Effect of the N/C Ratios of Ammonia Added to Process Gas Mixtures on the Morphology and Structure of MPCVD Diamond Films
Abstract
:1. Introduction
2. Experiment Details
3. Results and Discussion
3.1. Effect of Nitrogen on the Film Morphology
3.2. AFM Analysis
3.3. X-ray Diffraction
3.4. Raman Spectroscopy
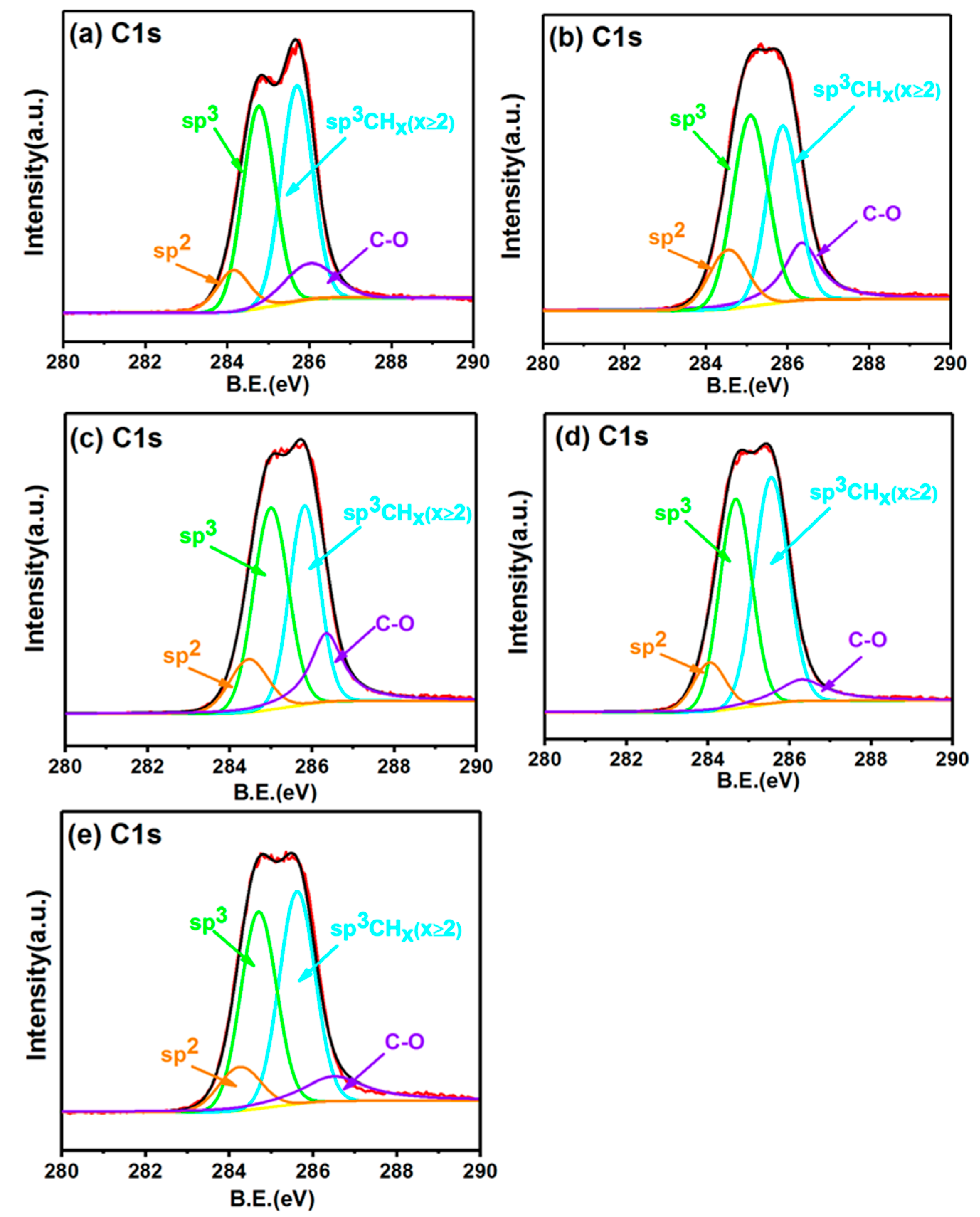
3.5. XPS Analysis
3.6. Discussion
4. Conclusions
- The addition of trace amounts of NH3 to the standard CH4/H2 process gas mixture has a significant effect on the surface morphology and preferred orientation of the resulting crystals.
