Assessing of New Coatings for Iron Artifacts Conservation by Recurrence Plots Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
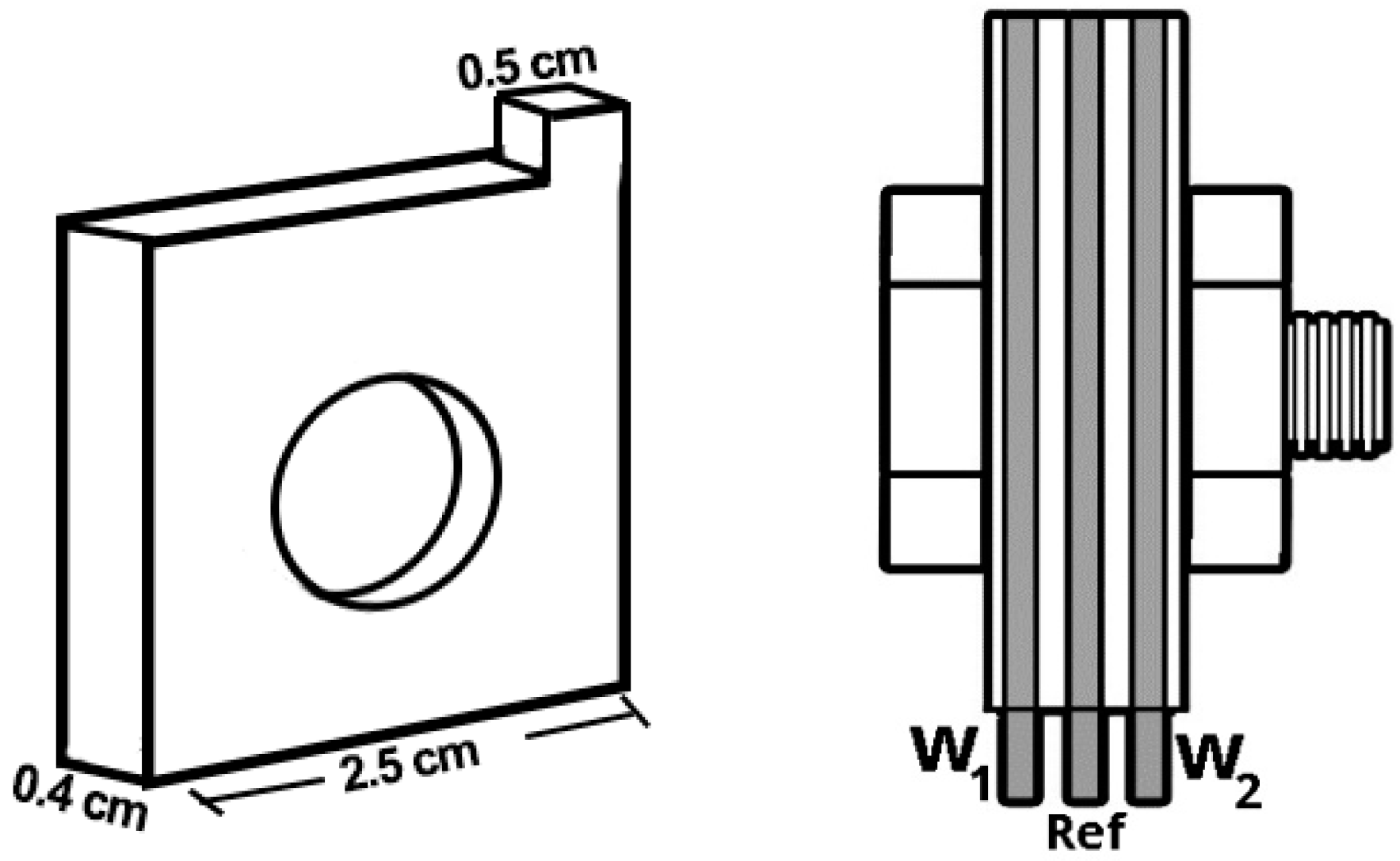
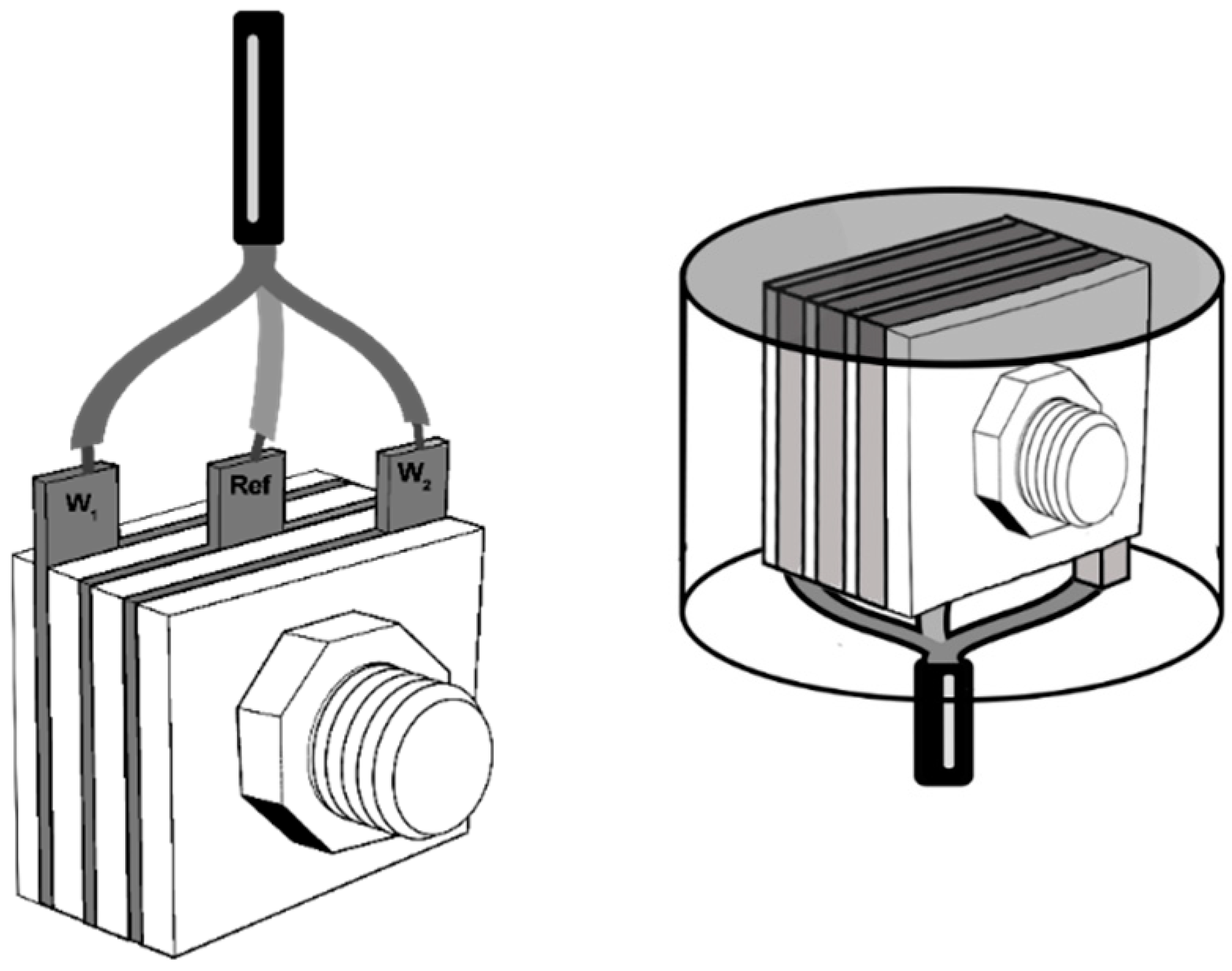
2.2. Sensor Construction (Atmospheric Corrosion Monitor, ACM)
2.3. Relative Humidity
2.4. Electrochemical Noise Measurements
3. Results
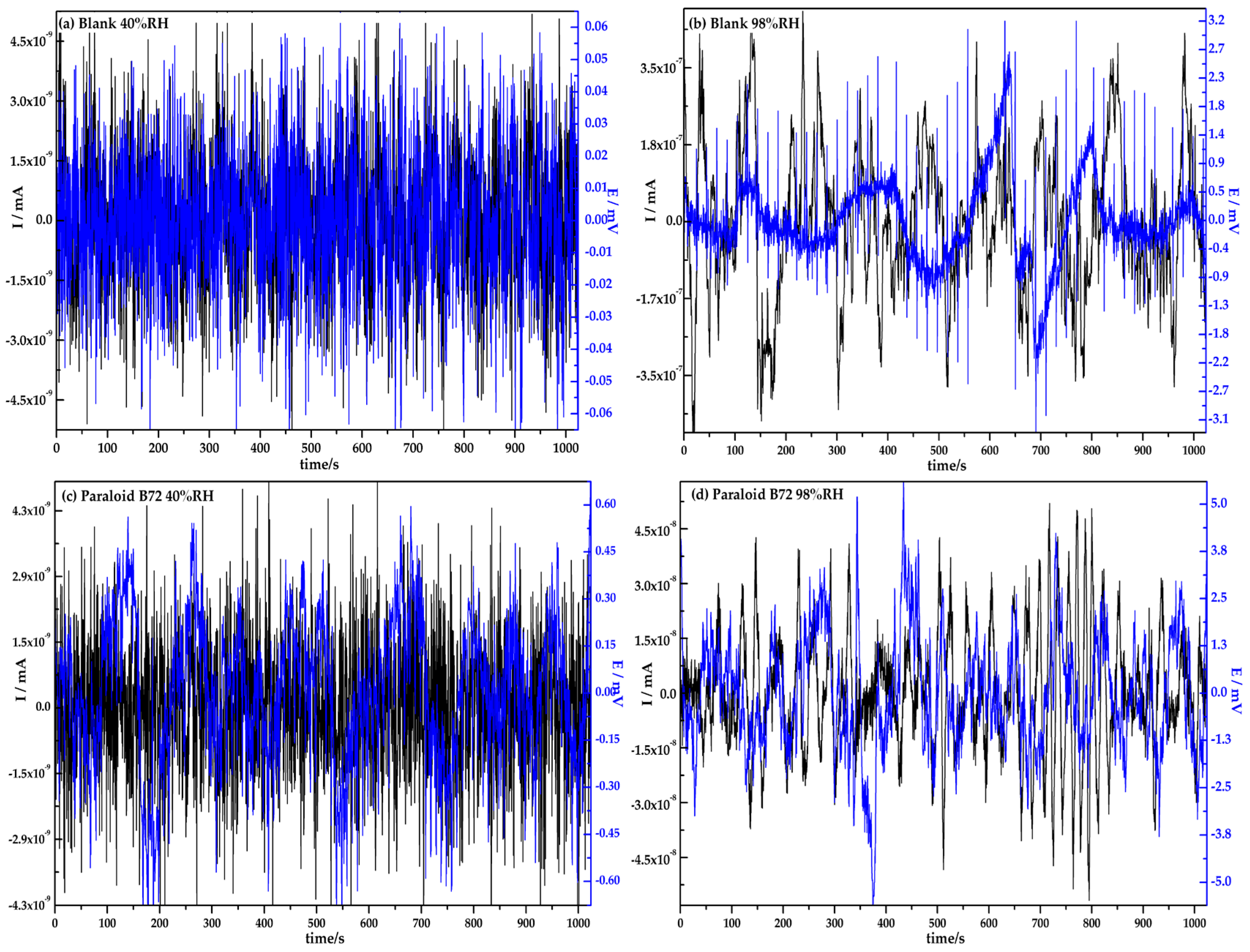
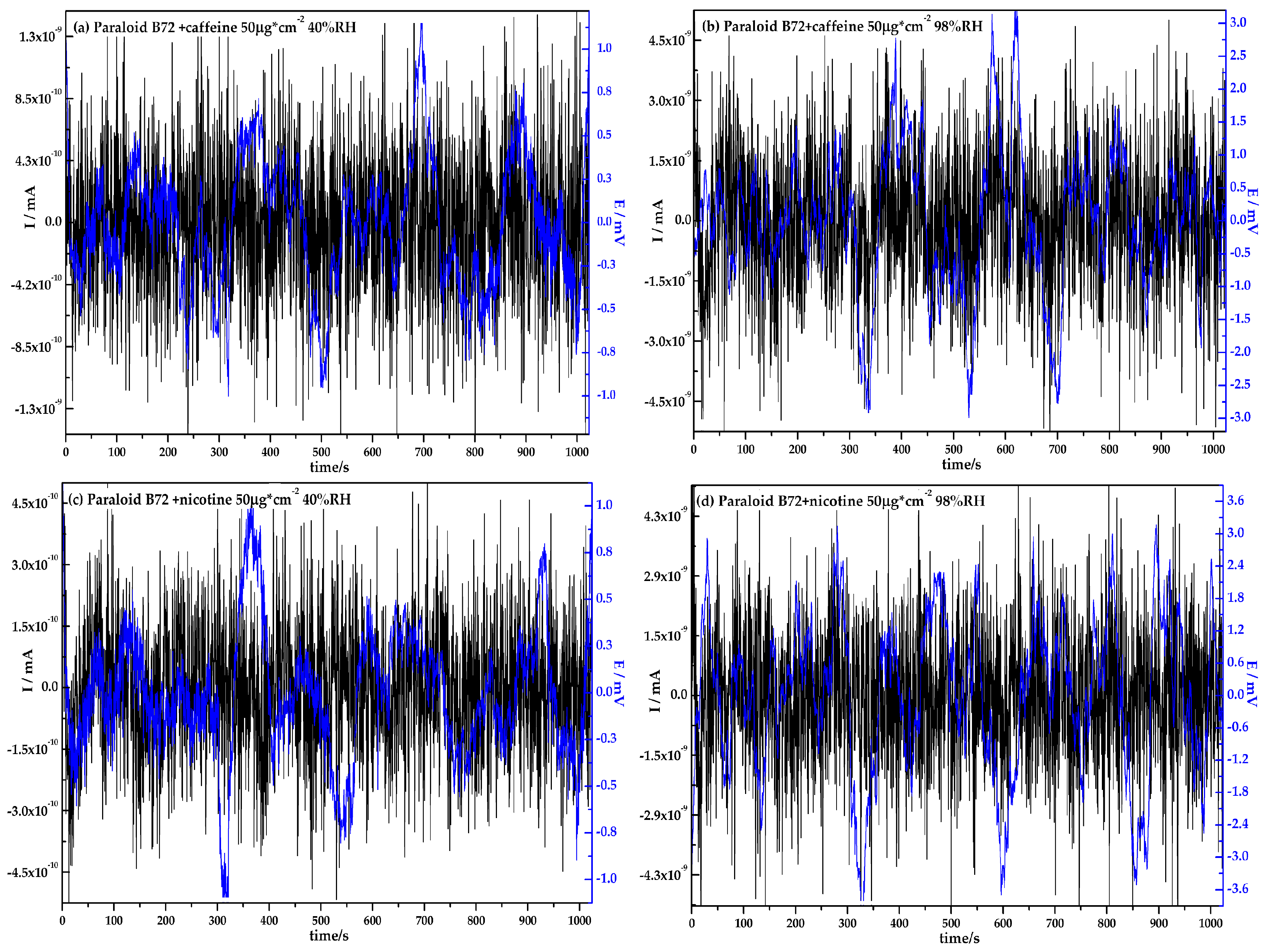
3.1. Electrochemical Noise Analysis
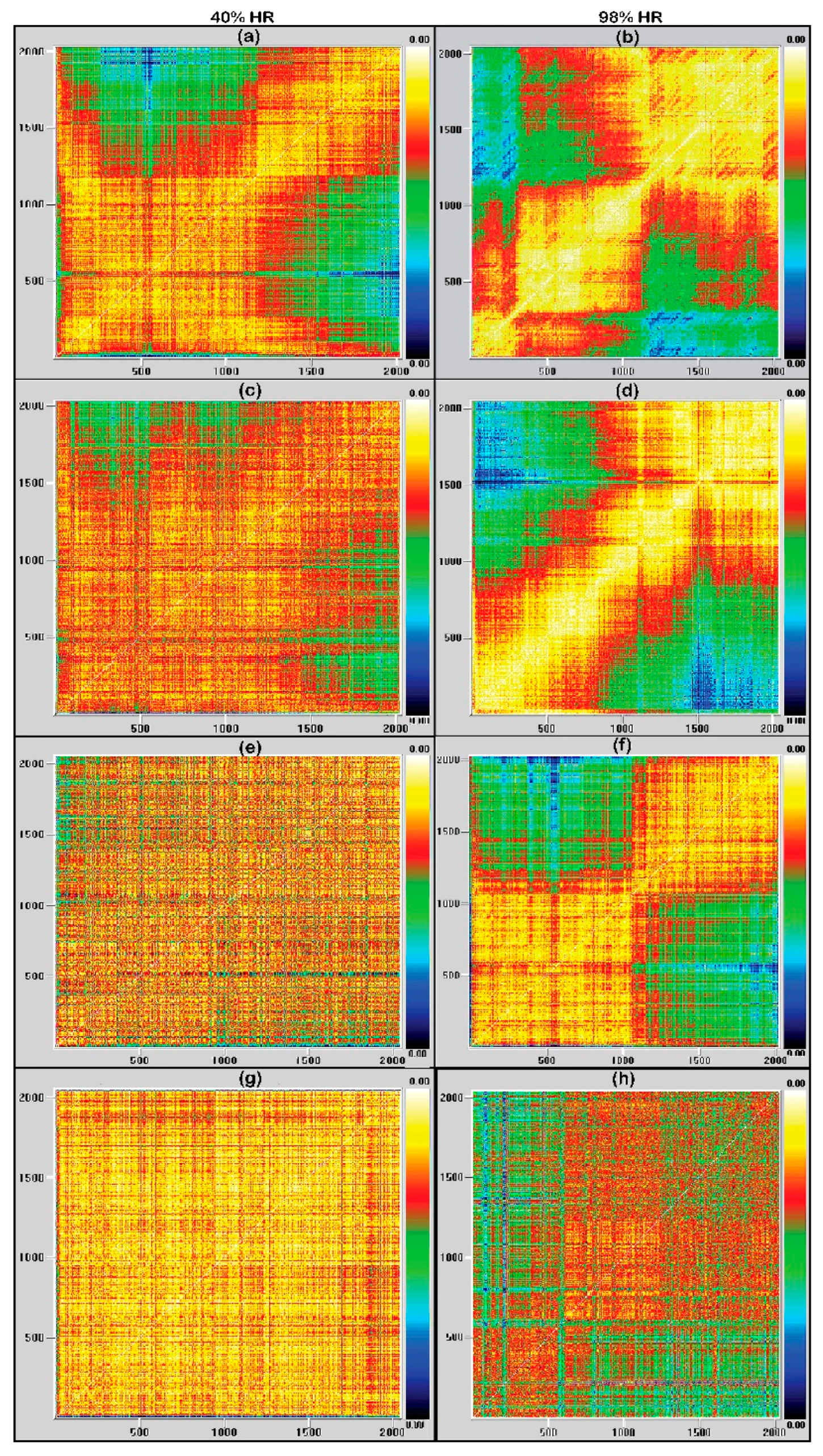
3.2. Recurrence Plots Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caple, C. Conservation Skills: Judgement, Method and Decision Making; Routledge: Abingdon, UK, 2012. [Google Scholar]
- Feller, R.L. Aspects of chemical research in conservation: The deterioration process. J. Am. Inst. Conserv. 1994, 33, 91–99. [Google Scholar] [CrossRef]
- González, M.L.G.; Gomez, M.L. La Restauración: Examen Científico Aplicado a la Conservación de Obras de arte; Cátedra: Madrid, Spain, 1998. (In Spanish) [Google Scholar]
