Coupling the Microscopic Healing Behaviour of Coatings to the Thermoreversible Diels-Alder Network Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.2.1. Dynamic Rheology
2.2.2. Dynamic Mechanical Analysis
2.2.3. Atomic Force Microscopy
3. Results
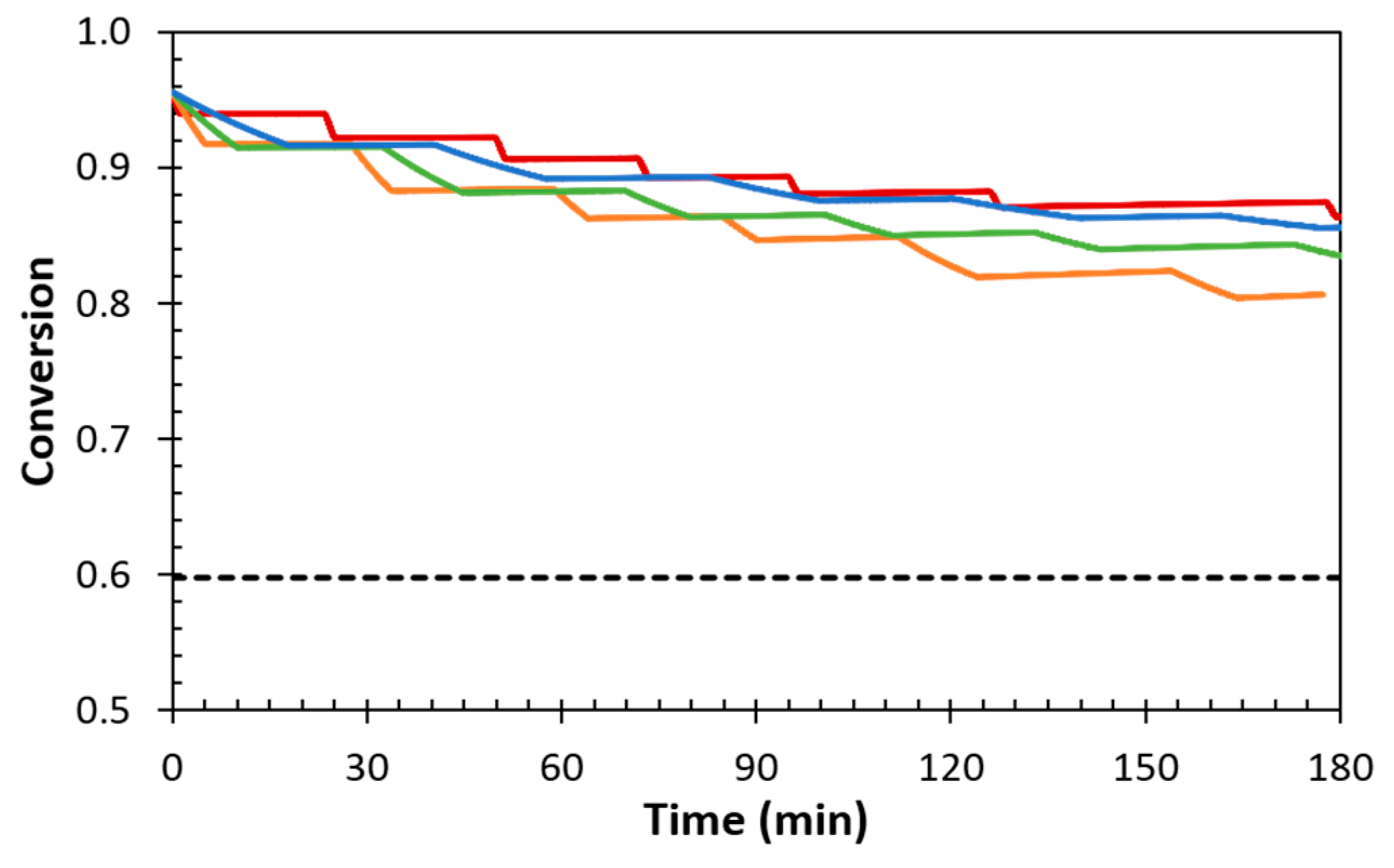
3.1. Dynamic Reversibility of Diels-Alder Cycloadducts
3.1.1. Thermomechanical Behaviour
3.2. Dynamically Reversible Polymer Network Coatings
3.2.1. Thermally Reversible Thermoset Coatings
3.2.2. Thermally Reversible Elastomeric Coatings
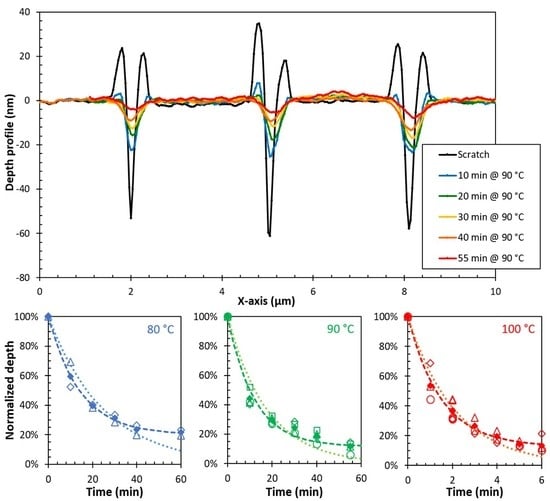
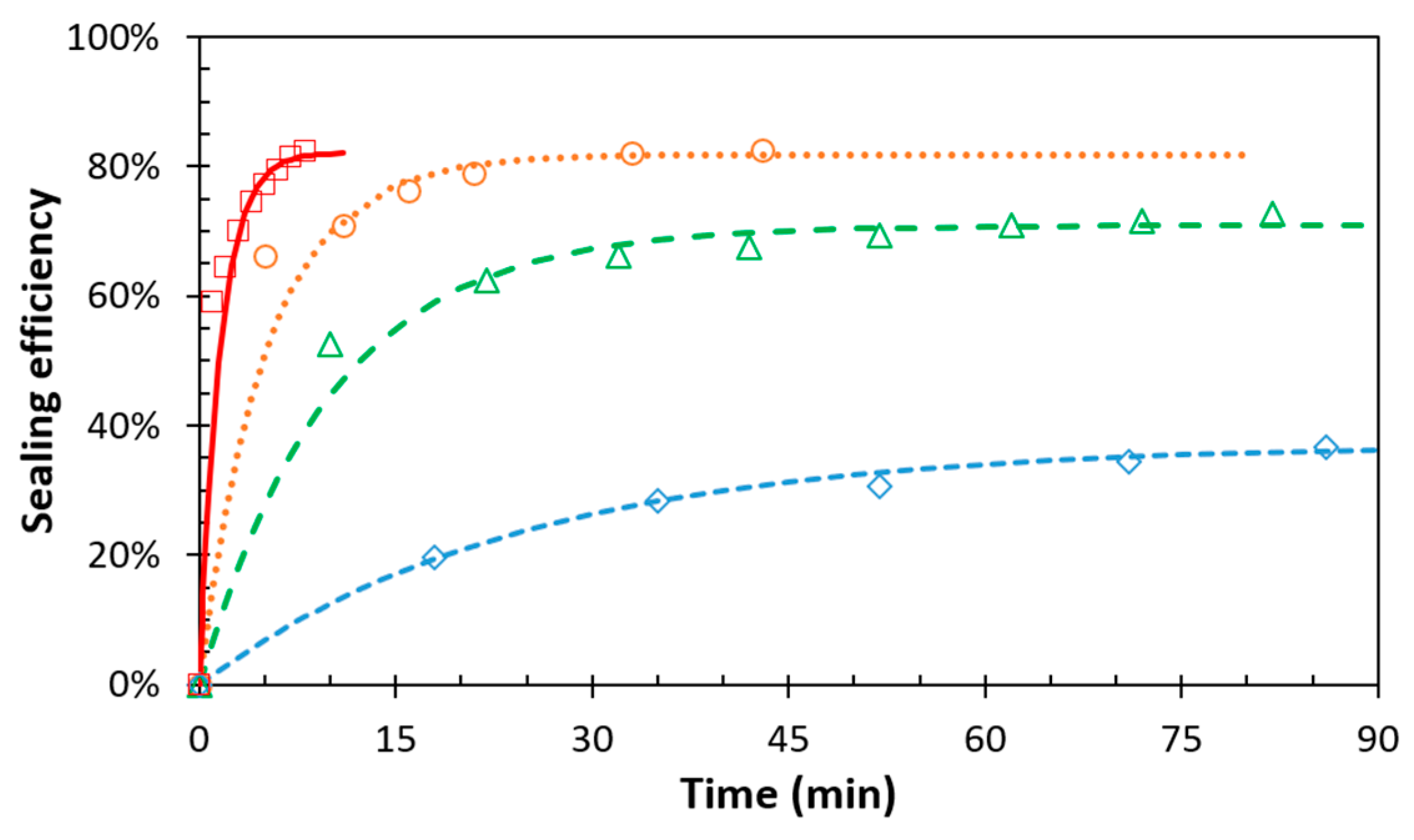
3.3. Temperature Dependence of the Sealing Mechanism
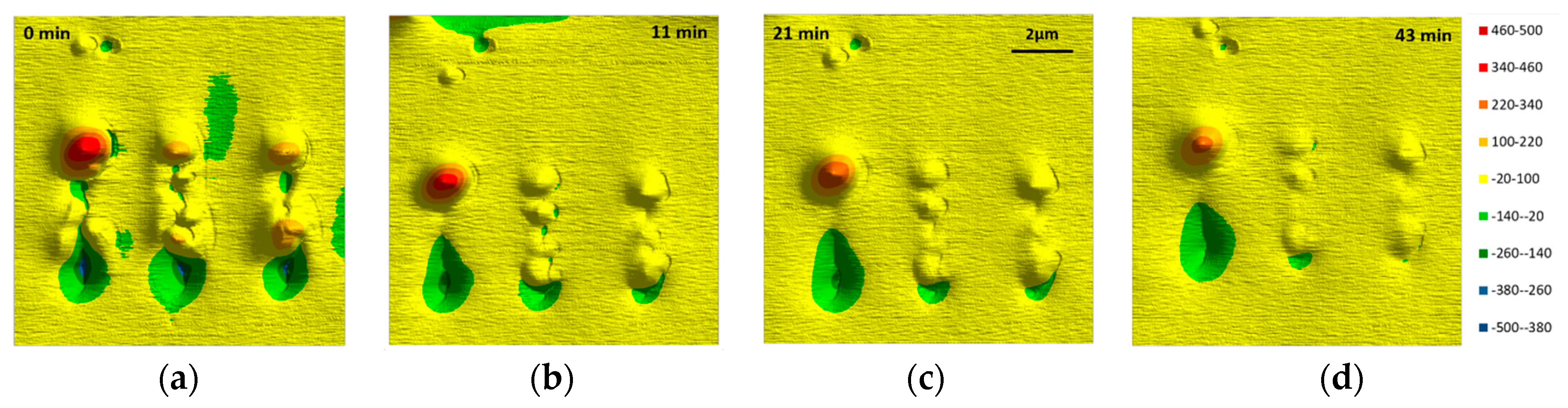
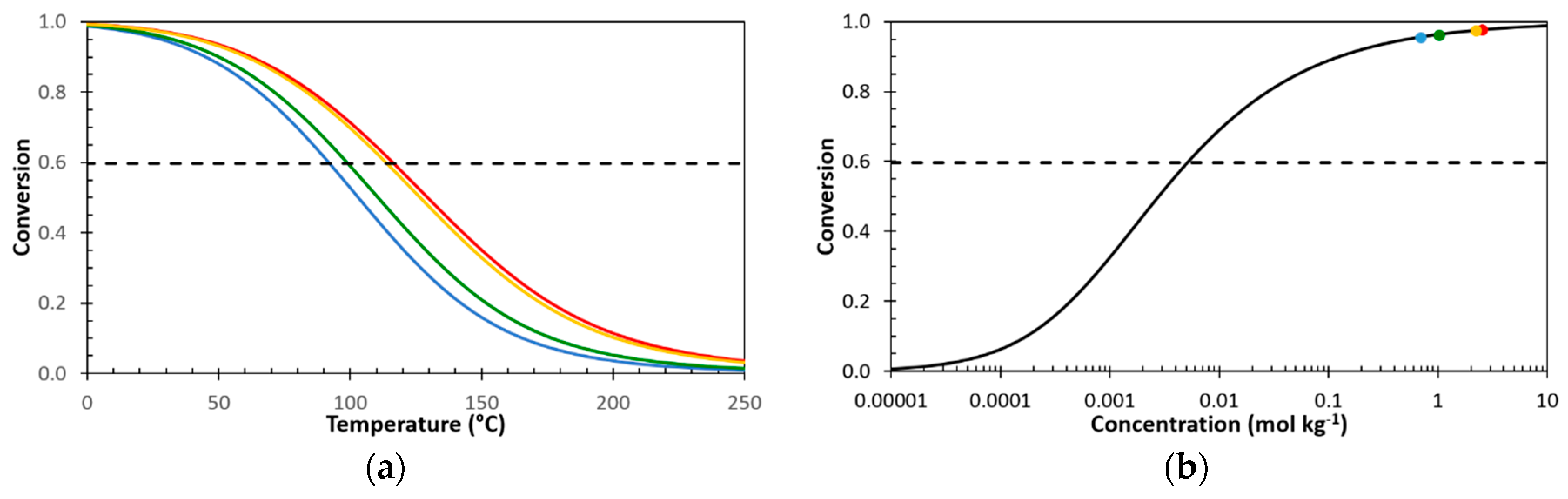
3.4. Structure-Property Relation of the Sealing Mechanism
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Kinetic Parameter | Endo Isomer | Exo Isomer |
|---|---|---|
| ln ADA (kg·mol−1·s−1) | 15.8 | 16.4 |
| EDA (kJ·mol−1) | 65.1 | 73.7 |
| ln ArDA (s−1) | 27.9 | 37.1 |
| ErDA (kJ·mol−1) | 101.3 | 137.1 |
| ΔrH° (kJ·mol−1) | −36.2 | −63.4 |
| ΔrS° (kJ·mol−1·K−1) | −100.5 | −172.3 |



References
- Esteves, C. Self-healing functional surfaces. Adv. Mater. Interfaces 2018, 5, 1800293. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Möhwald, H. Self-repairing coatings containing active nanoreservoirs. Small 2007, 3, 926–943. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; White, S.R.; Braun, P.V. Self-healing polymer coatings. Adv. Mater. 2009, 21, 645–649. [Google Scholar] [CrossRef]
