Effect of Particle Size on the Corrosion Behaviour of Gold in the Presence of Chloride Impurities: An EFC-ICP-MS Potentiodynamic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Au/C Synthesis
2.2. Transmission Electron Microscopy
2.3. Electrochemical Measurements
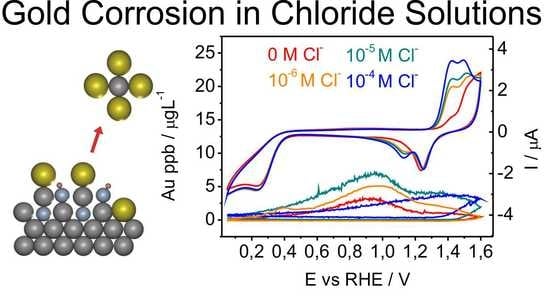
3. Results
3.1. Structural Characterization of Au/C Nanoparticles Composite
3.2. Electrochemical Dissolution of Polycrystalline Au Disk
3.2.1. Dissolution of Polycrystalline Au Disk in the Absence of Chlorides
3.2.2. Dissolution of Au Polycrystalline in the Presence of Chlorides
3.3. Electrochemical Dissolution of Au Nanoparticles
3.3.1. Dissolution of Au Nanoparticles in the Absence of Chlorides
3.3.2. Dissolution of Au Nanoparticles in the Presence of Chlorides
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hammer, B.; Norskov, J.K. Why gold is the noblest of all the metals. Nature 1995, 376, 238–240. [Google Scholar] [CrossRef]
- Wittstock, A.; Zielasek, V.; Biener, J.; Friend, C.M.; Baumer, M. Nanoporous gold catalysts for selective gas-phase oxidative coupling of methanol at low temperature. Science 2010, 327, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Cherevko, S.; Chung, C.-H. Gold nanowire array electrode for non-enzymatic voltammetric and amperometric glucose detection. Sens. Actuators B Chem. 2009, 142, 216–223. [Google Scholar] [CrossRef]
- Segura, R.; Pizarro, J.; Díaz, K.; Placencio, A.; Godoy, F.; Pino, E.; Recio, F. Development of electrochemical sensors for the determination of selenium using gold nanoparticles modified electrodes. Sens. Actuators B Chem. 2015, 220, 263–269. [Google Scholar] [CrossRef]
- Cherevko, S.; Kulyk, N.; Chung, C. Nanoporous Pt@AuxCu100−x by hydrogen evolution assisted electrodeposition of AuxCu100−x and galvanic replacement of Cu with Pt: Electrocatalytic properties. Langmuir 2012, 28, 3306–3315. [Google Scholar] [CrossRef] [PubMed]
- Hartl, K.; Mayrhofer, K.J.J.J.; Lopez, M.; Goia, D.; Arenz, M. AuPt core–shell nanocatalysts with bulk Pt activity. Electrochem. Commun. 2010, 12, 1487–1489. [Google Scholar] [CrossRef]
- Gatalo, M.; Jovanovič, P.; Polymeros, G.; Grote, J.-P.; Pavlišič, A.; Ruiz-Zepeda, F.; Šelih, V.S.; Šala, M.; Hočevar, S.; Bele, M.; et al. Positive effect of surface doping with Au on the stability of Pt-based electrocatalysts. ACS Catal. 2016, 6, 1630–1634. [Google Scholar] [CrossRef]
- Selvaganesh, S.V.; Selvarani, G.; Sridhar, P.; Pitchumani, S.; Shukla, A.K. Durable electrocatalytic-activity of Pt–Au/C cathode in PEMFCs. Phys. Chem. Chem. Phys. 2011, 13. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Dongguo, L.; Yong, D.; Zhu, W.; Shaojun, G.; Zhong, L.W.; Sun, S. Core/shell Au/CuPt nanoparticles and their dual electrocatalysis for both reduction and oxidation reactions. J. Am. Chem. Soc. 2014, 136, 5745–5749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sasaki, K.; Sutter, E.; Adzic, R.R. Stabilization of platinum oxygen-reduction electrocatalysts using gold clusters. Science 2007, 315, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Xu, B.