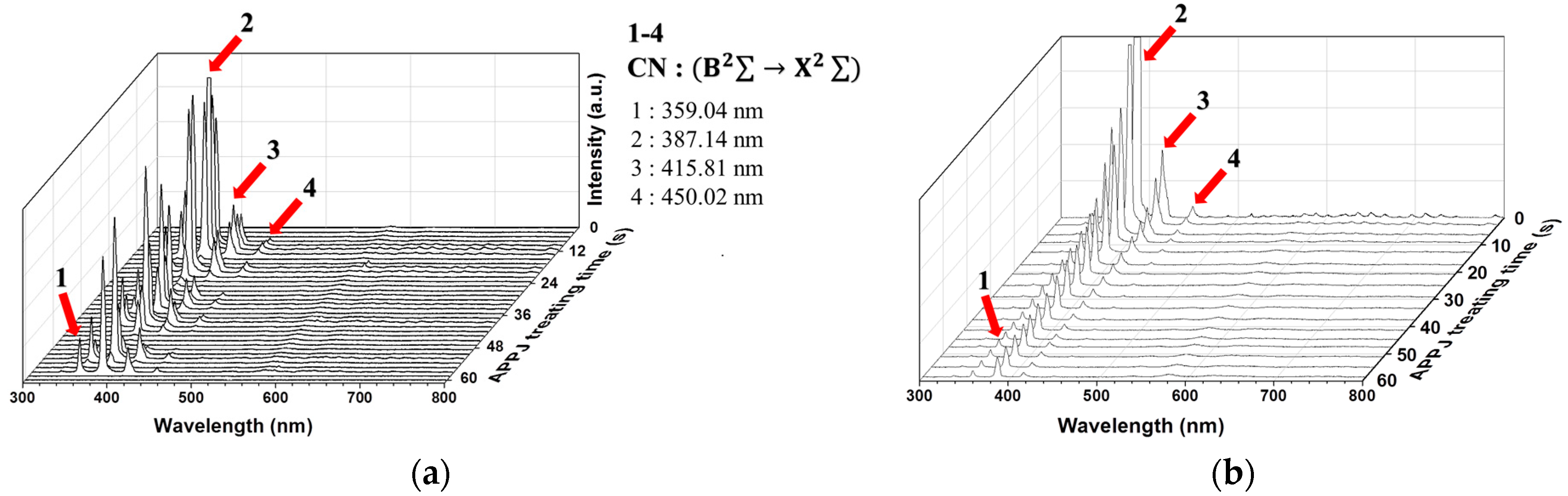
3.1. OES of Plasma during APPJ Processing
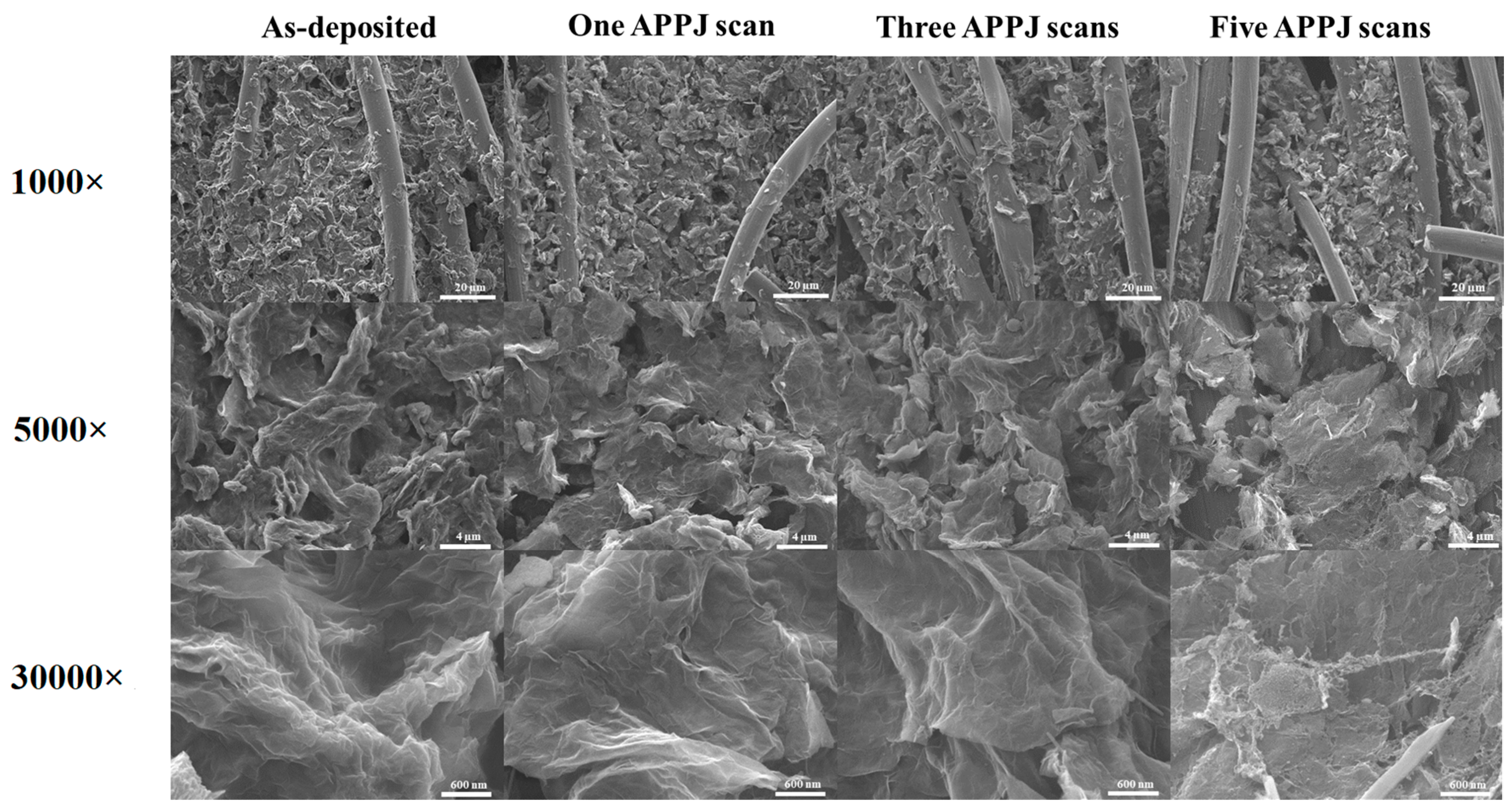
3.2. SEM Images of APPJ-Processed rGOs on Carbon Cloth
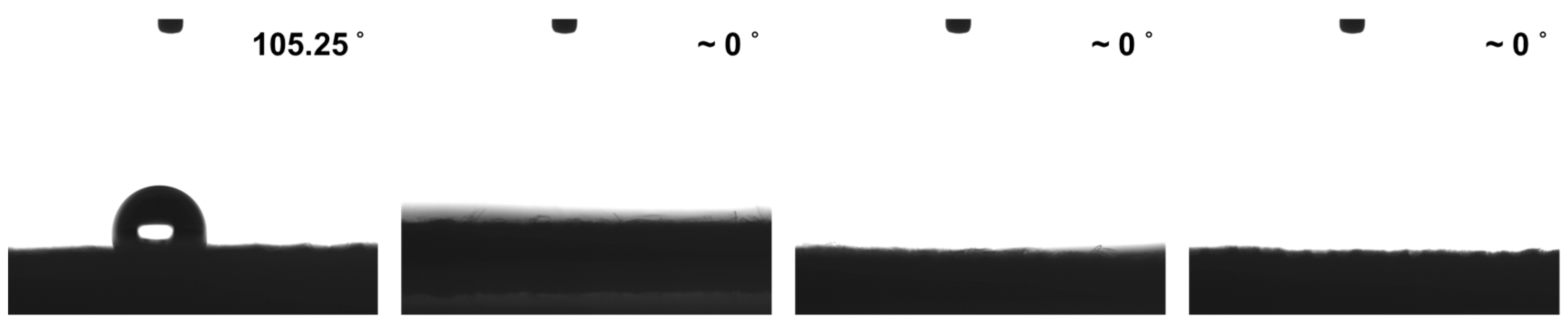
3.3. Water Contact Angle Measurement of APPJ-Processed rGOs on Carbon Cloth
3.4. XPS Results of APPJ-Processed rGOs on Carbon Cloth
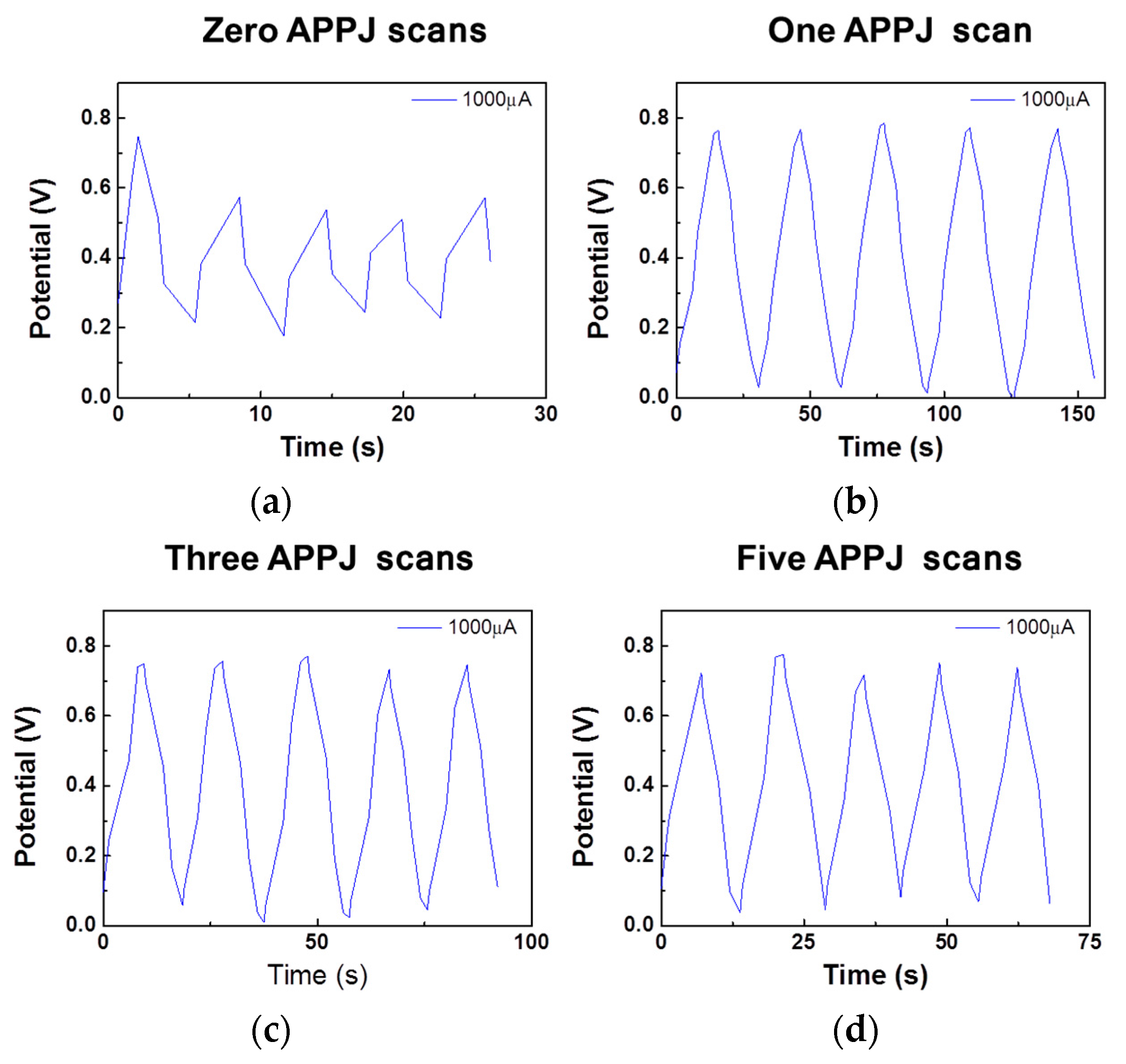
3.5. GCD Results of Gel-Electrolyte Supercapacitor
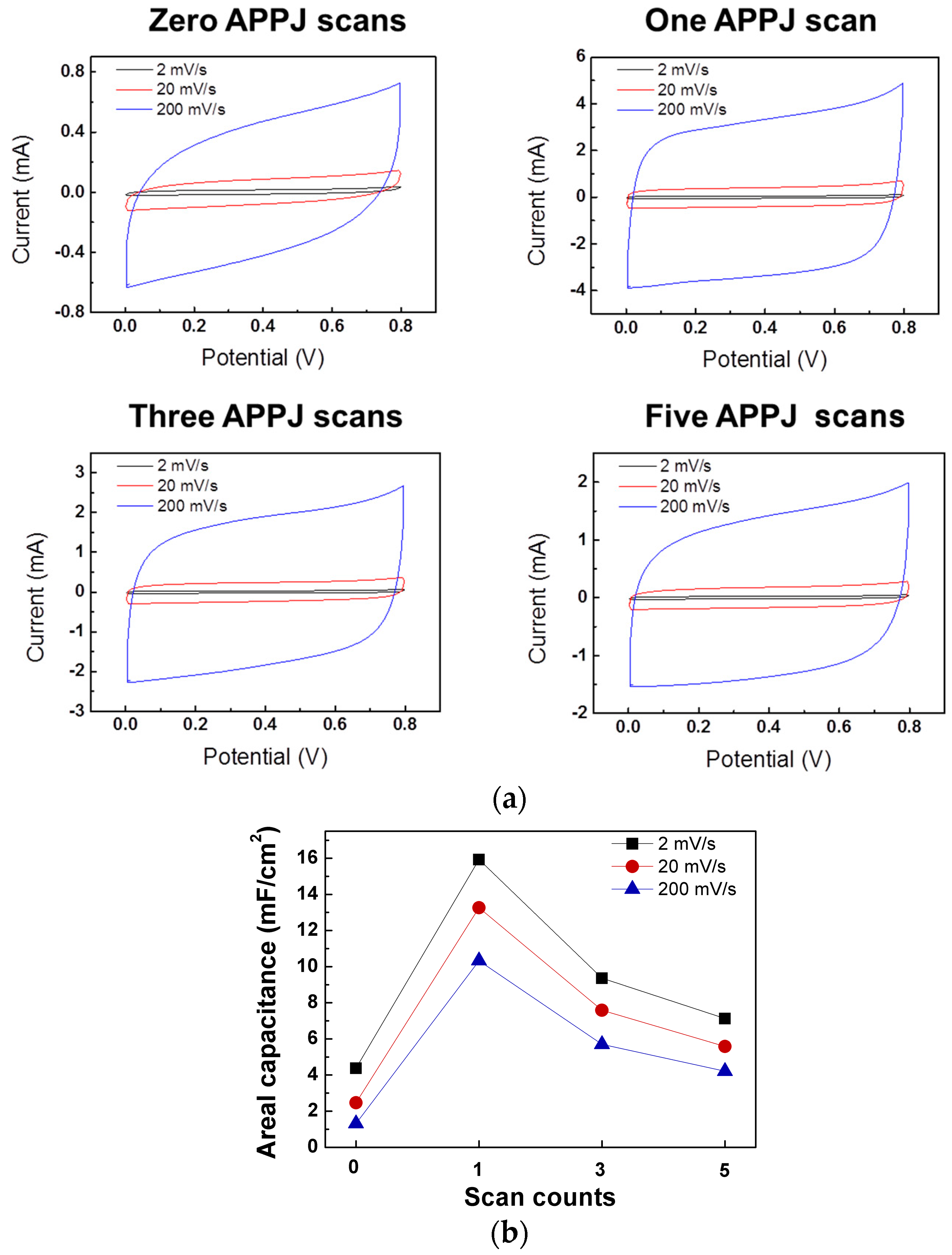
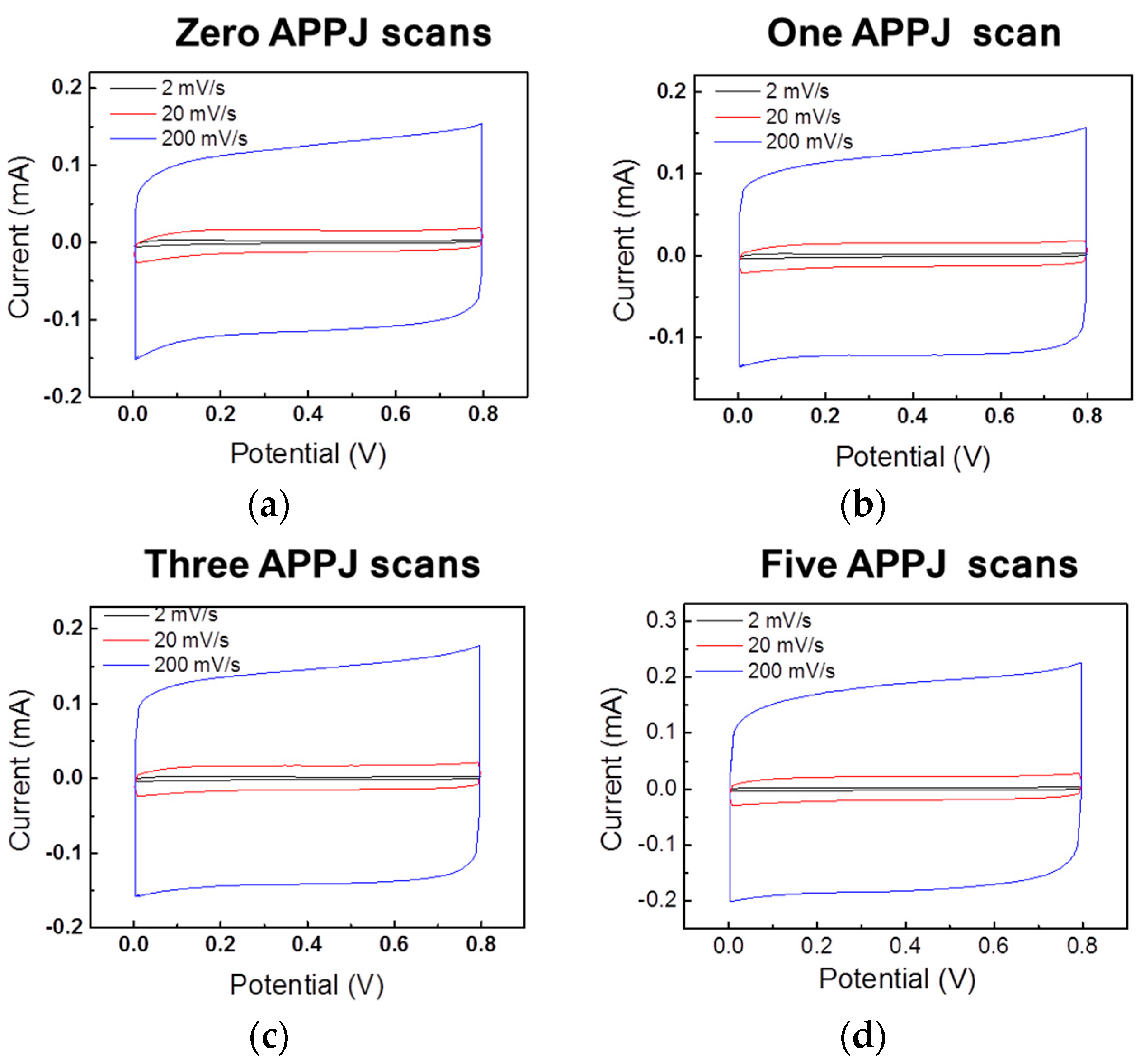
3.6. CV Results of Gel-Electrolyte Supercapacitor
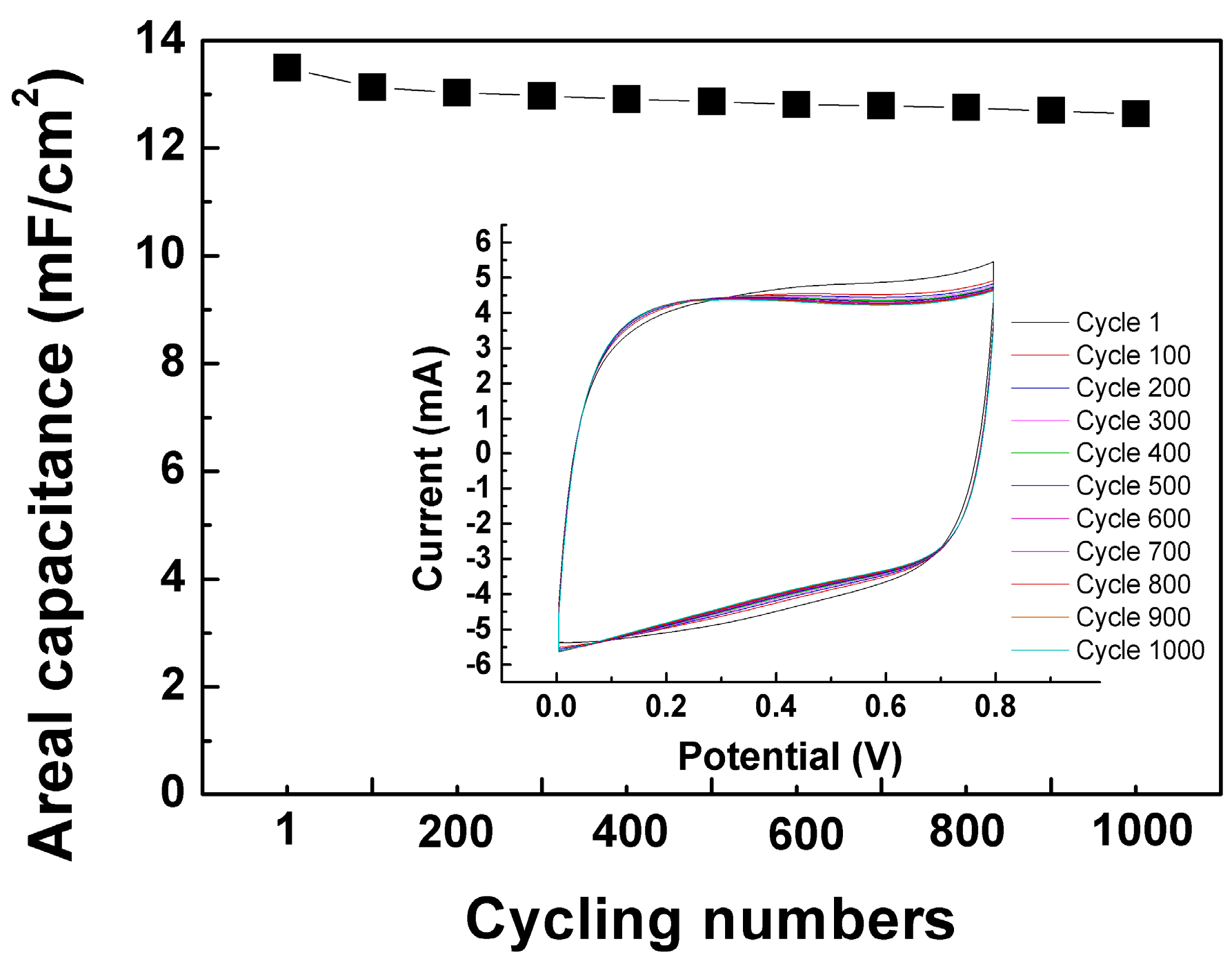
3.7. CV Cycling Stability Test Results of Gel-Electrolyte Supercapacitor
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schutze, A.; Jeong, J.Y.; Babayan, S.E.; Park, J.; Selwyn, G.S.; Hicks, R.F. The atmospheric-pressure plasma jet: A review and comparison to other plasma sources. IEEE Trans. Plasma Sci. 1998, 26, 1685–1694. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta B 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Park, G.Y.; Park, S.J.; Choi, M.Y.; Koo, I.G.; Byun, J.H.; Hong, J.W.; Sim, J.Y.; Collins, G.J.; Lee, J.K. Atmospheric-pressure plasma sources for biomedical applications. Plasma Sources Sci. Technol. 2012, 21, 043001. [Google Scholar] [CrossRef]
- Winter, J.; Brandenburg, R.; Weltmann, K.D. Atmospheric pressure plasma jets: An overview of devices and new directions. Plasma Sources Sci. Technol. 2015, 24, 064001. [Google Scholar] [CrossRef]
- Nassour, K.; Brahami, M.; Nemmich, S.; Hammadi, N.; Zouzou, N.; Tilmatine, A. New hybrid surface-volume dielectric barrier discharge reactor for ozone generation. IEEE Trans. Ind. Appl. 2017, 53, 2477–2484. [Google Scholar] [CrossRef]
- Zhang, H.B.; Li, H.; Fang, M.; Wang, Z.D.; Sang, L.J.; Yang, L.Z.; Chen, Q. Roll-to-roll DBD plasma pretreated polyethylene web for enhancement of al coating adhesion and barrier property. App. Surf. Sci. 2016, 388, 539–545. [Google Scholar] [CrossRef]
