Corrosion Behavior of Fe/Zr Composite Coating on ZK60 Mg Alloy by Ion Implantation and Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate Pretreatment
2.2. Coating Preparation
2.3. Microstructure Characterization
2.4. Electrochemical Measurements
2.5. Immersion Tests
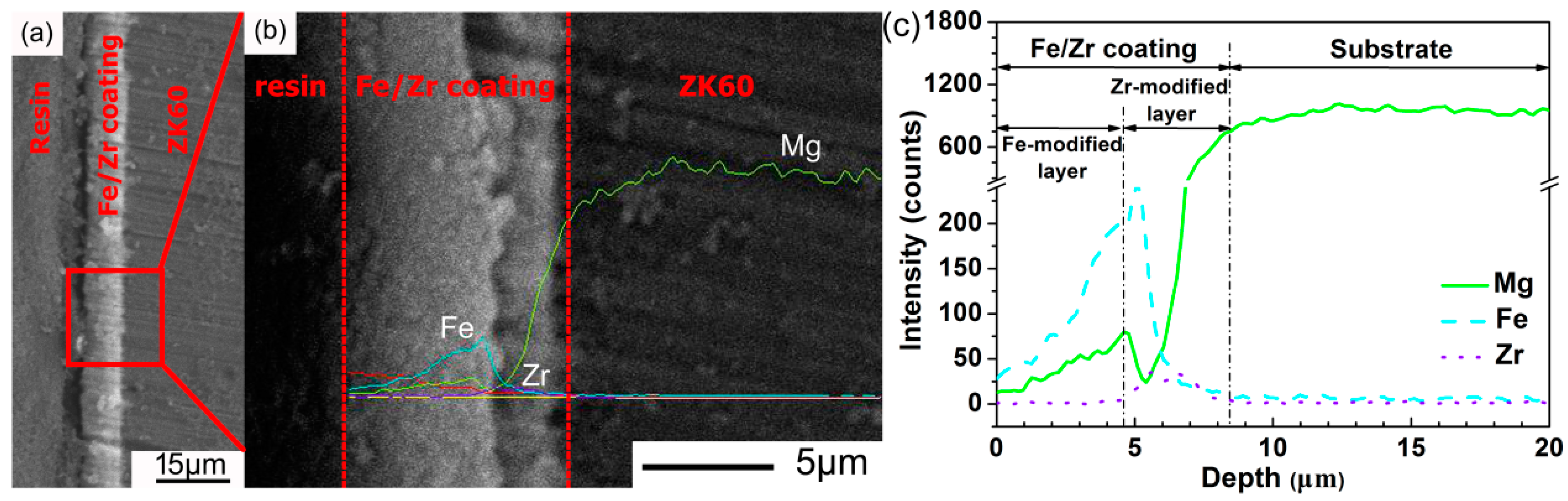
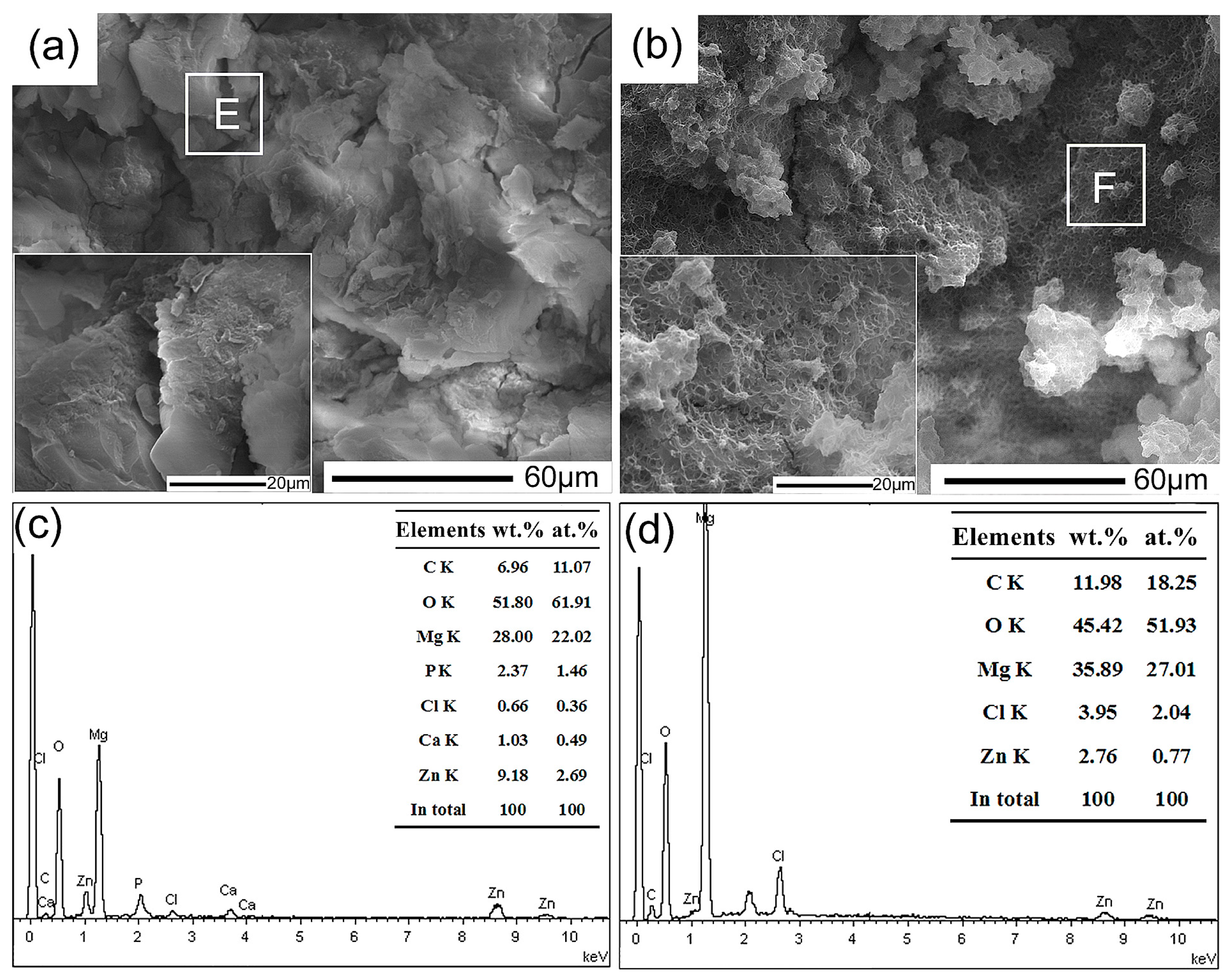
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kraus, T.; Fischerauer, S.; Treichler, S.; Martinelli, E.; Eichler, J.; Myrissa, A.; Zötsch, S.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. The influence of biodegradable magnesium implants on the growth plate. Acta Biomater. 2017, 66, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Im, S.H.; Jung, Y.; Kim, S.H. Current status and future direction of biodegradable metallic and polymeric vascular scaffolds for next-generation stents. Acta Biomater. 2017, 60, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Janning, C.; Willbold, E.; Vogt, C.; Nellesen, J.; Meyer-Lindenberg, A.; Windhagen, H.; Thorey, F.; Witte, F. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodeling. Acta Biomater. 2010, 6, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Person, M.; Torgersen, J.; Berto, F. Mg and its alloys for biomedical applications: Exploring corrosion and its interplay with mechanical failure. Metals 2017, 7, 252. [Google Scholar] [CrossRef]
- Pezzato, L.; Vranescu, D.; Sinico, M.; Gennari, C.; Settimi, A.G.; Pranovi, P.; Brunelli, K.; Dabalà, M. Tribocorrosion properties of PEO coatings produced on AZ91 magnesium alloy with silicate- or phosphate-based electrolytes. Coatings 2018, 8, 202. [Google Scholar] [CrossRef]
- Upadhyay, V.; Bergseth, Z.; Kelly, B.; Battocchi, D. Silica-based sol-gel coating on magnesium alloy with green inhibitors. Coatings 2017, 7, 86. [Google Scholar] [CrossRef]
