Modulating the Partitioning of Microparticles in a Polyethylene Glycol (PEG)-Dextran (DEX) Aqueous Biphasic System by Surface Modification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
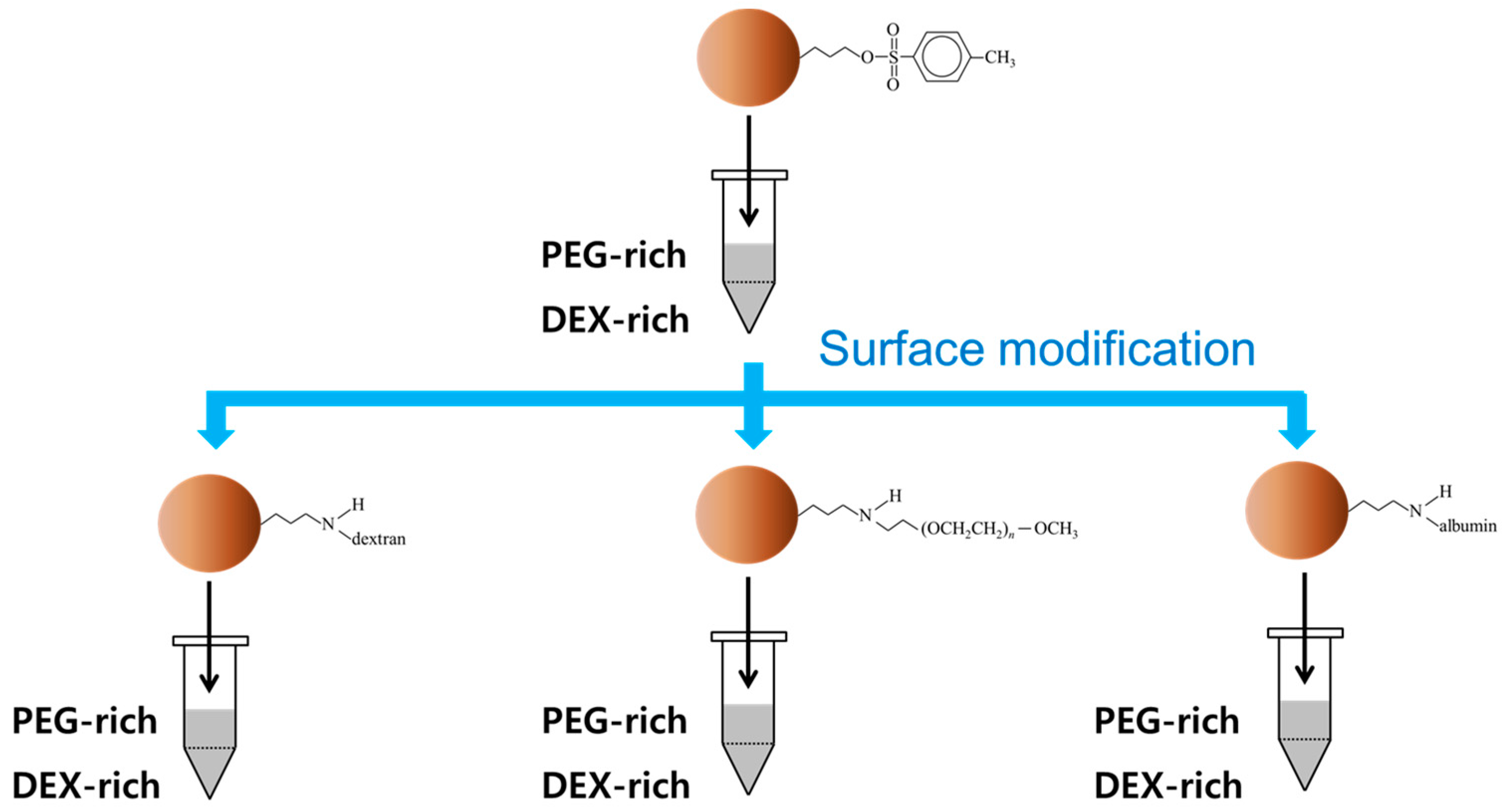
2.2. Preparation of Polymer-Bound Magnetic Particles
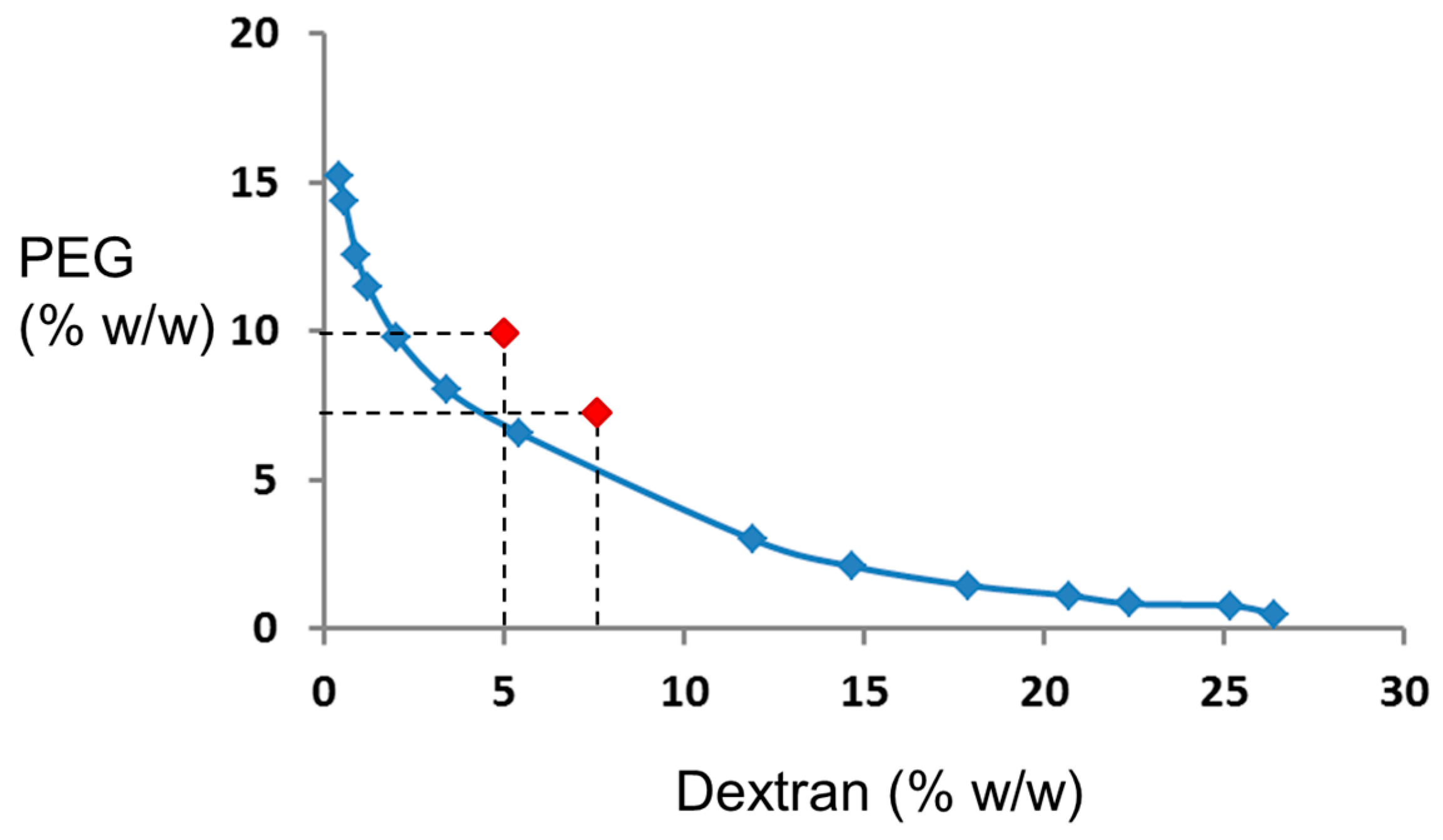
2.3. Preparation of the ATPS Solutions
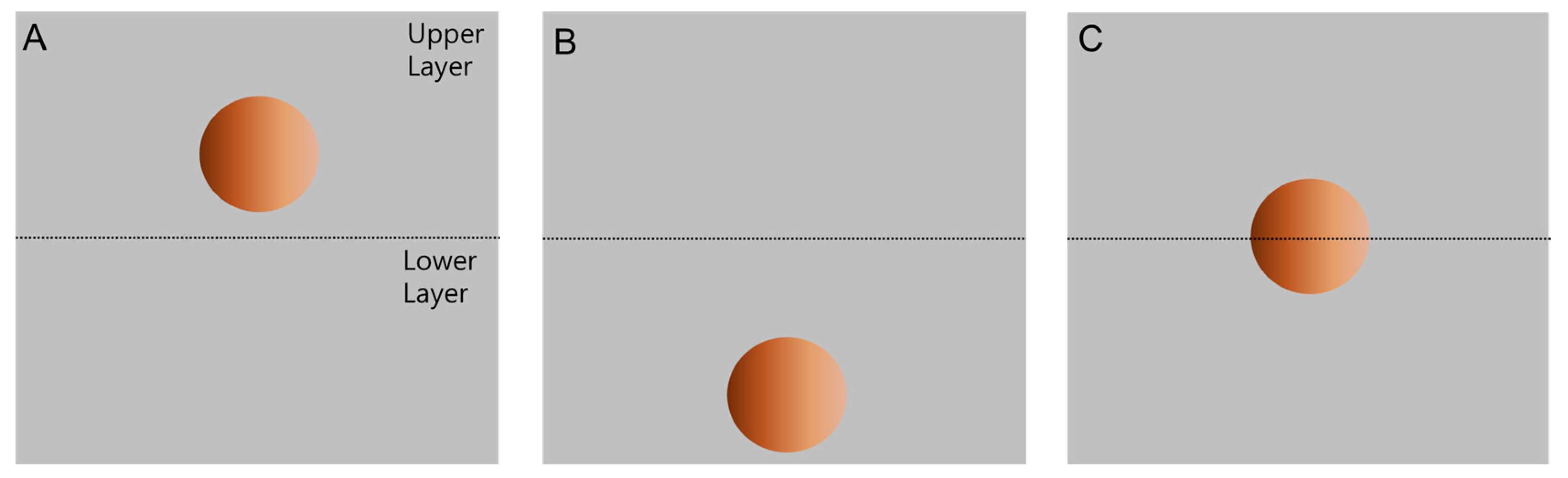
2.4. Partition Observation of Polymer-Bound Magnetic Particles
3. Results
3.1. Theoretical Considerations
3.2. Partitioning of Unmodified PS Microbeads in PEG-DEX ATPS
3.3. Partitioning of Amino-Dextran (aDEX)-Modified Microbeads in PEG-DEX ATPS
3.4. Partitioning of Methoxy-Polyethylene Glycol Amine (mPEG)-Modified Microbeads in PEG-DEX ATPS
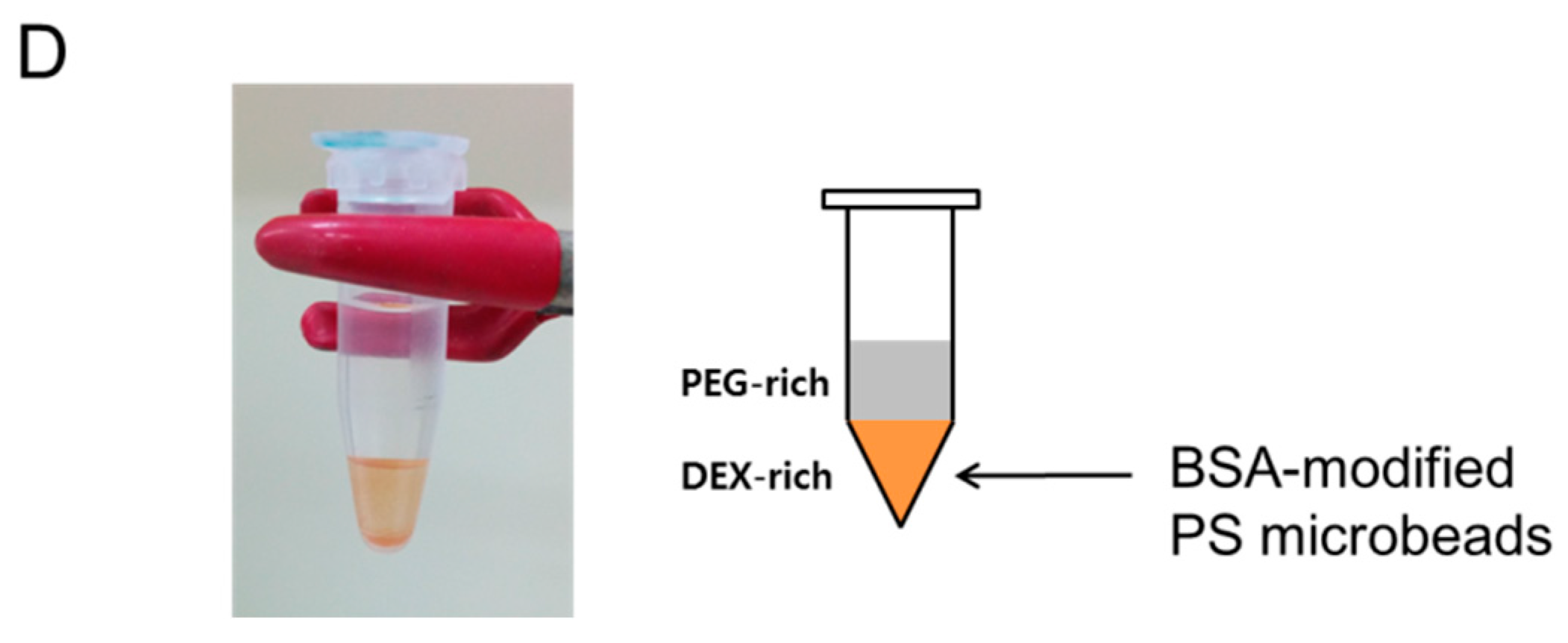
3.5. Partitioning of Bovine Serum Albumin (BSA)-Modified Microbeads in PEG-DEX ATPS
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, P.-C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for physicochemical characterization of nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Holmes, M.; Schaefer, E.; Mulchandani, A.; Ge, X. Highly active spore biocatalyst by self-assembly of co-expressed anchoring scaffoldin and multimeric enzyme. Biotechnol. Bioeng. 