- Homola, T.; Shekargoftar, M.; Dzik, P.; Krumpolec, R.; Durasova, Z.; Vesely, M.; Cernak, M. Low-temperature (70 °C) ambient air plasma-fabrication of inkjet-printed mesoporous TiO2 flexible photoanodes. Flex. Print. Electron. 2017, 2, 035010. [Google Scholar] [CrossRef]
- Kelly-Wintenberg, K.; Sherman, D.M.; Tsai, P.P.; Gadri, R.B.; Karakaya, F.; Chen, Z.; Roth, J.R.; Montie, T.C. Air filter sterilization using a one atmosphere uniform glow discharge plasma (the volfilter). IEEE Trans. Plasma Sci. 2000, 28, 64–71. [Google Scholar] [CrossRef]
- Gaunt, L.F.; Beggs, C.B.; Georghiou, G.E. Bactericidal action of the reactive species produced by gas-discharge nonthermal plasma at atmospheric pressure: A review. IEEE Trans. Plasma Sci. 2006, 34, 1257–1269. [Google Scholar] [CrossRef]
- Homma, T.; Furuta, M.; Takemura, Y. Inactivation of Escherichia coli using the atmospheric pressure plasma jet of Ar gas. Jpn. J. Appl. Phys. 2013, 52, 036201. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, M.S.; Won, I.H.; Yun, G.S.; Lee, J.K. Self-prevention of instability in a low-power microwave Ar plasma jet for biomedical applications. J. Phys. D Appl. Phys. 2015, 48, 155203. [Google Scholar] [CrossRef]
- Liu, H.-W.; Liang, S.-P.; Wu, T.-J.; Chang, H.; Kao, P.-K.; Hsu, C.-C.; Chen, J.-Z.; Chou, P.-T.; Cheng, I.-C. Rapid atmospheric pressure plasma jet processed reduced graphene oxide counter electrodes for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2014, 6, 15105–15112. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-H.; Cheng, I.-C.; Hsu, C.-C.; Chen, J.-Z. UV photocurrent responses of ZnO and MgZnO/ZnO processed by atmospheric pressure plasma jets. J. Alloy. Compd. 2015, 628, 68–74. [Google Scholar] [CrossRef]
- Wu, T.-J.; Chou, C.-Y.; Hsu, C.-M.; Hsu, C.-C.; Chen, J.-Z.; Cheng, I.-C. Ultrafast synthesis of continuous au thin films from chloroauric acid solution using an atmospheric pressure plasma jet. RSC Adv. 2015, 5, 99654–99657. [Google Scholar] [CrossRef]