- Samadzadeh, M.; Boura, S.H.; Peikari, M.; Kasiriha, S.M.; Ashrafi, A. A review on self-healing coatings based on micro/nanocapsules. Prog. Org. Coat. 2010, 68, 159–164. [Google Scholar] [CrossRef]
- Toohey, K.S.; Sottos, N.R.; Lewis, J.A.; Moore, J.S.; White, S.R. Self-healing materials with microvascular networks. Nat. Mater. 2007, 6, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Torre-Muruzabal, A.; Daelemans, L.; van Assche, G.; de Clerck, K.; Rahier, H. Creation of a nanovascular network by electrospun sacrificial nanofibers for self-healing applications and its effect on the flexural properties of the bulk material. Polym. Test. 2016, 54, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Wietor, J.L.; Dimopoulos, A.; Govaert, L.E.; van Benthem, R.A.T.M.; de With, G.; Sijbesma, R.P. Preemptive healing through supramolecular cross-links. Macromolecules 2009, 42, 6640–6646. [Google Scholar] [CrossRef]
- Li, Y.; Fang, X.; Wang, Y.; Ma, B.; Sun, J. Highly transparent and water-enabled healable antifogging and frost-resisting films based on poly(vinyl alcohol)-Nafion complexes. Chem. Mater. 2016, 28, 6975–6984. [Google Scholar] [CrossRef]
- Wouters, M.; Craenmehr, E.; Tempelaars, K.; Fischer, H.; Stroeks, N.; van Zanten, J. Preparation and properties of a novel remendable coating concept. Prog. Org. Coat. 2009, 64, 156–162. [Google Scholar] [CrossRef]
