Development and Characterization of Anticorrosion and Antifriction Properties for High Performance Polyurethane/Graphene Composite Coatings
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
3. Results and Discussion
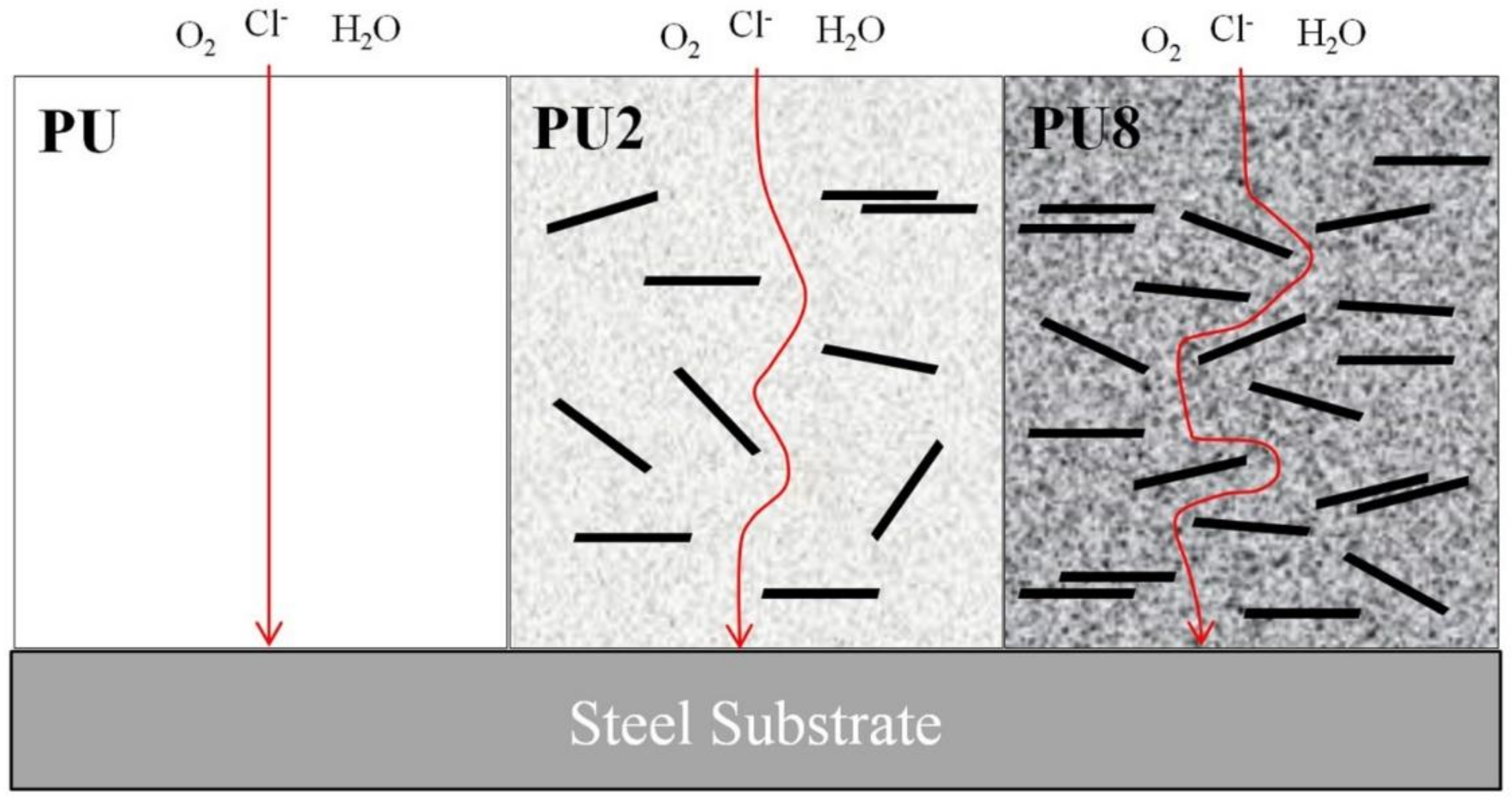
3.1. Morphologies of PU/Gr Composite Coatings
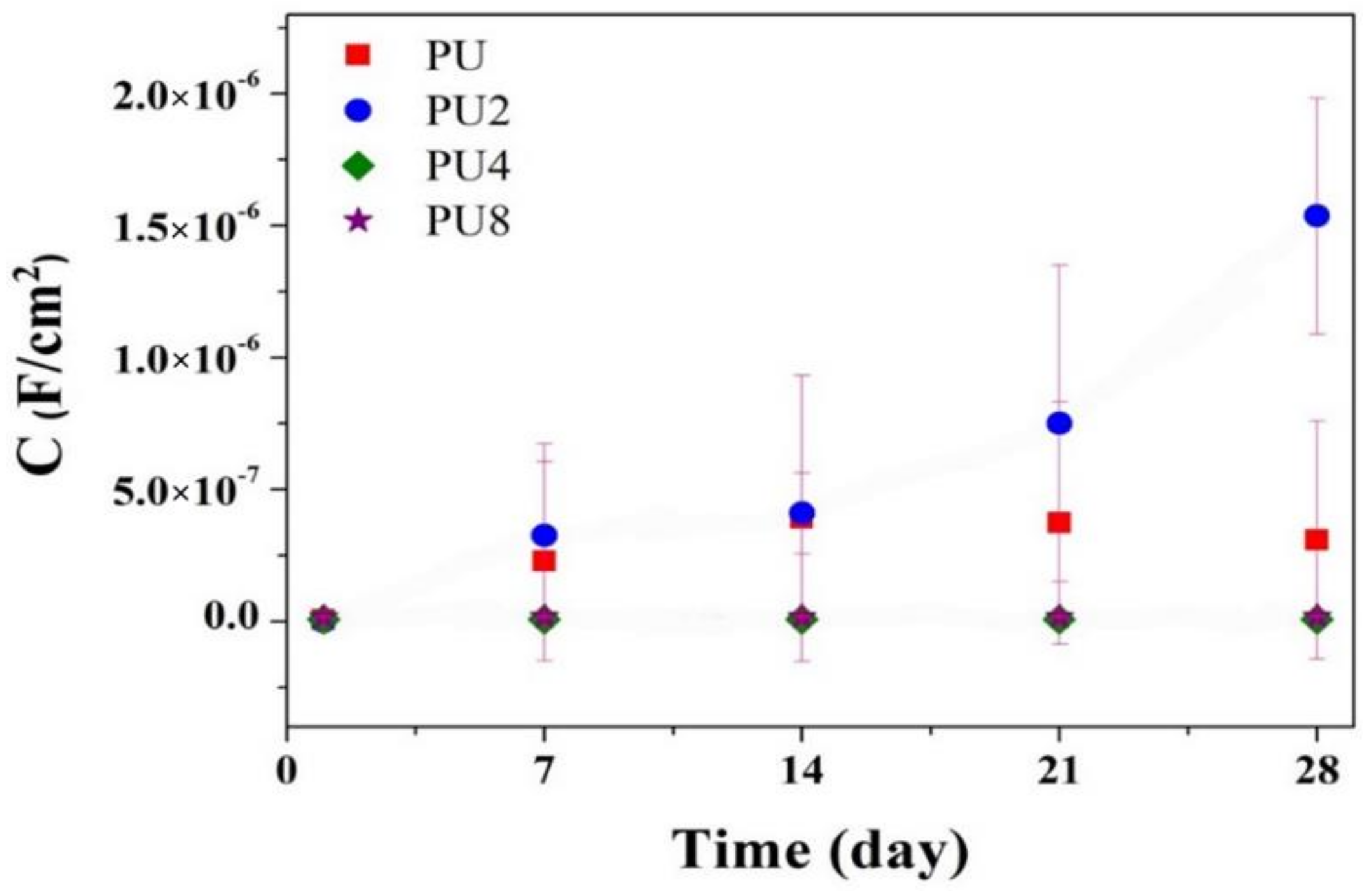
3.2. Corrosion Resistance of PU/Gr Composite Coatings
3.3. SST
3.4. Adhesion of Composite Coatings by the Cross-Cut Tape Test
3.5. Antifriction Properties and COF Analysis
4. Conclusions
- Corrosion results obtained using EIS indicated that the |Z|0.01Hz value, pore resistance and coating capacitance of the PU coatings containing 4 and 8 wt.% of Gr exhibited stable evolution during 28-day immersion in 3.5 wt.% of NaCl solution.