- Garcia, E.; Díaz, S. Técnicas Metodológicas Aplicadas a la Conservación-Restauración del Patrimonio Metálico; Ministerio de Cultura: Madrid, Spain, 2011. (In Spanish) [Google Scholar]
- Mirambet, F.; Reguer, S.; Rocca, E.; Hollner, S.; Testemale, D. A complementary set of electrochemical and X-ray synchrotron techniques to determine the passivation mechanism of iron treated in a new corrosion inhibitor solution specifically developed for the preservation of metallic artefacts. Appl. Phys. A 2010, 99, 341–349. [Google Scholar] [CrossRef]
- Dillmann, P.; Beranger, G.; Piccardo, P.; Matthiessen, H. Corrosion of Metallic Heritage Artefacts: Investigation, Conservation and Prediction of Long Term Behaviour; Elsevier: New York, NY, USA, 2014. [Google Scholar]
- Cano, E.; Lafuente, D. Corrosion inhibitors for the preservation of metallic heritage artefacts. In Corrosion and Conservation of Cultural Heritage Metallic Artefacts; Elsevier: New York, NY, USA, 2013; pp. 570–594. [Google Scholar]
- Liu, A.M.; Ren, X.F.; Wang, B.; Zhang, J.; Yang, P.X.; Zhang, J.Q.; An, M.Z. Complexing agent study via computational chemistry for environmentally friendly silver electrodeposition and the application of a silver deposit. RSC Adv. 2014, 4, 40930–40940. [Google Scholar] [CrossRef]
- Hollner, S.; Mirambet, F.; Rocca, E.; Reguer, S. Evaluation of new non-toxic corrosion inhibitors for conservation of iron artefacts. Corros. Eng. Sci. Technol. 2010, 45, 362–366. [Google Scholar] [CrossRef]
- Cano, E.; Bastidas, D.M.; Argyropoulos, V.; Fajardo, S.; Siatou, A.; Bastidas, J.; Degrigny, C. Electrochemical characterization of organic coatings for protection of historic steel artefacts. J. Solid State Electr. 2010, 14, 453. [Google Scholar] [CrossRef]
- Satri, V. Green Corrosion Inhibitors: Theory and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Rahmouni, K.; Takenouti, H. Struggle against