- Chen, J.-Z.; Wang, C.; Hsu, C.-C.; Cheng, I.-C. Ultrafast synthesis of carbon-nanotube counter electrodes for dye-sensitized solar cells using an atmospheric-pressure plasma jet. Carbon 2016, 98, 34–40. [Google Scholar] [CrossRef]
- Xu, C.-H.; Chen, J.-Z. Atmospheric-pressure plasma jet processed SnO2/CNT nanocomposite for supercapacitor application. Ceram. Int. 2016, 42, 14287–14291. [Google Scholar] [CrossRef]
- Xu, C.-H.; Shen, P.-Y.; Chiu, Y.-F.; Yeh, P.-W.; Chen, C.-C.; Chen, L.-C.; Hsu, C.-C.; Cheng, I.-C.; Chen, J.-Z. Atmospheric pressure plasma jet processed nanoporous Fe2O3/CNT composites for supercapacitor application. J. Alloy. Compd. 2016, 676, 469–473. [Google Scholar] [CrossRef]
- Kuok, F.-H.; Liao, C.-Y.; Wan, T.-H.; Yeh, P.-W.; Cheng, I.-C.; Chen, J.-Z. Atmospheric pressure plasma jet processed reduced graphene oxides for supercapacitor application. J. Alloy. Compd. 2017, 692, 558–562. [Google Scholar] [CrossRef]
- Yang, C.-H.; Kuok, F.-H.; Liao, C.-Y.; Wan, T.-H.; Chen, C.-W.; Hsu, C.-C.; Cheng, I.-C.; Chen, J.-Z. Flexible reduced graphene oxide supercapacitor fabricated using a nitrogen DC-pulse atmospheric-pressure plasma jet. Mater. Res. Express 2017, 4, 025504. [Google Scholar] [CrossRef]
- Kuok, F.-H.; Kan, K.-Y.; Yu, I.-S.; Chen, C.-W.; Hsu, C.-C.; Cheng, I.-C.; Chen, J.-Z. Application of atmospheric-pressure plasma jet processed carbon nanotubes to liquid and quasi-solid-state gel electrolyte supercapacitors. Appl. Surf. Sci. 2017, 425, 321–328. [Google Scholar] [CrossRef]
- Wan, T.-H.; Chiu, Y.-F.; Chen, C.-W.; Hsu, C.-C.; Cheng, I.-C.; Chen, J.-Z. Atmospheric-pressure plasma jet processed Pt-decorated reduced graphene oxides for counter-electrodes of dye-sensitized solar cells. Coatings 2016, 6, 44. [Google Scholar] [CrossRef]
- Chen, J.-Z.; Hsu, C.-C.; Wang, C.; Liao, W.-Y.; Wu, C.-H.; Wu, T.-J.; Liu, H.-W.; Chang, H.; Lien, S.-T.; Li, H.-C. Rapid atmospheric-pressure-plasma-jet processed porous materials for energy harvesting and storage devices. Coatings 2015, 5, 26–38. [Google Scholar] [CrossRef]