- Zhu, B.W.; Wang, S.M.; Wang, L.; Yang, Y.; Liang, J.; Cao, B.C. Preparation of hydroxyapatite/tannic acid coating to enhance the corrosion resistance and cytocompatibility of AZ31 magnesium alloys. Coatings 2017, 7, 105. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C 2017, 77, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.F.; Wei, B.B.; Wu, Z.T.; Qi, Z.B.; Wang, Z.C. A comparative study on the corrosion behavior of Al, Ti, Zr and Hf metallic coatings deposited on AZ91D magnesium alloys. Surf. Coat. Technol. 2016, 303, 94–102. [Google Scholar] [CrossRef]
- Jin, W.H.; Wang, G.M.; Lin, Z.J.; Feng, H.Q.; Li, W.; Peng, X.; Qasim, A.M.; Chu, P.K. Corrosion resistance and cytocompatibility of tantalum-surface-functionalized biomedical ZK60 Mg alloy. Corros. Sci. 2016, 114, 45–56. [Google Scholar] [CrossRef]
- Ba, Z.X.; Dong, Q.S.; Yin, J.H.; Wang, J.X.; Ma, B.; Zhang, X.B.; Wang, Z.Z. Surface properties of Mg-Gd-Zn-Zr alloy modified by Sn ion implantation. Mater. Lett. 2016, 190, 90–94. [Google Scholar] [CrossRef]
- Guo, S.F.; Pan, F.S.; Zhang, H.J.; Zhang, D.F.; Wang, J.F.; Miao, J.; Su, C.; Zhang, C. Fe-based amorphous coating for corrosion protection of magnesium alloy. Mater. Des. 2016, 108, 624–631. [Google Scholar] [CrossRef]
- Fu, Z.X.; Chen, X.; Liu, B.; Liu, J.; Han, X.P.; Deng, Y.D.; Hu, W.B.; Zhong, C. One-step fabrication and localized electrochemical characterization of continuous Al-alloyed intermetallic surface layer on magnesium alloy. Coatings 2018, 8, 148. [Google Scholar] [CrossRef]
- Anders, A. Metal plasma immersion ion implantation and deposition: A review. Surf. Coat. Technol. 1997, 93, 158–167. [Google Scholar] [CrossRef]
- Jabbari, Y.S.A.; Fehrman, J.; Barnes, A.C.; Zapf, A.M.; Zinelis, S.; Berzins, D.W. Titanium nitride and nitrogen ion implanted coated dental materials. Coatings 2012, 2, 160–178. [Google Scholar] [CrossRef]