corrosion: Protection by triazoles compounds of ancient and modern bronzes covered with patina. Actual. Chim. 2009, 327–328, 38–44. [Google Scholar]
- Al-Otaibi, M.S.; Al-Mayouf, A.M.; Khan, M.; Mousa, A.A.; Al-Mazroa, S.A.; Alkhathlan, H.Z. Corrosion inhibitory action of some plant extracts on the corrosion of mild steel in acidic media. Arab. J. Chem. 2014, 7, 340–346. [Google Scholar] [CrossRef]
- Okafor, P.; Ikpi, M.; Uwaha, I.; Ebenso, E.; Ekpe, E.; Umoren, S. Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media. Corros. Sci. 2008, 50, 2310–2317. [Google Scholar] [CrossRef]
- Zajec, B.; Leban, M.B.; Lenart, S.; Gavin, K.; Legat, A. Electrochemical impedance and electrical resistance sensors for the evaluation of anticorrosive coating degradation. Corros. Rev. 2017, 35, 65–74. [Google Scholar] [CrossRef]
- Xia, D.H.; Ma, C.; Song, S.Z.; Ma, L.L.; Wang, J.H.; Gao, Z.M.; Zhong, C.; Hu, W.B. Assessing atmospheric corrosion of metals by a novel electrochemical