- Based on the XRD analysis results, ammonia addition to the process gas at a 0.4% to 1% N/C ratio causes an increase in the intensity ratios of the (220) to (111) peaks with [I(220)/I(111)] values from 17.4% to 35.4%, indicating that a certain amount of ammonia addition to the process gas is beneficial for the growth of the (110) faceted grains.
- The XPS spectra analysis indicates that the incorporation of ammonia into the process gas can influence the sp3 fraction of the films. The ammonia addition to the process gas at a 0.4% N/C ratio results in a decrease in the sp3/sp2 ratios from 8.5 to 5.5. However, upon a further increase of the ammonia addition in the N/C ratio from 0.6% to 1%, the sp3/sp2 ratios increase from 5.5 to 9.0, and the amount of the sp3 phase fraction at a 1% N/C ratio exceeds the amount of the sp3 phase fraction of undoped diamond films. This finding indicates that the ammonia addition to the process gas enhances the sp3 fraction in the deposited diamond films.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Okumura, Y.; Kanayama, K.; Nishiguchi, H. Synthesis of n-type semiconducting diamond films in acetylene flame with nitrogen doping. Proc. Combust. Inst. 2017, 36, 4409–4417. [Google Scholar] [CrossRef]
- Nemanič, V.; Žumer, M.; Kovač, J.; Koeck, F.A.M.; Nemanich, R.J. In situ reactivation of low-temperature thermionic electron emission from nitrogen doped diamond films by hydrogen exposure. Diam. Relat. Mater. 2014, 50, 151–156. [Google Scholar] [CrossRef]
- Elfimchev, S.; Chandran, M.; Akhvlediani, R.; Hoffman, A. Visible sub-band gap photoelectron emission from nitrogen doped and undoped polycrystalline diamond films. Appl. Surf. Sci. 2017, 410, 414–422. [Google Scholar] [CrossRef]
- Kudo, Y.; Sato, Y.; Masuzawa, T.; YamadaI, T.; Saito, I.; Yoshino, T.; Chun, W.J.; Yamasaki, S.; Okano, K. Field emission from N-doped diamond doped with dimethylurea. J. Vac. Sci. Technol. B 2009, 28, 506–510. [Google Scholar] [CrossRef]
- Skoog, S.A.; Miller, P.R.; Boehm, R.D.; Sumant, A.V.; Polsky, R.; Narayan, R.J. Nitrogen-incorporated ultrananocrystalline diamond microneedle arrays for electrochemical biosensing. Diam. Relat. Mater. 2015, 54, 39–46. [Google Scholar] [CrossRef]
- Tong, W.; Tran, P.A.; Turnley, A.M.; Aramesh, M.; Prawer, S.; Brandt, M.; Fox, K. The influence of sterilization on nitrogen-included ultrananocrystalline diamond for biomedical applications. Mater. Sci. Eng. C 2016, 61, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Tallaire, A.; Lesik, M.; Jacques, V.; Pezzagna, S.; Mille, V.; Brinza, O.; Meijer, J.; Abel, B.; Roch, J.F.; Gicquel, A.; et al. Temperature dependent creation of nitrogen-vacancy centers in single crystal CVD diamond layers. Diam. Relat. Mater. 2015, 51, 55–60. [Google Scholar] [CrossRef]
- Sebert, W.M.; Wörner, E.; Fuchs, F.; Wild, C.; Koidl, P. Nitrogen induced increase of growth rate in chemical vapor deposition of diamond. Appl. Phys. Lett. 1996, 68, 759–760. [Google Scholar] [CrossRef]
- Liu, T.; Raabe, D. Influence of nitrogen doping on growth rate and texture evolution of chemical vapor deposition diamond films. Appl. Phys. Lett. 2009, 94, 021119. [Google Scholar] [CrossRef]