- SST results revealed that the PU4 and PU8 coatings decelerated the corrosion rate and enhanced long-term corrosion protection.
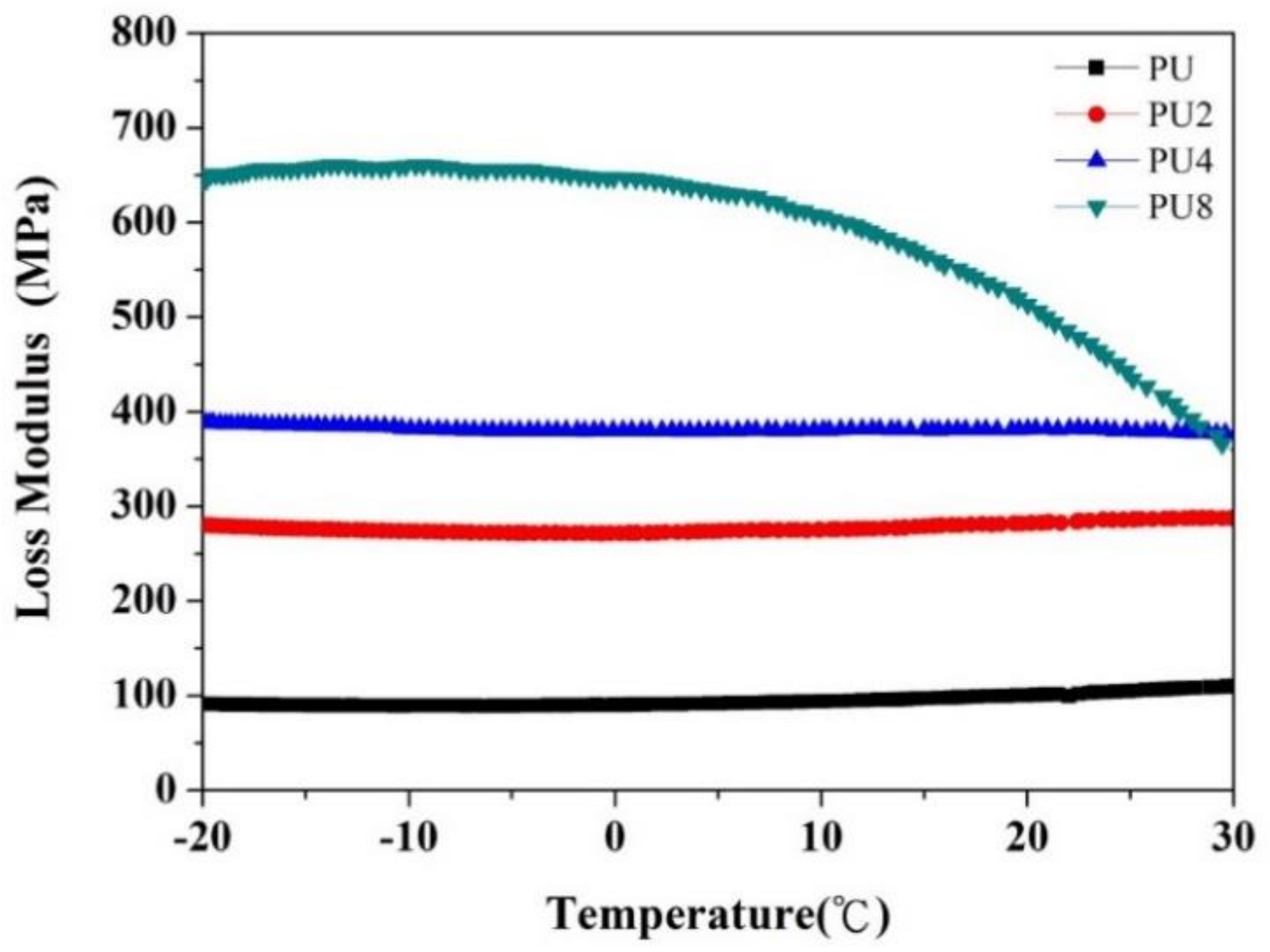
- The loss modulus and thermal conductivity of the PU coating increased with the Gr content owing to the strong frictional energy dissipation effect and the superior thermal conductivity of Gr.