sensor combining with a thin insulating net using electrochemical noise technique. Sens. Actuators B Chem. 2017, 252, 353–358. [Google Scholar] [CrossRef]
- Ma, C.; Song, S.Z.; Gao, Z.M.; Wang, J.H.; Hu, W.B.; Behnamian, Y.; Xia, D.H. Electrochemical noise monitoring of the atmospheric corrosion of steels: Identifying corrosion form using wavelet analysis. Corros. Eng. Sci. Technol. 2017, 52, 432–440. [Google Scholar] [CrossRef]
- Schaefer, K.; Mills, D.J. The application of organic coatings in conservation of archaeological objects excavated from the sea. Prog. Org. Coat. 2017, 102, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Bierwagen, G.P.; Wang, X.; Tallman, D.E. In situ study of coatings using embedded electrodes for ENM measurements. Prog. Org. Coat. 2003, 46, 163–175. [Google Scholar] [CrossRef]
- Puget, Y.; Trethewey, K.; Wood, R.J.K. Electrochemical noise analysis of polyurethane-coated steel subjected to erosion-corrosion. Wear 1999, 233, 552–567. [Google Scholar] [CrossRef]
- Mansfeld, F.; Han, L.T.; Lee, C.C.; Chen, C.; Zhang, G.; Xiao, H. Analysis of electrochemical impedance and noise data for polymer coated metals. Corros. Sci. 1997, 39, 255–279. [Google Scholar] [CrossRef]
- Skerry, B.S.; Eden, D.A. Characterization of coatings performance using electrochemical noise-analysis. Prog. Org. Coat. 1991, 19, 379–396. [Google Scholar] [CrossRef]
- Greisiger, H.; Schauer, T. On the interpretation of the electrochemical noise data for coatings. Prog. Org. Coat. 2000, 39, 31–36. [Google Scholar] [CrossRef]
- Kiele, E.; Lukseniene, J.; Griguceviciene, A.; Selskis, A.; Senvaitiene, J.; Ramanauskas, R.; Raudonis, R.; Kareiva, A. Methyl-modified hybrid organic-inorganic coatings for the conservation of copper. J. Cult. Herit. 2014, 15, 242–249. [Google Scholar] [CrossRef]
- Mohamed, W.A.; Mohamed, N.M. Testing coatings for enameled metal artifacts. Int. J. Conserv. Sci. 2017, 8, 15–24. [Google Scholar]
- Mills, D.; Picton, P.; Mularczyk, L. Developments in the electrochemical noise method (ENM) to make it more practical for assessment of anti-corrosive coatings. Electrochim. Acta 2014, 124, 199–205. [Google Scholar] [CrossRef]
- ASTM b499–09 Standard Test Method for Measurement of Coating Thicknesses by the Magnetic Method: Nonmagnetic Coatings on Magnetic Basis Metals; ASTM International: West Conshohocken, PA, USA, 2014.
- Gonzalez, J.A.; Otero, E.; Cabanas, C.; Bastidas, J.M. Electrochemical sensors for atmospheric corrosion rates—A new design. Br. Corros. J. 1984, 19, 89–94. [Google Scholar] [CrossRef]
- Lecuyer, C.; Barreau, C.; Thierry, D. Electrochemical sensor for in-situ monitoring of coated metals degradation under atmospheric conditions. Mem. Etud. Sci. Rev. Met. 1991, 88, 691–701. [Google Scholar]
- ASTM e104–02 Standard Practice for Maintaining Constant Relative Humidity by Means of Aqueous Solutions; ASTM International: West Conshohocken, PA, USA, 2012.