- Liu, L.; Ye, D.; Yu, Y.; Liu, L.; Wu, Y. Carbon-based flexible micro-supercapacitor fabrication via mask-free ambient micro-plasma-jet etching. Carbon 2017, 111, 121–127. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Babayan, S.E.; Tu, V.J.; Park, J.; Henins, I.; Hicks, R.F.; Selwyn, G.S. Etching materials with an atmospheric-pressure plasma jet. Plasma Sources Sci. Technol. 1998, 7, 282–285. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Babayan, S.E.; Schutze, A.; Tu, V.J.; Park, J.; Henins, I.; Selwyn, G.S.; Hicks, R.F. Etching polyimide with a nonequilibrium atmospheric-pressure plasma jet. J. Vac. Sci. Technol. A 1999, 17, 2581–2585. [Google Scholar] [CrossRef]
- Surowsky, B.; Schluter, O.; Knorr, D. Interactions of non-thermal atmospheric pressure plasma with solid and liquid food systems: A review. Food Eng. Rev. 2015, 7, 82–108. [Google Scholar] [CrossRef]
- Misra, N.N.; Schluter, O.; Cullen, P.J. Cold Plasma in Food and Agriculture: Fundamentals and Applications; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Ramazzina, I.; Tappi, S.; Rocculi, P.; Sacchetti, G.; Berardinelli, A.; Marseglia, A.; Rizzi, F. Effect of cold plasma treatment on the functional properties of fresh-cut apples. J. Agric. Food Chem. 2016, 64, 8010–8018. [Google Scholar] [CrossRef] [PubMed]
- Kusano, Y. Atmospheric pressure plasma processing for polymer adhesion: A review. J. Adhes. 2014, 90, 755–777. [Google Scholar] [CrossRef]
- Homola, T.; Matousek, J.; Medvecka, V.; Zahoranova, A.; Kormunda, M.; Kovacik, D.; Cernak, M. Atmospheric pressure diffuse plasma in ambient air for ITO surface cleaning. Appl. Surf. Sci. 2012, 258, 7135–7139. [Google Scholar] [CrossRef]
- Kim, K.N.; Lee, S.M.; Mishra, A.; Yeom, G.Y. Atmospheric pressure plasmas for surface modification of flexible and printed electronic devices: A review. Thin Solid Films 2016, 598, 315–334. [Google Scholar] [CrossRef]
- Penkov, O.V.; Khadem, M.; Lim, W.S.; Kim, D.E. A review of recent applications of atmospheric pressure plasma jets for materials processing. J. Coat. Technol. Res. 2015, 12, 225–235. [Google Scholar] [CrossRef]