- The addition of Gr to neat PU coating reduced the COF of the PU/Gr composite coatings. Our results indicated that the PU 8 sample had excellent antifriction properties. The COF value of the PU8 coating was 61% lower than that of the neat PU coating.
- Incorporating 8 wt.% Gr into the PU coating led to enhanced anticorrosion properties and this coating exhibited the best antifriction behaviors.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dhoke, S.K.; Khanna, A.S.; Sinha, T.J.M. Effect of nano-ZnO particles on the corrosion behavior of alkyd-based waterborne coatings. Prog. Org. Coat. 2009, 64, 371–382. [Google Scholar] [CrossRef]
- Shi, X.; Nguyen, T.A.; Suo, Z.; Liu, Y.; Avci, R. Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating. Surf. Coat. Technol. 2009, 204, 237–245. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; Du, Y. Effect of nano-sized titanium powder addition on corrosion performance of epoxy coatings. Surf. Coat. Technol. 2007, 201, 7241–7245. [Google Scholar] [CrossRef]
- Shao, Y.; Jia, C.; Meng, G. The role of a zinc phosphate pigment in the corrosion of scratched epoxy-coated steel. Corros. Sci. 2009, 51, 371–379. [Google Scholar] [CrossRef]
- Sababi, M.; Pan, J.; Augustsson, P.-E.; Sundell, P.-E.; Claesson, P.M. Influence of polyaniline and ceria nanoparticle additives on corrosion protection of a UV-cure coating on carbon steel. Corros. Sci. 2014, 84, 189–197. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, S.; Chen, M.; Zhang, W.; Mao, J.; Zhao, Y.; Maitz, M.F.; Huang, N.; Wan, G. Sandwiched polydopamine (PDA) layer for titanium dioxide (TiO2) coating on magnesium to enhance corrosion protection. Corros. Sci. 2015, 96, 67–73. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Niroumandrad, S.; Ahmadi, A.; Mahdavian, M.; Moghadam, M.M. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]
- Sun, W.; Wang, L.; Wu, T.; Pan, Y.; Liu, G. Communication—Multi-Layer Boron Nitride Nanosheets as Corrosion-Protective Coating Fillers. J. Electrochem. Soc. 2016, 163, C16–C18. [Google Scholar] [CrossRef]
- Niroumandrada, S.; Rostamib, M.; Ramezanzadeh, B. Effects of combined surface treatments of aluminium nanoparticle on its corrosion resistance before and after inclusion into an epoxy coating. Prog. Org. Coat. 2016, 101, 486–501. [Google Scholar] [CrossRef]
- Yeh, J.M.; Yao, C.T.; Hsieh, C.F.; Lin, L.H.; Chen, P.L.; Wu, J.C.; Yang, H.C.; Wu, C.P. Preparation, characterization and electrochemical corrosion studies on environmentally friendly waterborne polyurethane/Na+-MMT clay nanocomposite coatings. Eur. Polym. J. 2008, 44, 3046–3056. [Google Scholar] [CrossRef]
- Deyaba, M.A.; Ouarsalb, R.; Al-Sabagha, A.M.; Lachkarb, M.; el Balib, B. Enhancement of corrosion protection performance of epoxy coating by introducing new hydrogenphosphate compound. Prog. Org. Coat. 2017, 107, 37–42. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Chen, J.; Liu, S.; Zhang, G.; Zhao, H.; Wang, L.; Xuea, Q. Anticorrosive performance of waterborne epoxy coatings containing water-dispersible hexagonal boron nitride (h-BN) nanosheets. Appl. Surf. Sci. 2017, 397, 77–86. [Google Scholar] [CrossRef]
- Caldona, E.B.; de Leon, A.C.C.; Pajarito, B.B.; Advincula, R.C. Novel anti-corrosion coatings from rubber-modified polybenzoxazine-based polyaniline composites. Appl. Surf. Sci. 2017, 422, 162–171. [Google Scholar] [CrossRef]