- Scheltjens, G.; Brancart, J.; de Graeve, I.; van Mele, B.; Terryn, H.; van Assche, G. Self-healing property characterization of reversible thermoset coatings. J. Therm. Anal. Calorim. 2011, 105, 805–809. [Google Scholar] [CrossRef]
- Imbesi, P.M.; Fidge, C.; Raymond, J.E.; Cauët, S.I.; Wooley, K.L. Model Diels-Alder studies for the creation of amphiphilic cross-linked networks as healable, antibiofouling coatings. ACS Macro Lett. 2012, 1, 473–477. [Google Scholar] [CrossRef]
- Kötteritzsch, J.; Stumpf, S.; Hoeppener, S.; Vitz, J.; Hager, M.D.; Schubert, U.S. One-component intrinsic self-healing polymer for coatings based on reversible crosslinking by Diels-Alder-cycloaddition, macromol. Chem. Phys. 2013, 214, 1636–1649. [Google Scholar]
- Barthel, M.J.; Rudolph, T.; Teichler, A.; Paulus, R.M.; Vitz, J.; Hoeppener, S.; Hager, M.D.; Schacher, F.H.; Schubert, U.S. Self-healing materials via reversible crosslinking of poly(ethylene oxide)-block-poly(furfuryl glycidyl ether) (PEO-b-PFGE) block copolymer films. Adv. Funct. Mater. 2013, 23, 4921–4932. [Google Scholar] [CrossRef]
- Turkenburg, D.H.; Durant, Y.; Fischer, H.R. Bio-based self-healing coatings based on thermo-reversible Diels-Alder reaction. Prog. Org. Coat. 2017, 111, 38–46. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Tedim, J.; Ferreira, M.G.S. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Lutz, A.; van den Berg, O.; Wielant, J.; de Graeve, I.; Terryn, H. A Multiple-action self-healing coating. Front. Mater. 2016, 2, 73. [Google Scholar] [CrossRef]