2018, 115, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, P.-A. Partition of Cell Particles and Macromolecules, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Byun, C.K.; Hwang, H.; Choi, W.S.; Yaguchi, T.; Park, J.; Kim, D.; Mitchell, R.J.; Kim, T.; Cho, Y.-K.; Takayama, S. Productive chemical interaction between a bacterial microcolony couple is enhanced by periodic relocation. J. Am. Chem. Soc. 2013, 135, 2242–2247. [Google Scholar] [CrossRef] [PubMed]
- Byun, C.K. Investigation of chemical modification on tosyl-activated polystyrene microsphere magnetic particle surface by infrared microscopy. Anal. Sci. Technol. 2016, 29, 225–233. [Google Scholar] [CrossRef]
- Iqbal, M.; Tao, Y.; Xie, S.; Zhu, Y.; Chen, D.; Wang, X.; Huang, L.; Peng, D.; Satter, A.; Shabbir, M.A.B.; et al. Aqueous two-phase system (ATPS): An overview and advances in its applications. Biol. Proced. Online 2016, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Croll, T.; Munro, P.D.; Winzor, D.J.; Trau, M.; Nielsen, L.K. Quantitative prediction of phase diagrams for polymer partitioning in aqueous two-phase systems. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 437–443. [Google Scholar] [CrossRef]