- Gharagozloua, M.; Ramezanzadehb, B.; Baradarana, Z. Synthesize and characterization of a novel anticorrosive cobalt ferrite nanoparticles dispersed in silica matrix (CoFe2O4-SiO2) to improve the corrosion protection performance of epoxy coating. Appl. Surf. Sci. 2016, 377, 86–98. [Google Scholar] [CrossRef]
- Palimi, M.J.; Alibakhshi, E.; Bahlakeh, G.; Ramezanzadeh, B.; Mahdavian, M. Electrochemical Investigations of the Corrosion Protection Properties of an Epoxy-Ester Coating Filled with Cerium Acetyl Acetonate Anticorrosive Pigment. J. Electrochem. Soc. 2017, 164, C709–C716. [Google Scholar] [CrossRef]
- Song, D.; Yin, Z.; Liu, F.; Wan, H.; Gao, J.; Zhang, D.; Li, X. Effect of carbon nanotubes on the corrosion resistance of water-borne acrylic coatings. Prog. Org. Coat. 2017, 110, 182–186. [Google Scholar] [CrossRef]
- Shen, W.; Feng, L.; Liu, X.; Luo, H.; Liu, Z.; Tong, P.; Zhang, W. Multiwall carbon nanotubes-reinforced epoxy hybrid coatings with high electrical conductivity and corrosion resistance prepared via electrostatic spraying. Prog. Org. Coat. 2016, 90, 139–146. [Google Scholar] [CrossRef]
- Deyab, M.A. Effect of carbon nano-tubes on the corrosion resistance of alkyd coating immersed in sodium chloride solution. Prog. Org. Coat. 2015, 85, 146–150. [Google Scholar] [CrossRef]
- Yu, Y.H.; Lin, Y.Y.; Lin, C.H.; Chana, C.C.; Huang, Y.C. High-performance polystyrene/graphene-based nanocomposites with excellent anti-corrosion properties. Polym. Chem. 2014, 5, 535–550. [Google Scholar] [CrossRef]
- Chang, C.H.; Huang, T.C.; Peng, C.W.; Yeh, T.C.; Lu, H.I.; Hung, W.I.; Weng, C.J.; Yang, T.I.; Yeh, J.M. Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 2012, 50, 5044–5051. [Google Scholar] [CrossRef]
- Chang, K.C.; Ji, W.F.; Lai, M.C.; Hsiao, Y.R.; Hsu, C.H.; Chuang, T.L.; Wei, H.; Yeh, J.M.; Liu, W.R. Synergistic effects of hydrophobicity and gas barrier properties on the anticorrosion property of PMMA nanocomposite coatings embedded with graphene nanosheets. Polym. Chem. 2014, 5, 1049–1056. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Qiu, H.; Dai, Y.; Zheng, Q.; Li, J.; Yang, J. Self-aligned graphene as anticorrosive barrier in waterborne polyurethane composite coatings. J. Mater. Chem. A 2014, 2, 14139–14145. [Google Scholar] [CrossRef]
- Hayatgheib, Y.; Ramezanzadeh, B.; Kardar, P.; Mahdavian, M. A comparative study on fabrication of a highly effective corrosion protective system based on graphene oxide-polyaniline nanofibers/epoxy composite. Corros. Sci. 2018, 133, 358–373. [Google Scholar] [CrossRef]
- Liu, S.; Gu, L.; Zhao, H.; Chen, J.; Yu, H. Corrosion Resistance of Graphene-Reinforced Waterborne Epoxy Coatings. J. Mater. Sci. Technol. 2016, 32, 425–431. [Google Scholar] [CrossRef]
- Li, J.; Cui, J.; Yang, J.; Li, Y.; Qiu, H.; Yang, J. Reinforcement of graphene and its derivatives on the anticorrosive properties of waterborne polyurethane coatings. Compos. Sci. Technol. 2016, 129, 30–37. [Google Scholar] [CrossRef]
- Li, J.; Cui, J.; Yang, J.; Ma, Y.; Qiu, H.; Yang, J. Silanized graphene oxide reinforced organofunctional silane composite coatings for corrosion protection. Prog. Org. Coat. 2016, 99, 443–451. [Google Scholar] [CrossRef]