-Q. Platinum covering of gold nanoparticles for utilization enhancement of Pt in electrocatalysts. Phys. Chem. Chem. Phys. 2006, 8, 5106–5114. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Kahlich, M.; Gasteiger, H.; Behm, R. Correlation between CO surface coverage and selectivity/kinetics for the preferential CO oxidation over Pt/γ-Al2O3 and Au/α-Fe2O3: An in-situ DRIFTS study. J. Power Sources 1999, 84, 175–182. [Google Scholar] [CrossRef]
- Fujita, T.; Guan, P.; McKenna, K.; Lang, X.; Hirata, A.; Zhang, L.; Tokunaga, T.; Arai, S.; Yamamoto, Y.; Tanaka, N.; et al. Atomic origins of the high catalytic activity of nanoporous gold. Nat. Mater. 2012, 11, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Morales, O.; Calle-Vallejo, F.; de Munck, C.; Koper, M.T.M. Electrochemical water splitting by gold: Evidence for an oxide decomposition mechanism. Chem. Sci. 2013, 4. [Google Scholar] [CrossRef]
- Jeyabharathi, C.; Hasse, U.; Ahrens, P.; Scholz, F. Oxygen electroreduction on polycrystalline gold electrodes and on gold nanoparticle-modified glassy carbon electrodes. J. Solid State Electrochem. 2014, 18, 3299–3306. [Google Scholar] [CrossRef]
- Määttänen, A.; Ihalainen, P.; Pulkkinen, P.; Wang, S.; Tenhu, H.; Peltonen, J. Inkjet-printed gold electrodes on paper: Characterization and functionalization. ACS Appl. Mater. Interfaces 2012, 4, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Y.; Lu, X.; Ma, Z.; Xie, C.; Zheng, Z. Chemical formation of soft metal electrodes for flexible and wearable electronics. Chem. Soc. Rev. 2018, 47, 4611–4641. [Google Scholar] [CrossRef] [PubMed]
- Shariq, M.; Friedrich, B.; Budic, B.; Hodnik, N.; Ruiz-Zepeda, F.; Majerič, P.; Rudolf, R. Successful synthesis of gold nanoparticles through ultrasonic spray pyrolysis from a gold(III) nitrate precursor and Their interaction with a high electron beam. ChemistryOpen 2018, 7, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Harsányi, G. Irregular effect of chloride impurities on migration failure reliability: Contradictions or understandable? Microelectron. Reliab. 1999, 39, 1407–1411. [Google Scholar] [CrossRef]
- Frankenthal, R.P. The anodic corrosion of gold in concentrated chloride solutions. J. Electrochem. Soc. 1982, 129, 1192–1196. [Google Scholar] [CrossRef]
- Gaur, J.N.; Schmid, G.M. Electrochemical behavior of gold in acidic chloride solutions. J. Electroanal. Chem. Interfacial Electrochem. 1970, 24, 279–286. [Google Scholar] [CrossRef]
- Klemm, S.O.; Topalov, A.A.; Laska, C.A.; Mayrhofer, K.J.J. Coupling of a high throughput microelectrochemical cell with online multielemental trace analysis by ICP-MS. Electrochem. Commun. 2011, 13, 1533–1535. [Google Scholar] [CrossRef]
- Cherevko, S.; Topalov, A.A.; Katsounaros, I.; Mayrhofer, K.J.J. Electrochemical dissolution of gold in acidic medium. Electrochem. Commun. 2013, 28, 44–46. [Google Scholar] [CrossRef]