- Amrhein, S.; Schwab, M.-L.; Hoffmann, M.; Hubbuch, J. Characterization of aqueous two phase systems by combining lab-on-a-chip technology with robotic liquid handling stations. J. Chromatogr. A 2014, 1367, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Atefi, E.; Fyffe, D.; Kaylan, K.B.; Tavana, H. Characterization of aqueous two-phase systems from volume and density measurements. J. Chem. Eng. Data 2016, 61, 1531–1539. [Google Scholar] [CrossRef]
- Asenjo, J.A.; Andrews, B.A. Aqueous two-phase systems for protein separation: A perspective. J. Chromatogr. A 2011, 1218, 8826–8835. [Google Scholar] [CrossRef] [PubMed]
- Jauregi, P.; Hoeben, M.A.; van der Lans, R.G.J.; Kwant, G.; van der Wielen, L.A.M. Selective separation of physically near-identical microparticle mixtures by interfacial partitioning. Ind. Eng. Chem. Res. 2001, 40, 5815–5821. [Google Scholar] [CrossRef]
- Cakmak, F.P.; Keating, C.D. Combining catalytic microparticles with droplets formed by phase coexistence: adsorption and activity of natural clays at the aqueous/aqueous interface. Sci. Rep. 2017, 7, 3215. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.F.; Zhang, D.L.; Zhu, L.L.; Long, T.; Chen, J. Applying aqueous biphasic systems for partitioning N-methylimidazolium grafted Merrifield resin microparticles. Solv. Extr. Ion Exch. 2010, 28, 653–664. [Google Scholar] [CrossRef]
- Mace, C.R.; Akbulut, O.; Kumar, A.A.; Shapiro, N.D.; Derda, R.; Patton, M.R.; Whitesides, G.M. Aqueous multiphase systems of polymers and surfactants provide self-assembling step-gradients in density. J. Am. Chem. Soc. 2012, 134, 9094–9097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Hong, R.Y. Synthesis, surface modification and characterization of nanoparticles. In Advances in Nanocomposites—Synthesis, Characterization and Industrial Applications; Reddy, B., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Yu, Y.; Addai-Mensah, J.; Losic, D. Surface modification of diatomaceous earth silica microparticles with functional silanes for metal ions sorption. Chemeca 2010, 38, 379–388. [Google Scholar]
- Bhayani, K.R.; Kale, S.N.; Arora, S.; Rajagopal, R.; Mamgain, H.; Kaul-Ghanckar, R.; Kundaliya, D.C.; Kulkarni, S.D.; Parsricha, R.; Dhole, S.D.; et al. Protein and polymer immobilized La0.7Sr0.3MnO3 nanoparticles for possible biomedical applications. Nanotechnology 2007, 18, 345101. [Google Scholar] [CrossRef]
- Yang, C.; Guan, Y.; Xing, J.; Liu, H. Surface functionalization and characterization of magnetic polystyrene microbeads. Langmuir 2008, 24, 9006–9010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Luijten, E.; Granick, S. Toward design rules of directional Janus colloidal assembly. Annu. Rev. Phys. Chem. 2015, 66, 581–600. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byun, C.K.; Kim, M.; Kim, D. Modulating the Partitioning of Microparticles in a Polyethylene Glycol (PEG)-Dextran (DEX) Aqueous Biphasic System by Surface Modification. Coatings 2018, 8, 85. https://doi.org/10.3390/coatings8030085
Byun CK, Kim M, Kim D. Modulating the Partitioning of Microparticles in a Polyethylene Glycol (PEG)-Dextran (DEX) Aqueous Biphasic System by Surface Modification. Coatings. 2018; 8(3):85. https://doi.org/10.3390/coatings8030085
Chicago/Turabian StyleByun, Chang Kyu, Minkyung Kim, and Daehee Kim. 2018. "Modulating the Partitioning of Microparticles in a Polyethylene Glycol (PEG)-Dextran (DEX) Aqueous Biphasic System by Surface Modification" Coatings 8, no. 3: 85. https://doi.org/10.3390/coatings8030085