- Yu, Z.; Di, H.; Ma, Y.; Pan, L.L.Y.; Zhang, C.; He, Y. Fabrication of graphene oxide–alumina hybrids to reinforce the anti-corrosion performance of composite epoxy coatings. Appl. Surf. Sci. 2015, 351, 986–996. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Distinctive roles of silane coupling agents on the corrosion inhibition performance of graphene oxide in epoxy coatings. Prog. Org. Coat. 2017, 111, 47–56. [Google Scholar] [CrossRef]
- Pourhashem, S.; Rashidi, A.; Vaezi, M.R.; Bagherzadeh, M.R. Excellent corrosion protection performance of epoxy composite coatings filled with amino-silane functionalized graphene oxide. Surf. Coat. Technol. 2017, 317, 1–9. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A. Investigating the effect of SiO2-graphene oxide hybrid as inorganic nanofiller on corrosion protection properties of epoxy coatings. Surf. Coat. Technol. 2017, 311, 282–294. [Google Scholar] [CrossRef]
- Xue, B.; Yu, M.; Liu, J.; Li, S.; Xiong, L.; Kong, X. Corrosion Protective Properties of Silane Functionalized Graphene Oxide Film on AA2024-T3 Aluminum Alloy. J. Electrochem. Soc. 2016, 163, C798–C806. [Google Scholar] [CrossRef]
- Zhou, P.; Li, W.; Zhu, X.; Li, Y.; Jin, X.; Chen, J. Graphene Containing Composite Coatings as a Protective Coatings against Hydrogen Embrittlement in Quenching & Partitioning High Strength Steel. J. Electrochem. Soc. 2016, 163, D160–D166. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, Q.; Zhou, Q. Corrosion behavior of Cu during graphene growth by CVD. Corros. Sci. 2014, 89, 214–219. [Google Scholar] [CrossRef]
- Yoo, B.M.; Shin, H.J.; Yoon, H.W.; Park, H.B. Graphene and graphene oxide and their uses in barrier polymers. J. Appl. Polym. Sci. 2014, 131, 39628. [Google Scholar] [CrossRef]
- Xiong, J.; Zheng, Z.; Qin, X.; Li, M.; Li, H.; Wang, X. The thermal and mechanical properties of a polyurethane/multi-walled carbon nanotube composite. Carbon 2006, 44, 2701–2707. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Ren, Z.; Chou, T.-W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef]
- Khun, N.W.; Frankel, G.S. Cathodic delamination of polyurethane/multiwalled carbon nanotube composite coatings from steel substrates. Prog. Org. Coat. 2016, 99, 55–60. [Google Scholar] [CrossRef]
- Mo, M.; Zhao, W.; Chen, Z.; Yu, Q.; Zeng, Z.; Wu, X.; Xue, Q. Excellent tribological and anti-corrosion performance of polyurethane composite coatings reinforced with functionalized graphene and graphene oxide nanosheets. RSC Adv. 2015, 5, 56486–56497. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, W.; Liu, S.; Cen, Q.; Xue, Q. Comparative tribological and corrosion resistance properties of epoxy composite coatings reinforced with functionalized fullerene C60 and grapheme. Surf. Coat. Technol. 2016, 286, 354–364. [Google Scholar] [CrossRef]
- Chen, C.; Qiu, S.; Cui, M.; Qin, S.; Yan, G.; Zhao, H.; Wang, L.; Xue, Q. Achieving high performance corrosion and wear resistant epoxy coatings via incorporation of noncovalent functionalized grapheme. Carbon 2017, 114, 356–366. [Google Scholar] [CrossRef]
- Bandeira, P.; Monteiro, J.; Baptista, A.M.; Magalhaes, F.D. Influence of oxidized graphene nanoplatelets and [DMIM][NTf2] ionic liquid on the tribological performance of an epoxy-PTFE coating. Tribol. Int. 2016, 97, 478–489. [Google Scholar] [CrossRef]