- Li, F.; Song, J.; Yang, H.; Gan, S.; Zhang, Q.; Han, D.; Ivaska, A.; Niu, L. One-step synthesis of graphene/SnO2 nanocomposites and its application in electrochemical supercapacitors. Nanotechnology 2009, 20, 455602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.C.; Zhang, X.; Chen, Y.; Wang, C.H.; Ma, Y.W. An environment-friendly route to synthesize reduced graphene oxide as a supercapacitor electrode material. Electrochim. Acta 2012, 69, 364–370. [Google Scholar] [CrossRef]
- Hu, Z.A.; Xie, Y.L.; Wang, Y.X.; Mo, L.P.; Yang, Y.Y.; Zhang, Z.Y. Polyaniline/SnO2 nanocomposite for supercapacitor applications. Mater. Chem. Phys. 2009, 114, 990–995. [Google Scholar] [CrossRef]
- Lu, X.H.; Zheng, D.Z.; Zhai, T.; Liu, Z.Q.; Huang, Y.Y.; Xie, S.L.; Tong, Y.X. Facile synthesis of large-area manganese oxide nanorod arrays as a high-performance electrochemical supercapacitor. Energy Environ. Sci. 2011, 4, 2915–2921. [Google Scholar] [CrossRef]
- Davies, A.; Audette, P.; Farrow, B.; Hassan, F.; Chen, Z.; Choi, J.-Y.; Yu, A. Graphene-based flexible supercapacitors: Pulse-electropolymerization of polypyrrole on free-standing graphene films. J. Phys. Chem. C 2011, 115, 17612–17620. [Google Scholar] [CrossRef]
- Choi, B.G.; Chang, S.-J.; Kang, H.-W.; Park, C.P.; Kim, H.J.; Hong, W.H.; Lee, S.; Huh, Y.S. High performance of a solid-state flexible asymmetric supercapacitor based on graphene films. Nanoscale 2012, 4, 4983–4988. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liang, J.; Chen, Y. An overview of the applications of graphene-based materials in supercapacitors. Small 2012, 8, 1805–1834. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-S.; Liang, X.-Q.; Song, W.-G.; Wu, Z.-Y. Identification of the nitrogen species on N-doped graphene layers and Pt/NG composite catalyst for direct methanol fuel cell. Phys. Chem. Chem. Phys. 2010, 12, 12055–12059. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Chen, C.-W.; Lin, Y.-K.; Yeh, Y.-C.; Hsu, C.-C.; Fan, Y.-J.; Yu, S.; Chen, J.-Z. Atmospheric-pressure plasma jet processed carbon-based electrochemical sensor integrated with a 3D-printed microfluidic channel. J. Electrochem. Soc. 2017, 164, B534–B541. [Google Scholar] [CrossRef]