- Topalov, A.A.; Katsounaros, I.; Auinger, M.; Cherevko, S.; Meier, J.C.; Klemm, S.O.; Mayrhofer, K.J.J. Dissolution of platinum: Limits for the deployment of electrochemical energy conversion? Angew. Chem. Int. Ed. Engl. 2012, 51, 12613–12615. [Google Scholar] [CrossRef] [PubMed]
- Schuppert, A.K.; Topalov, A.A.; Katsounaros, I.; Klemm, S.O.; Mayrhofer, K.J.J. A scanning flow cell system for fully automated screening of electrocatalyst materials. J. Electrochem. Soc. 2012, 159, F670–F675. [Google Scholar] [CrossRef]
- Cherevko, S.; Zeradjanin, A.R.; Topalov, A.A.; Kulyk, N.; Katsounaros, I.; Mayrhofer, K.J.J. Dissolution of noble metals during oxygen evolution in acidic media. ChemCatChem. 2014, 6, 2219–2223. [Google Scholar] [CrossRef]
- Cherevko, S.; Topalov, A.A.; Zeradjanin, A.R.; Katsounaros, I.; Mayrhofer, K.J.J. Gold dissolution: Towards understanding of noble metal corrosion. RSC Adv. 2013, 3, 16516–16527. [Google Scholar] [CrossRef]
- Jovanovič, P.; Šelih, V.S.; Šala, M.; Hočevar, S.; Ruiz-Zepeda, F.; Hodnik, N.; Bele, M.; Gaberšček, M. Potentiodynamic dissolution study of PtRu/C electrocatalyst in the presence of methanol. Electrochim. Acta 2016, 211, 851–859. [Google Scholar] [CrossRef]
- Hodnik, N.; Jovanovič, P.; Pavlišič, A.; Jozinović, B.; Zorko, M.; Bele, M.; Šelih, V.S.; Šala, M.; Hočevar, S.; Gaberšček, M. New insights into corrosion of ruthenium and ruthenium oxide nanoparticles in acidic media. J. Phys. Chem. C 2015, 119, 10140–10147. [Google Scholar] [CrossRef]
- Jovanovič, P.; Šelih, V.S.; Šala, M.; Hočevar, S.B.; Pavlišič, A.; Gatalo, M.; Bele, M.; Ruiz-Zepeda, F.; Čekada, M.; Hodnik, N.; Gaberšček, M. Electrochemical in-situ dissolution study of structurally ordered, disordered and gold doped PtCu3 nanoparticles on carbon composites. J. Power Sources 2016, 327, 675–680. [Google Scholar] [CrossRef]
- Jovanovič, P.; Pavlišič, A.; Šelih, V.S.; Šala, M.; Hodnik, N.; Bele, M.; Hočevar, S.; Gaberšček, M. New insight into platinum dissolution from nanoparticulate platinum-based electrocatalysts using highly sensitive in situ concentration measurements. ChemCatChem 2014, 6, 449–453. [Google Scholar] [CrossRef]
- Pavlišič, A.; Jovanovič, P.; Šelih, V.S.; Šala, M.; Hodnik, N.; Hočevar, S.; Gaberšček, M. The influence of chloride impurities on Pt/C fuel cell catalyst corrosion. Chem. Commun. 2014, 50, 3732–3734. [Google Scholar] [CrossRef] [PubMed]
- Nicol, M.J. The anodic behaviour of gold—Part II–Oxidation in alkaline solutions. Gold Bull. 1980, 13, 105–106. [Google Scholar] [CrossRef]
- Cadle, S.H.; Bruckenstein, S. A ring-disk study of the effect of trace chloride ion on the anodic behavior of gold in 0.2 M H2SO4. J. Electroanal. Chem. Interfacial Electrochem. 1973, 48, 325–331. [Google Scholar] [CrossRef]
- Nowicka, A.M.; Hasse, U.; Sievers, G.; Donten, M.; Stojek, Z.; Fletcher, S.; Scholz, F. Selective knockout of gold active sites. Angew. Chem. Int. Ed. 2010, 49, 3006–3009. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.D.; O’Mullane, A.P. Generation of active surface states of gold and the role of such states in electrocatalysis. J. Solid State Electrochem. 2000, 4, 285–297. [Google Scholar] [CrossRef]