- Sanchez-Amaya, J.M.; Cottis, R.A.; Botana, F.J. Shot noise and statistical parameters for the estimation of corrosion mechanisms. Corros. Sci. 2005, 47, 3280–3299. [Google Scholar] [CrossRef]
- Bertocci, U.; Gabrielli, C.; Huet, F.; Keddam, M. Noise resistance applied to corrosion measurements I. Theoretical analysis. J. Electrochem. Soc. 1997, 144, 31–37. [Google Scholar] [CrossRef]
- Kearns, J.R.; Metals, A.C.G. Electrochemical Noise Measurement for Corrosion Applications; ASTM: West Conshohocken, PA, USA, 1996. [Google Scholar]
- Cottis, R.; Turgoose, S. Electrochemical Impedance and Noise; NACE International: Houston, TX, USA, 1999. [Google Scholar]
- Mabbutt, S.; Mills, D.J.; Woodcock, C.P. Developments of the electrochemical noise method (ENM) for more practical assessment of anti-corrosion coatings. Prog. Org. Coat. 2007, 59, 192–196. [Google Scholar] [CrossRef] [Green Version]
- Mansfeld, F.; Sun, Z. Localization index obtained from electrochemical noise analysis. Corrosion 1999, 55, 915–918. [Google Scholar] [CrossRef]
- Cottis, R.A.; Al-Awadhi, M.A.A.; Al-Mazeedi, H.; Turgoose, S. Measures for the detection of localized corrosion with electrochemical noise. Electrochim. Acta 2001, 46, 3665–3674. [Google Scholar] [CrossRef]
- Mansfeld, F. The electrochemical noise technique—Applications in corrosion research. AIP Conf. Proc. 2005, 780, 625–630. [Google Scholar]
- Pham, T.D. From fuzzy recurrence plots to scalable recurrence networks of time series. EPL-Europhys. Lett. 2017, 118, 20003. [Google Scholar] [CrossRef]
- Eckmann, J.-P.; Kamphorst, S.O.; Ruelle, D. Recurrence plots of dynamical systems. EPL-Europhys. Lett. 1987, 4, 973. [Google Scholar] [CrossRef]
- Zbilut, J.P.; Webber, C.L. Embeddings and delays as derived from quantification of recurrence plots. Phys. Lett. A 1992, 171, 199–203. [Google Scholar] [CrossRef]
- Trulla, L.L.; Giuliani, A.; Zbilut, J.P.; Webber, C.L. Recurrence quantification analysis of the logistic equation with transients. Phys. Lett. A 1996, 223, 255–260. [Google Scholar] [CrossRef]
- Mitschke, F.; Dämmig, M. Chaos versus noise in experimental data. Int. J. Bifurcat. Chaos 1993, 3, 693–702. [Google Scholar] [CrossRef]
- Cazares-Ibanez, E.; Vazquez-Coutino, G.A.; Garcia-Ochoa, E. Application of recurrence plots as a new tool in the analysis of electrochemical oscillations of copper. J. Electroanal. Chem. 2005, 583, 17–33. [Google Scholar] [CrossRef]
- Webber, C.L., Jr.; Ioana, C.; Marwan, N. Recurrence Plots and Their Quantifications: Expanding Horizons; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Webber, C.L., Jr.; Marwan, N. Recurrence Quantification Analysis: Theory and Best Practices; Springer: Berlin, Germany, 2014. [Google Scholar]



| Code | Fe | C | Si | Mn | P | S |
|---|---|---|---|---|---|---|
| Concentration % | 92.645 | 3.65 | 2.5 | 0.6 | 0.9 | 0.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roncagliolo Barrera, P.; Rodríguez Gómez, F.J.; García Ochoa, E. Assessing of New Coatings for Iron Artifacts Conservation by Recurrence Plots Analysis. Coatings 2019, 9, 12. https://doi.org/10.3390/coatings9010012
Roncagliolo Barrera P, Rodríguez Gómez FJ, García Ochoa E. Assessing of New Coatings for Iron Artifacts Conservation by Recurrence Plots Analysis. Coatings. 2019; 9(1):12. https://doi.org/10.3390/coatings9010012
Chicago/Turabian StyleRoncagliolo Barrera, Paola, Francisco Javier Rodríguez Gómez, and Esteban García Ochoa. 2019. "Assessing of New Coatings for Iron Artifacts Conservation by Recurrence Plots Analysis" Coatings 9, no. 1: 12. https://doi.org/10.3390/coatings9010012