- Xia, S.; Liu, Y.; Pei, F.; Zhang, L.; Gao, Q.; Zou, W.; Peng, J.; Cao, S. Identical steady tribological performance of graphene-oxide-strengthened polyurethane/epoxy interpenetrating polymer networks derived from graphene nanosheet. Polymer 2015, 64, 62–68. [Google Scholar] [CrossRef]
- Li, G.; Feng, L.; Tong, P.; Zhai, Z. The properties of MWCNT/polyurethane conductive composite coating prepared by electrostatic spraying. Prog. Org. Coat. 2016, 90, 284–290. [Google Scholar] [CrossRef]
- Park, C.; Ounaies, Z.; Watson, K.A.; Crooks, R.E.; Smith, J., Jr.; Lowther, S.E.; Connell, J.W.; Siochi, E.J.; Harrison, J.S.; St Clair, T.L. Dispersion of single wall carbon nanotubes by in situ polymerization under sonication. Chem. Phys. Lett. 2002, 364, 303–308. [Google Scholar] [CrossRef]
- ASTM International. ASTM Standard, B117-03, Standard Practice for Operating Salt Spray (Fog) Apparatus; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar] [CrossRef]
- McCafferty, E. Introduction to Corrosion Science; Springer: New York, NY, USA, 2010. [Google Scholar]
- Amirudin, A.; Thierry, D. Application of electrochemical impedance spectroscopy to study the degradation of polymer-coated metals. Prog. Org. Coat. 1995, 26, 1–28. [Google Scholar] [CrossRef]
- Li, J.; Gan, L.; Liu, Y.; Mateti, S.; Lei, W.; Chen, Y.; Yang, J. Boron nitride nanosheets reinforced waterborne polyurethane coatings for improving corrosion resistance and antifriction properties. Eur. Polym. J. 2018, 104, 57–63. [Google Scholar] [CrossRef]
- Potvin, E.; Brossard, L.; Larochelle, G. Corrosion protective performances of commercial low-VOC epoxy/urethane coatings on hot-rolled 1010 mild steel. Prog. Org. Coat. 1997, 31, 363–373. [Google Scholar] [CrossRef]
- Lee, Y.L.; Luo, X.L.; Hu, S.J.; Li, Y.B.; Buchheit, R. Corrosion Protection Studies of Crude Glycerol-Based Waterborne Polyurethane Coating on Steel Substrate. J. Electrochem. Soc. 2016, 163, C54–C61. [Google Scholar] [CrossRef]
- Hu, J.M.; Zhang, J.Q.; Cao, C.N. Determination of water uptake and diffusion of Cl− ion in epoxy primer on aluminum alloys in NaCl solution by electrochemical impedance spectroscopy. Prog. Org. Coat. 2003, 46, 273–279. [Google Scholar] [CrossRef]
- Deflorian, F.; Fedrizzi, L.; Rossi, S.; Bonora, P.L. Organic coating capacitance measurement by EIS: Ideal and actual trends. Electrochim. Acta 1999, 44, 4243–4249. [Google Scholar] [CrossRef]
- Wong, F.; Buchheit, R.G. Utilizing the structural memory effect of layered double hydroxides for sensing water uptake in organic coatings. Prog. Org. Coat. 2004, 51, 91–102. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Carneiro, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Influence of sol-gel process parameters on the protection properties of sol–gel coatings applied on AA2024. Surf. Coat. Technol. 2014, 246, 6–16. [Google Scholar] [CrossRef]
- Ghaffari, M.S.; Naderi, R.; Sayehbani, M. The effect of mixture of mercaptobenzimidazole and zinc phosphate on the corrosion protection of epoxy/polyamide coating. Prog. Org. Coat. 2015, 86, 117–124. [Google Scholar] [CrossRef]
- Mansfeld, F. Use of electrochemical impedance spectroscopy for the study of corrosion protection by polymer coatings. J. Appl. Electrochem. 1995, 25, 187–202. [Google Scholar] [CrossRef]