- Zhang, H.; Su, H.; Zhang, L.; Zhang, B.; Chun, F.; Chu, X.; He, W.; Yang, W. Flexible supercapacitors with high areal capacitance based on hierarchical carbon tubular nanostructures. J. Power Sources 2016, 331, 332–339. [Google Scholar] [CrossRef]
- Ng, K.C.; Zhang, S.; Peng, C.; Chen, G.Z. Individual and bipolarly stacked asymmetrical aqueous supercapacitors of CNTs/SnO2 and CNTs/MnO2 nanocomposites. J. Electrochem. Soc. 2009, 156, A846–A853. [Google Scholar] [CrossRef]
- Bo, Z.; Zhu, W.; Tu, X.; Yang, Y.; Mao, S.; He, Y.; Chen, J.; Yan, J.; Cen, K. Instantaneous reduction of graphene oxide paper for supercapacitor electrodes with unimpeded liquid permeation. J. Phys. Chem. C 2014, 118, 13493–13502. [Google Scholar] [CrossRef]
- Alotaibi, F.; Tung, T.T.; Nine, M.J.; Kabiri, S.; Moussa, M.; Tran, D.N.H.; Losic, D. Scanning atmospheric plasma for ultrafast reduction of graphene oxide and fabrication of highly conductive graphene films and patterns. Carbon 2018, 127, 113–121. [Google Scholar] [CrossRef]
- Hsu, C.C.; Yang, Y.J. The increase of the jet size of an atmospheric-pressure plasma jet by ambient air control. IEEE Trans. Plasma Sci. 2010, 38, 496–499. [Google Scholar]
- Hsu, Y.-W.; Yang, Y.-J.; Wu, C.-Y.; Hsu, C.-C. Downstream characterization of an atmospheric pressure pulsed arc jet. Plasma Chem. Plasma Process. 2010, 30, 363–372. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Wu, C.-Y. Electrical characterization of the glow-to-arc transition of an atmospheric pressure pulsed arc jet. J. Phys. D Appl. Phys. 2009, 42, 215202. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Wu, C.-Y.; Chen, C.-W.; Cheng, W.-C. Mode transition of an atmospheric pressure arc plasma jet sustained by pulsed DC power. Jpn. J. Appl. Phys. 2009, 48, 076002. [Google Scholar] [CrossRef]