- Liu, C.L.; Xin, Y.C.; Tian, X.B.; Chu, P.K. Corrosion behavior of AZ91 magnesium alloy treated by plasma immersion ion implantation and deposition in artificial physiological fluids. Thin Solid Films 2007, 516, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zheng, Y.; Bi, Y.Z.; Li, Y.; Zheng, Y.F. Improved cytocompatibility of Mg-1Ca alloy modified by Zn ion implantation and deposition. Mater. Lett. 2017, 205, 87–89. [Google Scholar] [CrossRef]
- Schinhammer, M.; Hänzi, A.C.; Löffler, J.F.; Uggowitzer, P.J. Design strategy for biodegradable Fe-based alloys for medical applications. Acta Biomater. 2009, 6, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Jamesh, M.I.; Wu, G.S.; Zhao, Y.; McKenzie, D.R.; Bilek, M.M.M.; Chu, P.K. Effects of zirconium and oxygen plasma ion implantation on the corrosion behavior of ZK60 Mg alloy in simulated body fluids. Corros. Sci. 2014, 82, 7–26. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, L.; Feng, J.; Li, L.; Esmaeili, S.; Zhou, Y. Bonding of immiscible Mg and Fe via a nanoscale Fe2Al5 transition layer. Scr. Mater. 2011, 65, 982–985. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.Z.; Yang, X.B.; Li, P.H.; Suen, K.W.; Wu, G.S.; Chu, P.K. Electrochemical properties and corrosion resistance of carbon-ion-implanted magnesium. Corros. Sci. 2014, 82, 173–179. [Google Scholar] [CrossRef]
- Chen, X.B.; Nisbet, D.R.; Li, R.W.; Smith, P.N.; Abbott, T.B.; Easton, M.A.; Zhang, D.H.; Birbilis, N. Controlling initial biodegradation of magnesium by a biocompatible strontium phosphate conversion coating. Acta Biomater. 2014, 10, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- ASTM Standard G31-72 Standard Practice for Laboratory Immersion Corrosion Testing of Metals; ASTM International: West Conshohocken, PA, USA, 2004.