- Funke, W. The role of adhesion in corrosion protection by organic coatings. J. Oil Colour Chem. Assoc. 1985, 68, 229–232. [Google Scholar]
- Thomas, N.L. The barrier properties of paint coatings. Prog. Org. Coat. 1991, 19, 101–121. [Google Scholar] [CrossRef]
- Bajat, J.B.; Milosev, I.; Jovanovic, Z.; Miskovic-Stankovic, V.B. Studies on adhesion characteristics and corrosion behaviour of vinyltriethoxysilane/epoxy coating protective system on aluminium. Appl. Surf. Sci. 2010, 256, 3508–3517. [Google Scholar] [CrossRef]
- Lyon, S.B.; Bingham, R.; Mills, D.J. Advances in corrosion protection by organic coatings: What we know and what we would like to know. Prog. Org. Coat. 2017, 102, 2–7. [Google Scholar] [CrossRef] [Green Version]
- ASTM International. ASTM Standard, D3359, Standard Test Methods for Measuring Adhesion by Tape Test; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar] [CrossRef]
- Myshkin, N.K.; Petrokovet, M.I.; Kovalev, A.V. Tribology of polymers: Adhesion, friction, wear, and mass-transfer. Tribol. Int. 2005, 38, 910–921. [Google Scholar] [CrossRef]
- Gu, J.; Wu, G.; Zhang, Q. Effect of porosity on the damping properties of modified epoxy composites filled with fly ash. Scr. Mater. 2007, 57, 529–532. [Google Scholar] [CrossRef]
- Suhr, J.; Koratkar, N.; Keblinski, P.; Ajayan, P. Viscoelasticity in carbon nanotube composites. Nat. Mater. 2005, 4, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.; Cho, G. Sound absorption and viscoelastic property of acoustical automotive nonwovens and their plasma treatment. Fibers Polym. 2010, 11, 782–789. [Google Scholar] [CrossRef]
- Qiu, M.; Zhang, Y.Z.; Bao, S.; Du, S.M.; Yan, Z.W. The relationships between tribological behaviour and heat-transfer capability of Ti6Al4V alloys. Wear 2007, 263, 653–657. [Google Scholar] [CrossRef]
- Song, P.; Cao, Z.; Cai, Y.; Zhao, L.; Fang, Z.; Fu, S. Fabrication of exfoliated graphene-based polypropylene nanocomposites with enhanced mechanical and thermal properties. Polymer 2011, 52, 4001–4010. [Google Scholar] [CrossRef]







| Coatings | Thermal Conductivity (W·mK−1) |
|---|---|
| PU | 0.226 |
| PU2 | 0.356 |
| PU4 | 0.573 |
| PU8 | 1.151 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, P.-Y.; Chen, T.-E.; Lee, Y.-L. Development and Characterization of Anticorrosion and Antifriction Properties for High Performance Polyurethane/Graphene Composite Coatings. Coatings 2018, 8, 250. https://doi.org/10.3390/coatings8070250
Tsai P-Y, Chen T-E, Lee Y-L. Development and Characterization of Anticorrosion and Antifriction Properties for High Performance Polyurethane/Graphene Composite Coatings. Coatings. 2018; 8(7):250. https://doi.org/10.3390/coatings8070250
Chicago/Turabian StyleTsai, Pei-Ying, Tzu-En Chen, and Yueh-Lien Lee. 2018. "Development and Characterization of Anticorrosion and Antifriction Properties for High Performance Polyurethane/Graphene Composite Coatings" Coatings 8, no. 7: 250. https://doi.org/10.3390/coatings8070250
APA StyleTsai, P.-Y., Chen, T.-E., & Lee, Y.-L. (2018). Development and Characterization of Anticorrosion and Antifriction Properties for High Performance Polyurethane/Graphene Composite Coatings. Coatings, 8(7), 250. https://doi.org/10.3390/coatings8070250