- Kuok, F.-H.; Liao, C.-Y.; Chen, C.-W.; Hao, Y.-C.; Yu, I.-S.; Chen, J.-Z. Screen-printed SnO2/CNT quasi-solid-state gel-electrolyte supercapacitor. Mater Res Express 2017, 4, 115501. [Google Scholar] [CrossRef]
- Wright, A.N.; Wrinkler, C.A. Active Nitrogen; Academic Press: Cambridge, MA, USA, 1968. [Google Scholar]
- Jiang, Z.; Jiang, Z.; Chen, W. The role of holes in improving the performance of nitrogen-doped holey graphene as an active electrode material for supercapacitor and oxygen reduction reaction. J. Power Sources 2014, 251, 55–65. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.; Xu, H.; Xiong, X.; Jiang, Y.; Zou, F.; Hu, X.; Xin, Y.; Zhang, Z.; Huang, Y. Synthesis of functionalized 3D hierarchical porous carbon for high-performance supercapacitors. Energy Environ. Sci. 2013, 6, 2497–2504. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, X.; Chang, J.; Wu, D.; Xu, F.; Zhang, L.; Du, W.; Jiang, K. Graphene incorporated, N doped activated carbon as catalytic electrode in redox active electrolyte mediated supercapacitor. J. Power Sources 2017, 337, 25–35. [Google Scholar] [CrossRef]
- Wan, T.-H.; Lee, C.-C.; Chen, C.-W.; Hsu, C.-C.; Cheng, I.-C.; Chen, J.-Z. A comparison study of furnace and atmospheric-pressure-plasma jet calcined Pt-decorated reduced graphene oxides for dye-sensitized solar cell application. J. Electrochem. Soc. 2017, 164, H931–H935. [Google Scholar] [CrossRef]
- Luo, G.; Liu, L.; Zhang, J.; Li, G.; Wang, B.; Zhao, J. Hole defects and nitrogen doping in graphene: Implication for supercapacitor applications. ACS Appl. Mater. Interfaces 2013, 5, 11184–11193. [Google Scholar] [CrossRef] [PubMed]

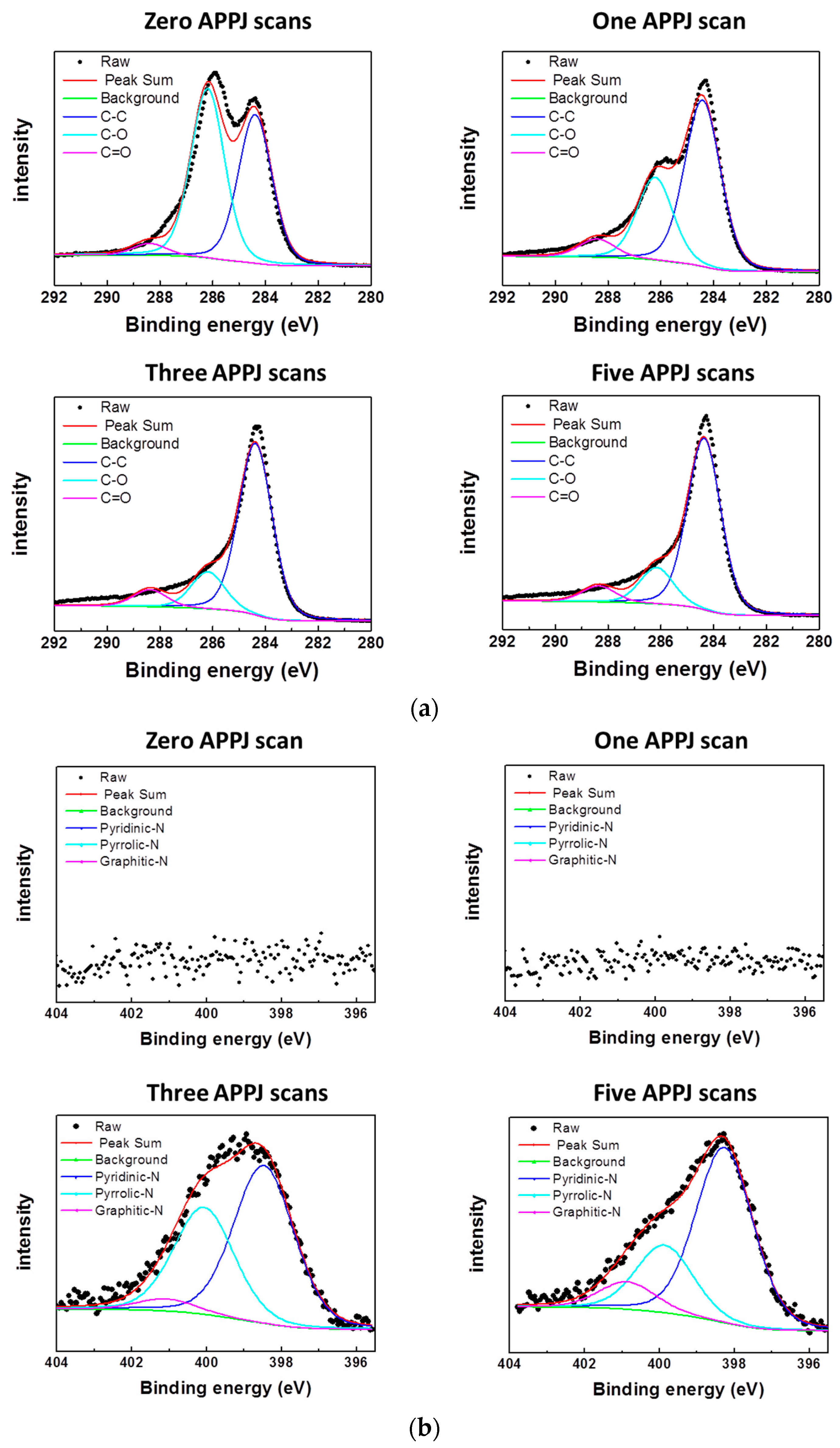
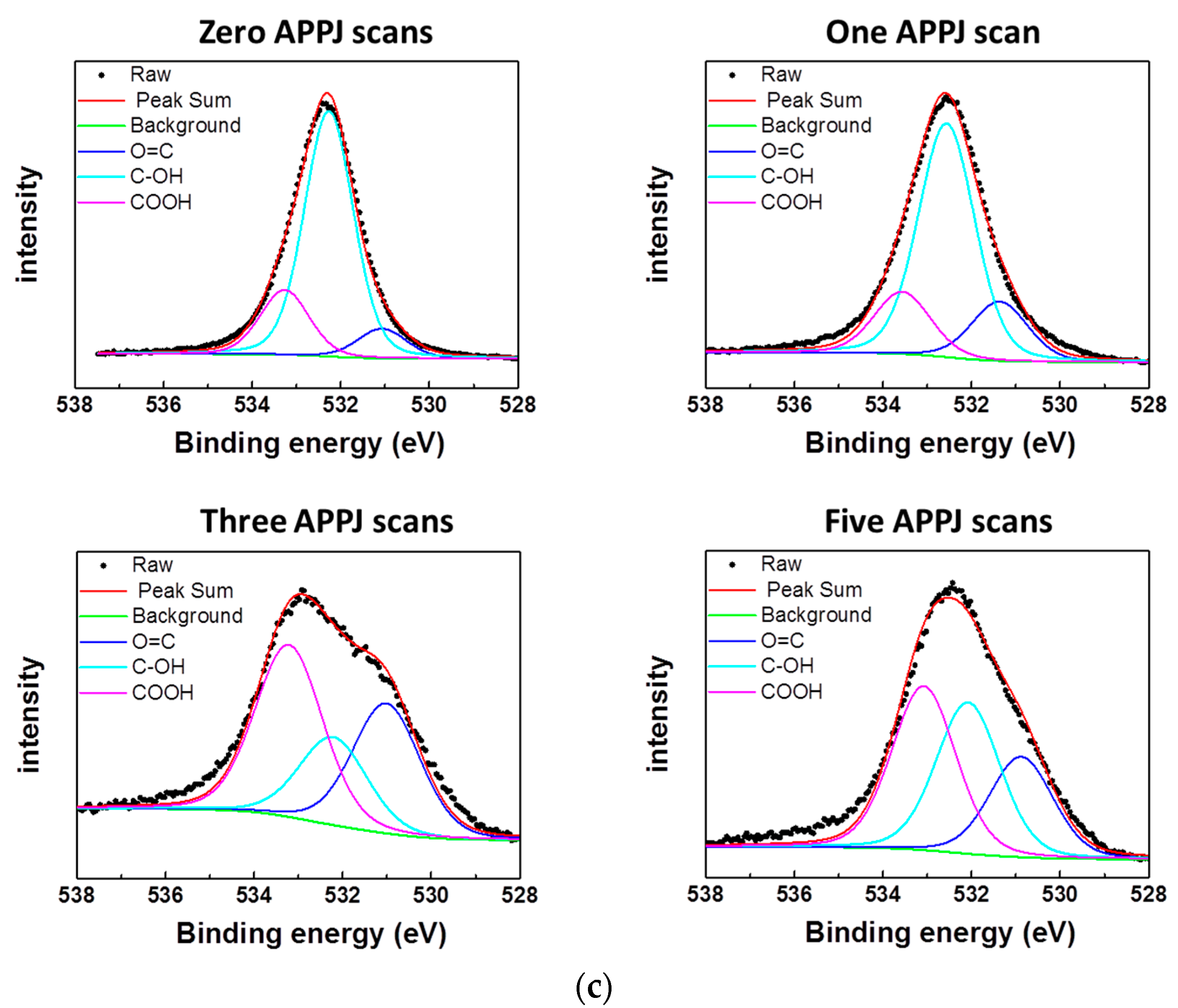
| Bonding Configuration | Bonding Content | |||
|---|---|---|---|---|
| Zero APPJ Scans | One APPJ Scan | Three APPJ Scans | Five APPJ Scans | |
| C 1s | – | – | – | – |
| C–C (284.5 eV) | 45.00% | 59.24% | 70.08% | 70.66% |
| C–O (286.3 eV) | 51.40% | 31.30% | 18.81% | 18.64% |