- Zheng, Y.; Li, Y.; Chen, J.H.; Zou, Z.Y. Effects of tensile and compressive deformation on corrosion behavior of a Mg-Zn alloy. Corros. Sci. 2015, 90, 445–450. [Google Scholar] [CrossRef]
- Li, C.X.; Yu, Y.D. The effect of solution heat treatments on the microstructure and hardness of ZK60 magnesium alloys prepared under low-frequency alternating magnetic fields. Mater. Sci. Eng. A 2013, 559, 22–28. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Y.F. Effects of alloying elements (Mn, Co, Al, W, Sn, B, C and S) on biodegradability and in vitro biocompatibility of pure iron. Acta Biomater. 2011, 7, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.T.; Li, Y.; Zhao, X.Q.; Chen, H.; Zhang, T. Ni ion release, osteoblasts-materials interactions and hemocompatibility of hafnium implanted NiTi alloy. J. Biomed. Mater. Res. B 2012, 100B, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.T.; Li, Y.; Xiang, Y.; Zhao, X.Q.; Zhang, T. Surface characteristics, nano-indentation and corrosion behavior of Nb implanted NiTi alloy. Surf. Coat. Technol. 2011, 205, 4404–4410. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Chen, J.H.; Zou, Z.Y. Surface characteristics and corrosion resistance of ZK60 magnesium alloy modified by Fe ion implantation and deposition. Prog. Nat. Sci. Mater. 2014, 24, 547–553. [Google Scholar] [CrossRef]
- He, W.W.; Zhang, E.L.; Yang, K. Effect of Y on the bio-corrosion behavior of extruded Mg-Zn-Mn alloy in Hank’s solution. Mater. Sci. Eng. C 2010, 30, 167–174. [Google Scholar] [CrossRef]
- Zhang, E.L.; Chen, H.Y.; Shen, F. Biocorrosion properties and blood and cell compatibility of pure iron as a biodegradable biomaterial. J. Mater. Sci. Mater. Med. 2010, 21, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Kasiri-Asgarani, M.; Jabbarzare, S.; Iqbal, N.; Abdul-kadir, M.R. Deposition of nanostructured fluorine-doped hydroxyapatite-polycaprolactone duplex coating to enhance the mechanical properties and corrosion resistance of Mg alloy for biomedical applications. Mater. Sci. Eng. C 2016, 60, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Daroonparvar, M.; Saud, S.N.; Abdul-kadir, M.R. Bi-layer nano-TiO2/FHA composite coatings on Mg-Zn-Ce alloy prepared by combined physical vapour deposition and electrochemical deposition methods. Vacuum 2014, 110, 127–135. [Google Scholar] [CrossRef]
- Jang, Y.; Collins, B.; Sankar, J.; Yun, Y. Effect of biologically relevant ions on the corrosion products formed on alloy AZ31B: An improved understanding of magnesium corrosion. Acta Biomater. 2013, 9, 8761–8770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.X.; Li, J.N.; Song, Y.; Zhao, C.L.; Zhang, X.N.; Xie, C.Y.; Zhang, Y.; Tao, H.R.; He, Y.H.; Jiang, Y.; et al. In vitro degradation, hemolysis and MC3T3-E1 cell adhesion of biodegradable Mg-Zn alloy. Mater. Sci. Eng. C 2009, 29, 1907–1912. [Google Scholar] [CrossRef]








| Source | Implantation Process | Deposition Process | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Voltage (kV) | Current (mA) | Dose (Ions/cm2) | Time (s) | Striking Current (A) | Bias Voltage (V) | Current (mA) | Electric Quantity (mC) | Time (s) | |
| Zr | 10 | 6 | 1 × 1016 | 160 | 150 | −150 | 500 | 6 × 105 | 1200 |
| Fe | 4 | 2 | 2.4 × 1017 | 6000 | 110 | −100 | 500 | 1.6 × 106 | 3200 |
| Sample | Ecorr (V/SCE) | icorr (μA·cm−2) | βc (V·decade−1) |
|---|---|---|---|
| ZK60 | −1.66 ± 0.03 | 112.3 ± 12.2 | −0.17 ± 0.03 |
| Fe-Zr-ZK60 | −1.56 ± 0.02 | 0.34 ± 0.02 | −0.22 ± 0.01 |
| Samples | Area Fraction (%) | ||
|---|---|---|---|
| 1 Day | 3 Days | 7 Days | |
| ZK60 | 9.74 | 4.97 | 2.55 |
| Fe-Zr-ZK60 | 5.85 | 1.98 | 0.97 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Zang, L.; Bi, Y.; Li, Y.; Chen, Y. Corrosion Behavior of Fe/Zr Composite Coating on ZK60 Mg Alloy by Ion Implantation and Deposition. Coatings 2018, 8, 261. https://doi.org/10.3390/coatings8080261
Zheng Y, Zang L, Bi Y, Li Y, Chen Y. Corrosion Behavior of Fe/Zr Composite Coating on ZK60 Mg Alloy by Ion Implantation and Deposition. Coatings. 2018; 8(8):261. https://doi.org/10.3390/coatings8080261
Chicago/Turabian StyleZheng, Yang, Libin Zang, Yanze Bi, Yan Li, and Yong Chen. 2018. "Corrosion Behavior of Fe/Zr Composite Coating on ZK60 Mg Alloy by Ion Implantation and Deposition" Coatings 8, no. 8: 261. https://doi.org/10.3390/coatings8080261