- Andreeva, D.V.; Shchukin, D.G. Smart self-repairing protective coatings. Mater. Today 2008, 11, 24–30. [Google Scholar] [CrossRef]
- Chuo, T.W.; Liu, Y.L. Furan-functionalized aniline trimer based self-healing polymers exhibiting high efficiency of anticorrosion. Polymer 2017, 125, 227–233. [Google Scholar] [CrossRef]
- Diaz, M.M.; Brancart, J.; van Assche, G.; van Mele, B. Room-temperature versus heating-mediated healing of a Diels-Alder crosslinked polymer network. Polymer 2018, 153, 453–463. [Google Scholar] [CrossRef]
- Brancart, J.; Scheltjens, G.; Muselle, T.; van Mele, B.; Terryn, H.; van Assche, G. Atomic force microscopy-based study of self-healing coatings based on reversible polymer network systems. J. Intell. Mater. Syst. Struct. 2014, 25, 40–46. [Google Scholar] [CrossRef]
- Yoon, J.A.; Kamada, J.; Koynov, K.; Mohin, J.; Nicola, R.; Zhang, Y.; Balazs, A.C.; Kowalewski, T.; Matyjaszewski, K. Self-healing polymer films based on thiol-disulfide exchange reactions and self-healing kinetics measured using atomic force microscopy. Macromolecules 2012, 45, 142–149. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, T.H.; Park, Y.I.; Nam, J.H.; Noh, S.M.; Cheong, I.W.; Kim, J.C. Influence of material properties on scratch-healing performance of polyacrylate-graft-polyurethane network that undergo thermally reversible crosslinking. Polymer 2017, 128, 135–146. [Google Scholar] [CrossRef]