- Tang, C.J.; Fernandes, A.J.S.; Granada, M.; Leitao, J.P.; Pereira, S.; Jiang, X.F.; Pinto, J.L.; Ye, H. High rate growth of nanocrystalline diamond films using high microwave power and pure nitrogen/methane/hydrogen plasma. Vacuum 2015, 122, 342–346. [Google Scholar] [CrossRef]
- Jin, S.; Moustakas, T.D. Effect of nitrogen on the growth of diamond films. Appl. Phys. Lett. 1994, 65, 403–405. [Google Scholar] [CrossRef]
- Samlenski, R.; Haug, C.; Brenn, R.; Wild, C.; Lecher, R.; Koidl, P. Characterisation and lattice location of nitrogen and boron in homoepitaxial CVD diamond. Diam. Relat. Mater. 1996, 5, 947–951. [Google Scholar] [CrossRef]
- Lecher, R.; Wild, C.; Herres, N.; Behr, D.; Koidl, P. Nitrogen stabilized (100) texture in chemical vapor deposited diamond films. Appl. Phys. Lett. 1994, 65, 34–36. [Google Scholar] [CrossRef]
- Bergmaier, A.; Dollinger, G.; Faestermann, T.; Frey, C.M.; Ferguson, M.; Giittler, H.; Schulz, G.; Willerscheid, H. Detection of nitrogen in CVD diamond. Diam. Relat. Mater. 1996, 5, 995–997. [Google Scholar] [CrossRef]
- Cao, G.Z.; Schermer, J.J.; Van Enckevort, W.J.P.; Elst, W.A.L.M.; Giling, L.J. Growth of {100} textured diamond films by the addition of nitrogen. J. Appl. Phys. 1996, 79, 1357–1364. [Google Scholar] [CrossRef]
- Chatei, H.; Bougdira, J.; Remy, M.; Alnot, P. Mechanisms of diamond films deposition from MPACVD in methane-hydrogen and nitrogen mixtures. Surf. Coat. Technol. 1998, 98, 1013–1019. [Google Scholar] [CrossRef]
- Tang, C.J.; Fernandes, A.J.S.; Costa, F.; Pinto, J.L. Effect of microwave power and nitrogen addition on the formation of (100) faceted diamond from microcrystalline to nanocrystalline. Vacuum 2011, 85, 1130–1134. [Google Scholar] [CrossRef]
- Othman, M.Z.; May, P.W.; Fox, N.A.; Heard, P.J. Incorporation of lithium and nitrogen into CVD diamond thin films. Diam. Relat. Mater. 2014, 44, 1–7. [Google Scholar] [CrossRef]
- Ullah, M.; Ahmed, E.; Welch, K.; Majdi, S.; Khalid, N.R.; Ahmad, M. Growth of Nitrogen-Incorporated Diamond Films Using Hot-Filament Chemical Vapor Deposition Technique. Adv. Sci. Lett. 2013, 19, 291–295. [Google Scholar] [CrossRef]
- Cherf, S.H.; Chandran, M.; Michaelson, S.H.; Elfimchev, S.; Akhvlediani, R.; Hoffman, A. Nitrogen and hydrogen content, morphology and phase composition of hot filament chemical vapor deposited diamond films from NH3/CH4/H2 gas mixtures. Thin Solid Films 2017, 638, 264–268. [Google Scholar] [CrossRef]
- Chatei, H.; Bougdira, J.; Remy, M.; Alnot, P.; Bruch, C.; Krueger, J.K. Effect of nitrogen concentration on plasma reactivity and diamond growth in a H2-CH4-N2 microwave discharge. Diam. Relat. Mater. 1997, 6, 107–119. [Google Scholar] [CrossRef]
- Liu, X.; Yin, Y.; Ren, Y.; Wei, H. Adsorption and evolution behavior of 4C1Si island configurations on diamond (001) surface: A first principle study. J. Alloy. Compd. 2015, 618, 516–521. [Google Scholar] [CrossRef]
- Liu, X.; Qiao, H.M.; Kang, C.J.; Ren, Y.; Tan, X.; Sun, S.Y. Adsorption and migration behavior of Si atoms on the hydrogen-terminated diamond (001) surface: A first principles study. Appl. Surf. Sci. 2017, 420, 542–549. [Google Scholar] [CrossRef]
- Liu, X.; Kang, C.J.; Qiao, H.M.; Ren, Y.; Tan, X.; Sun, S.Y. Theoretical Studies of the Adsorption and Migration Behavior of Boron Atoms on Hydrogen-Terminated Diamond (001) Surface. Coatings 2017, 7, 57. [Google Scholar]