- Angerstein-Kozlowska, H.; Conway, B.E.; Hamelin, A.; Stoicoviciu, L. Elementary steps of electrochemical oxidation of single-crystal planes of Au—I. Chemical basis of processes involving geometry of anions and the electrode surfaces. Electrochim. Acta 1986, 31, 1051–1061. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical oxide film formation at noble metals as a surface-chemical process. Prog. Surf. Sci. 1995, 49, 331–452. [Google Scholar] [CrossRef]
- Tian, M.; Pell, W.G.; Conway, B.E. Nanogravimetry study of the initial stages of anodic surface oxide film growth at Au in aqueous HClO4 and H2SO4 by means of EQCN. Electrochim. Acta 2003, 48, 2675–2689. [Google Scholar] [CrossRef]
- Tremiliosi-Filho, G.; Dall’Antonia, L.H.; Jerkiewicz, G. Limit to extent of formation of the quasi-two-dimensional oxide state on Au electrodes. J. Electroanal. Chem. 1997, 422, 149–159. [Google Scholar] [CrossRef]
- Jovanovič, P.; Šelih, V.S.; Šala, M.; Hodnik, N. In situ electrochemical dissolution of platinum and gold in organic-based solvent. Npj Mater. Degrad. 2018, 2, 9. [Google Scholar] [CrossRef]
- Pavlišič, A.; Jovanovič, P.; Šelih, V.S.; Šala, M.; Hodnik, N.; Gaberšček, M. Platinum dissolution and redeposition from Pt/C fuel cell electrocatalyst at potential cycling. J. Electrochem. Soc. 2018, 165, F3161–F3165. [Google Scholar] [CrossRef]
- Jovanovič, P.; Ruiz-Zepeda, F.; Šala, M.; Hodnik, N. Atomic scale insights into electrochemical dissolution of Janus Pt–SnO2 nanoparticles in the presence of ethanol in acidic media: An IL-STEM and EFC–ICP–MS study. J. Phys. Chem. C 2018, 122, 10050–10058. [Google Scholar] [CrossRef]
- Topalov, A.A.; Cherevko, S.; Zeradjanin, A.R.; Meier, J.C.; Katsounaros, I.; Mayrhofer, K.J.J.; Josef, C. Towards a comprehensive understanding of platinum dissolution in acidic media. Chem. Sci. 2014, 5, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Kasian, O.; Kulyk, N.; Mingers, A.; Zeradjanin, A.R.; Mayrhofer, K.J.J.; Cherevko, S. Electrochemical dissolution of gold in presence of chloride and bromide traces studied by on-line electrochemical inductively coupled plasma mass spectrometry. Electrochim. Acta 2016, 222, 1056–1063. [Google Scholar] [CrossRef]
- Cherevko, S.; Zeradjanin, A.R.; Keeley, G.P.; Mayrhofer, K.J.J. A comparative study on gold and platinum dissolution in acidic and alkaline media. J. Electrochem. Soc. 2014, 161, H822–H830. [Google Scholar] [CrossRef]
- Ye, S.; Ishibashi, C.; Shimazu, K.; Uosaki, K. An in situ electrochemical quartz crystal microbalance study of the dissolution process of a gold electrode in perchloric acid solution containing chloride ion. J. Electrochem. Soc. 1998, 145, 1614–1623. [Google Scholar] [CrossRef]
- Ye, S.; Ishibashi, C.; Uosaki, K. Anisotropic dissolution of an Au(111) electrode in perchloric acid solution containing chloride anion investigated by in situ STMThe important role of adsorbed chloride anion. Langmuir 1999, 15, 807–812. [Google Scholar] [CrossRef]