- González-García, Y.; Santana, J.J.; González-Guzmán, J.; Izquierdo, J.; González, S.; Souto, R.M. Scanning electrochemical microscopy for the investigation of localized degradation processes in coated metals. Prog. Org. Coat. 2010, 69, 110–117. [Google Scholar] [CrossRef]
- González, S.; Santana, J.J.; González-García, Y.; Fernández-Mérida, L.; Souto, R.M. Scanning electrochemical microscopy for the investigation of localized degradation processes in coated metals: Effect of oxygen. Corros. Sci. 2011, 53, 1910–1915. [Google Scholar] [CrossRef]
- Scheltjens, G.; Diaz, M.M.; Brancart, J.; van Assche, G.; van Mele, B. A self-healing polymer network based on reversible covalent bonding. React. Funct. Polym. 2013, 73, 413–420. [Google Scholar] [CrossRef]
- Diaz, M.M.; van Assche, G.; Maurer, F.H.J.; van Mele, B. Thermophysical characterization of a reversible dynamic polymer network based on kinetics and equilibrium of an amorphous furan-maleimide Diels-Alder cycloaddition. Polymer 2017, 120, 176–188. [Google Scholar] [CrossRef]
- Cuvellier, A.; Verhelle, R.; Brancart, J.; Vanderborght, B.; van Assche, G.; Rahier, H. The influence of stereochemistry on the reactivity of the Diels-Alder cycloaddition and the implications for reversible network polymerization. Polym. Chem. 2019, in press. [Google Scholar] [CrossRef]
- Winter, H.; Chambon, F. Analysis of linear viscoelasticity of a crosslinking polymer at the gel point. J. Rheol. 1986, 30, 367–382. [Google Scholar] [CrossRef]
- Miller, D.R.; Macosko, C.W. A New derivation of post gel properties of network polymers. Macromolecules 1976, 9, 206–211. [Google Scholar] [CrossRef]