- Liu, X.; Luo, H.; Ren, Y.; Xia, Q.; Li, W.; Tan, X.; Sun, S. Theoretical study of the migration behaviour of Y-C atoms on diamond (001) surface. Diam. Relat. Mater. 2016, 61, 102–108. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Ren, Y.; Luo, H.; Xia, Q.; Tan, X.; Sun, S. Adsorption and migration behaviours of Nb–C atoms on clean diamond (001) surface: A first principles study. Comp. Mater. Sci. 2016, 121, 159–166. [Google Scholar] [CrossRef]
- Liu, X.; Lu, P.; Wang, H.; Ren, Y.; Tan, X.; Sun, S. Morphology and structure of Ti-doped diamond films prepared by microwave plasma chemical vapor deposition. Appl. Surf. Sci. 2018, 442, 529–536. [Google Scholar] [CrossRef]
- Truscott, B.S.; Kelly, M.W.; Potter, K.J.; Johnson, M.; Ashfold, M.N.R. Microwave plasma-activated chemical vapour deposition of nitrogen-doped diamond. I. N2/H2 and NH3/H2 plasmas. J. Phys. Chem. A 2015, 120, 8537–8549. [Google Scholar] [CrossRef] [PubMed]
- Truscott, B.S.; Kelly, M.W.; Potter, K.J.; Ashfold, M.N.R. Microwave plasma-activated chemical vapor deposition of nitrogen-doped diamond. II: CH4/N2/H2 plasmas. J. Phys. Chem. A 2016, 120, 8537–8549. [Google Scholar] [CrossRef] [PubMed]
- May, P.W.; Burridge, P.R.; Rego, C.A.; Tsang, R.S.; Ashfold, M.N.R.; Rosser, K.N.; Tanner, R.E.; Cherns, D.; Vincent, R. Investigation of the addition of nitrogen-containing gases to a hot filament diamond chemical vapour deposition reactor. Diam. Relat. Mater. 1996, 5, 354–358. [Google Scholar] [CrossRef]
- Wang, Y.J.; Li, J.X.; Long, H.Y.; Luo, H.; Zhou, B.; Xie, Y.N.; Li, S.S.; Wei, Q.P.; Yu, Z.M. A periodic magnetic field assisted chemical vapor deposition technique to fabricate diamond film with preferred orientation. Surf. Coat. Technol. 2016, 292, 49–53. [Google Scholar] [CrossRef]
- Tang, C.J.; Neves, A.J.; Pereira, S.; Fernandes, A.J.S.; Grácio, J.; Carmo, M.C. Effect of nitrogen and oxygen addition on morphology and texture of diamond films (from polycrystalline to nanocrystalline). Diam. Relat. Mater. 2008, 17, 72–78. [Google Scholar] [CrossRef]
- Rakha, S.A.; Zhou, X.T.; Zhu, D.Z.; Yu, G.J. Effects of N2 addition on nanocrystalline diamond films by HFCVD in Ar/CH4, gas mixture. Curr. Appl. Phys. 2010, 10, 171–175. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamond like carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Klauser, F.; Steinmüller-Nethl, D.; Kaindl, R.; Bertel, E.; Memmel, N. Raman studies of nano- and ultra-nanocrystalline diamond films grown by hot-filament CVD. Chem. Vap. Depos. 2010, 16, 127–135. [Google Scholar] [CrossRef]
- Vlasov, I.I.; Goovaerts, E.; Ralchenko, V.G.; Konov, V.I.; Khomich, A.V.; Kanzyuba, M.V. Vibrational properties of nitrogen-doped ultrananocrystalline diamond films grown by microwave plasma CVD. Diam. Relat. Mater. 2007, 16, 2074–2077. [Google Scholar] [CrossRef]
- Prawer, S.; Nemanich, R.J. Raman spectroscopy of diamond and doped diamond. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2004, 362, 2537–2565. [Google Scholar] [CrossRef] [PubMed]
- Stuart, S.-A.; Prawer, S.; Weiser, P.S. Growth-sector dependence of fine structure in the first-order Raman diamond line from large isolated chemical-vapor-deposited diamond crystals. Appl. Phys. Lett. 1993, 62, 1227–1229. [Google Scholar] [CrossRef]