- Geiger, S.; Cherevko, S.; Mayrhofer, K.J.J.J. Dissolution of platinum in presence of chloride traces. Electrochim. Acta 2015, 179, 24–31. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.-W.; Popov, B.N. A review of gas diffusion layer in PEM fuel cells: Materials and designs. Int. J. Hydrogen Energ. 2012, 37, 5850–5865. [Google Scholar] [CrossRef]
- Kinoshita, K. Small-particle effects and structural considerations for electrocatalysis. In Modern Aspects of Electrochemistry; Springer: Boston, MA, USA, 1982. [Google Scholar]
- Maillard, F.; Pronkin, S.; Savinova, E.R. Size effects in electrocatalysis of fuel cell reactions on supported metal nanoparticles. In Fuel Cell Catalysis: A Surface Science Approach; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Lorenz, W.J.; Staikov, G.; Schindler, W.; Wiesbeck, W. The role of low-dimensional systems in electrochemical phase formation and dissolution processes. J. Electrochem. Soc. 2002, 149, K47–K59. [Google Scholar] [CrossRef]
- Plieth, W.J. Electrochemical properties of small clusters of metal atoms and their role in surface enhanced Raman scattering. J. Phys. Chem. 1982, 86, 3166–3170. [Google Scholar] [CrossRef]
- Tang, L.; Han, B.; Persson, K.; Friesen, C.; He, T.; Sieradzki, K.; Ceder, G. Electrochemical stability of nanometer-scale Pt particles in acidic environments. J. Am. Chem. Soc. 2010, 132, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, X.; Cammarata, R.C.; Friesen, C.; Sieradzki, K. Electrochemical stability of elemental metal nanoparticles. J. Am. Chem. Soc. 2010, 132, 11722–11726. [Google Scholar] [CrossRef] [PubMed]
- Mayrhofer, K.J.J.; Blizanac, B.B.; Arenz, M.; Stamenkovic, V.R.; Ross, P.N.; Markovic, N.M. The impact of geometric and surface electronic properties of Pt-catalysts on the particle size effect in electrocatalysis. J. Phys. Chem. B 2005, 109, 14433–14440. [Google Scholar] [CrossRef] [PubMed]
- Jovanovič, P.; Petek, U.; Hodnik, N.; Ruiz-Zepeda, F.; Gatalo, M.; Šala, M.; Šelih, V.S.; Fellinger, T.P.; Gaberšček, M. Importance of non-intrinsic platinum dissolution in Pt/C composite fuel cell catalysts. Phys. Chem. Chem. Phys. 2017, 19, 21446–21452. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanovič, P.; Može, M.; Gričar, E.; Šala, M.; Ruiz-Zepeda, F.; Bele, M.; Marolt, G.; Hodnik, N. Effect of Particle Size on the Corrosion Behaviour of Gold in the Presence of Chloride Impurities: An EFC-ICP-MS Potentiodynamic Study. Coatings 2019, 9, 10. https://doi.org/10.3390/coatings9010010
Jovanovič P, Može M, Gričar E, Šala M, Ruiz-Zepeda F, Bele M, Marolt G, Hodnik N. Effect of Particle Size on the Corrosion Behaviour of Gold in the Presence of Chloride Impurities: An EFC-ICP-MS Potentiodynamic Study. Coatings. 2019; 9(1):10. https://doi.org/10.3390/coatings9010010
Chicago/Turabian StyleJovanovič, Primož, Martina Može, Ema Gričar, Martin Šala, Francisco Ruiz-Zepeda, Marjan Bele, Gregor Marolt, and Nejc Hodnik. 2019. "Effect of Particle Size on the Corrosion Behaviour of Gold in the Presence of Chloride Impurities: An EFC-ICP-MS Potentiodynamic Study" Coatings 9, no. 1: 10. https://doi.org/10.3390/coatings9010010