| Network | [M]0 (mol kg−1) | Tg (°C) 1 | E′ (MPa) 2 | Tgel,eq (°C) | Tgel (°C) 3 | Tsoft (°C) |
|---|---|---|---|---|---|---|
| DPBM-F230 | 2.52 | 95 | >2 × 103 | 116 | 130 | 118 |
| DPBM-F400 | 2.22 | 79 | 2.3 × 103 | 114 | 120 | 114 |
| DPBM-F2000 | 1.02 | −47 | 65 | 99 | 97 | 104 |
| DPBM-F4000 | 0.69 | −60 | 6.4 | 92 | 91 | 85 |
| DPBM-F400 | Model | Temperature | τseal (min) | η∞ | DPBM-F4000 | Model | Temperature | τseal (min) | η∞ |
|---|---|---|---|---|---|---|---|---|---|
| scratches | improved | 80 °C | 14.3 | 80.0% | scratches | improved | 70 °C | 24.4 | 37% |
| 90 °C | 12.0 | 88.4% | 75 °C | 10.1 | 71% | ||||
| 100 °C | 1.5 | 87.1% | 80 °C | 5.2 | 82% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brancart, J.; Verhelle, R.; Mangialetto, J.; Van Assche, G. Coupling the Microscopic Healing Behaviour of Coatings to the Thermoreversible Diels-Alder Network Formation. Coatings 2019, 9, 13. https://doi.org/10.3390/coatings9010013
Brancart J, Verhelle R, Mangialetto J, Van Assche G. Coupling the Microscopic Healing Behaviour of Coatings to the Thermoreversible Diels-Alder Network Formation. Coatings. 2019; 9(1):13. https://doi.org/10.3390/coatings9010013
Chicago/Turabian StyleBrancart, Joost, Robrecht Verhelle, Jessica Mangialetto, and Guy Van Assche. 2019. "Coupling the Microscopic Healing Behaviour of Coatings to the Thermoreversible Diels-Alder Network Formation" Coatings 9, no. 1: 13. https://doi.org/10.3390/coatings9010013