| C=O (288.5 eV) | 3.60% | 9.46% | 11.11% | 10.70% |
| N 1s | – | – | – | – |
| Pyridinic-N (398.3 eV) | – | – | 54.18% | 62.62% |
| Pyrrolic-N (399.9 eV) | – | – | 38.65% | 25.76% |
| Graphitic-N (400.9 eV) | – | – | 7.17% | 11.62% |
| O 1s | – | – | – | – |
| O=C (531.1 eV) | 8.00% | 16.00% | 33.03% | 24.24% |
| C–OH (532.3 eV) | 73.50% | 67.20% | 23.23% | 36.30% |
| –COOH (533.3 eV) | 18.50% | 16.80% | 43.74% | 39.46% |
| Potential Scan Rate | Zero APPJ Scans | One APPJ Scan | Three APPJ Scans | Five APPJ Scans |
|---|---|---|---|---|
| 2 mV/s | 0.63 mF/cm2 | 0.58 mF/cm2 | 0.65 mF/cm2 | 0.83 mF/cm2 |
| 20 mV/s | 0.47 mF/cm2 | 0.46 mF/cm2 | 0.53 mF/cm2 | 0.68 mF/cm2 |
| 200 mV/s | 0.39 mF/cm2 | 0.40 mF/cm2 | 0.47 mF/cm2 | 0.59 mF/cm2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, A.R.; Chien, H.-H.; Liao, C.-Y.; Lee, C.-C.; Tsai, J.-H.; Hsu, C.-C.; Cheng, I.-C.; Chen, J.-Z. Scan-Mode Atmospheric-Pressure Plasma Jet Processed Reduced Graphene Oxides for Quasi-Solid-State Gel-Electrolyte Supercapacitors. Coatings 2018, 8, 52. https://doi.org/10.3390/coatings8020052
Hsu AR, Chien H-H, Liao C-Y, Lee C-C, Tsai J-H, Hsu C-C, Cheng I-C, Chen J-Z. Scan-Mode Atmospheric-Pressure Plasma Jet Processed Reduced Graphene Oxides for Quasi-Solid-State Gel-Electrolyte Supercapacitors. Coatings. 2018; 8(2):52. https://doi.org/10.3390/coatings8020052
Chicago/Turabian StyleHsu, Aliyah R., Hung-Hua Chien, Chen-Yu Liao, Chia-Chun Lee, Jui-Hsuan Tsai, Cheng-Che Hsu, I-Chun Cheng, and Jian-Zhang Chen. 2018. "Scan-Mode Atmospheric-Pressure Plasma Jet Processed Reduced Graphene Oxides for Quasi-Solid-State Gel-Electrolyte Supercapacitors" Coatings 8, no. 2: 52. https://doi.org/10.3390/coatings8020052
APA StyleHsu, A. R., Chien, H.-H., Liao, C.-Y., Lee, C.-C., Tsai, J.-H., Hsu, C.-C., Cheng, I.-C., & Chen, J.-Z. (2018). Scan-Mode Atmospheric-Pressure Plasma Jet Processed Reduced Graphene Oxides for Quasi-Solid-State Gel-Electrolyte Supercapacitors. Coatings, 8(2), 52. https://doi.org/10.3390/coatings8020052