- Safaie, P.; Eshaghi, A.; Bakhshi, S.R. Structure and mechanical properties of oxygen doped diamond-like carbon thin films. Diam. Relat. Mater. 2016, 70, 91–97. [Google Scholar] [CrossRef]
- Ferro, S.; Colle, M.D.; Battisti, A.D. Chemical surface characterization of electrochemically and thermally oxidized boron-doped diamond film electrodes. Carbon 2005, 43, 1191–1203. [Google Scholar] [CrossRef]
- Jia, F.; Bai, Y.; Qu, F.; Zhao, J.; Zhuang, C.; Jiang, X. Effect of B/C ratio on the physical properties of highly boron-doped diamond films. Vacuum 2010, 84, 930–934. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Shao, L.; Chen, J.J.; Bao, W.J.; Wang, F.B.; Xia, X.H. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and Its excellent electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, J.H.; Weng, J.; Liu, F. Surface structure and electric properties of nitrogen incorporated NCD films. Vacuum 2017, 137, 155–162. [Google Scholar] [CrossRef]
- Li, Y.L.; Li, J.J.; Xia, X.X.; Lu, C.; Jin, H.; Gu, C.Z. Effect of grain boundary on local surface conductivity of diamond film. J. Appl. Phys. 2009, 105, 013706. [Google Scholar] [CrossRef]
- Regemorter, T.V.; Larsson, K. A Theoretical study of nitrogen-induced effects on initial steps of diamond CVD growth. Chem. Vap. Depos. 2008, 14, 224–231. [Google Scholar] [CrossRef]
- Yiming, Z.; Larsson, F.; Larsson, K. Effect of CVD diamond growth by doping with nitrogen. Theor. Chem. Acc. 2014, 133, 1432. [Google Scholar] [CrossRef]





| Sample | (111) | (220) | Ratio | ||
|---|---|---|---|---|---|
| Position (2θ) | FWHM | Position (2θ) | FWHM | I(220)/I(111) | |
| a | 43.97 | 0.21 | 75.40 | 0.20 | 19.0% |
| b | 43.94 | 0.30 | 75.59 | 0.22 | 17.4% |
| c | 44.00 | 0.28 | 75.49 | 0.19 | 26.1% |
| d | 44.05 | 0.17 | 75.45 | 0.21 | 32.3% |
| e | 44.08 | 0.16 | 75.44 | 0.13 | 35.4% |
| Sample | Diamond Peak | G Band Peak | Ratio | ||||
|---|---|---|---|---|---|---|---|
| Center Position (cm−1) | FWHM | Center Position (cm−1) | FWHM | Center Position (cm−1) | FWHM | In/Id | |
| a | 1334 | 11 | 1527 | 90 | 1620 | 66 | 0.8 |
| b | 1333 | 15 | 1549 | 109 | 1638 | 67 | 7.8 |
| c | 1333 | 11 | 1543 | 115 | 1633 | 67 | 6 |
| d | 1333 | 10 | 1542 | 114 | 1632 | 64 | 2.3 |
| e | 1333 | 9 | 1542 | 110 | 1632 | 62 | 1.2 |
| N/C (%) | sp3/sp2 |
|---|---|
| 0 | 8.5 |
| 0.4 | 5.5 |
| 0.6 | 6.2 |
| 0.8 | 8.4 |
| 1.0 | 9.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, H.; Lu, P.; Ren, Y.; Tan, X.; Sun, S.; Jia, H. Effect of the N/C Ratios of Ammonia Added to Process Gas Mixtures on the Morphology and Structure of MPCVD Diamond Films. Coatings 2018, 8, 163. https://doi.org/10.3390/coatings8050163
Liu X, Wang H, Lu P, Ren Y, Tan X, Sun S, Jia H. Effect of the N/C Ratios of Ammonia Added to Process Gas Mixtures on the Morphology and Structure of MPCVD Diamond Films. Coatings. 2018; 8(5):163. https://doi.org/10.3390/coatings8050163
Chicago/Turabian StyleLiu, Xuejie, Hongchao Wang, Pengfei Lu, Yuan Ren, Xin Tan, Shiyang Sun, and Huiling Jia. 2018. "Effect of the N/C Ratios of Ammonia Added to Process Gas Mixtures on the Morphology and Structure of MPCVD Diamond Films" Coatings 8, no. 5: 163. https://doi.org/10.3390/coatings8050163




