Temperature-Induced Formation of Lubricous Oxides in Vanadium Containing Iron-Based Arc Sprayed Coatings
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
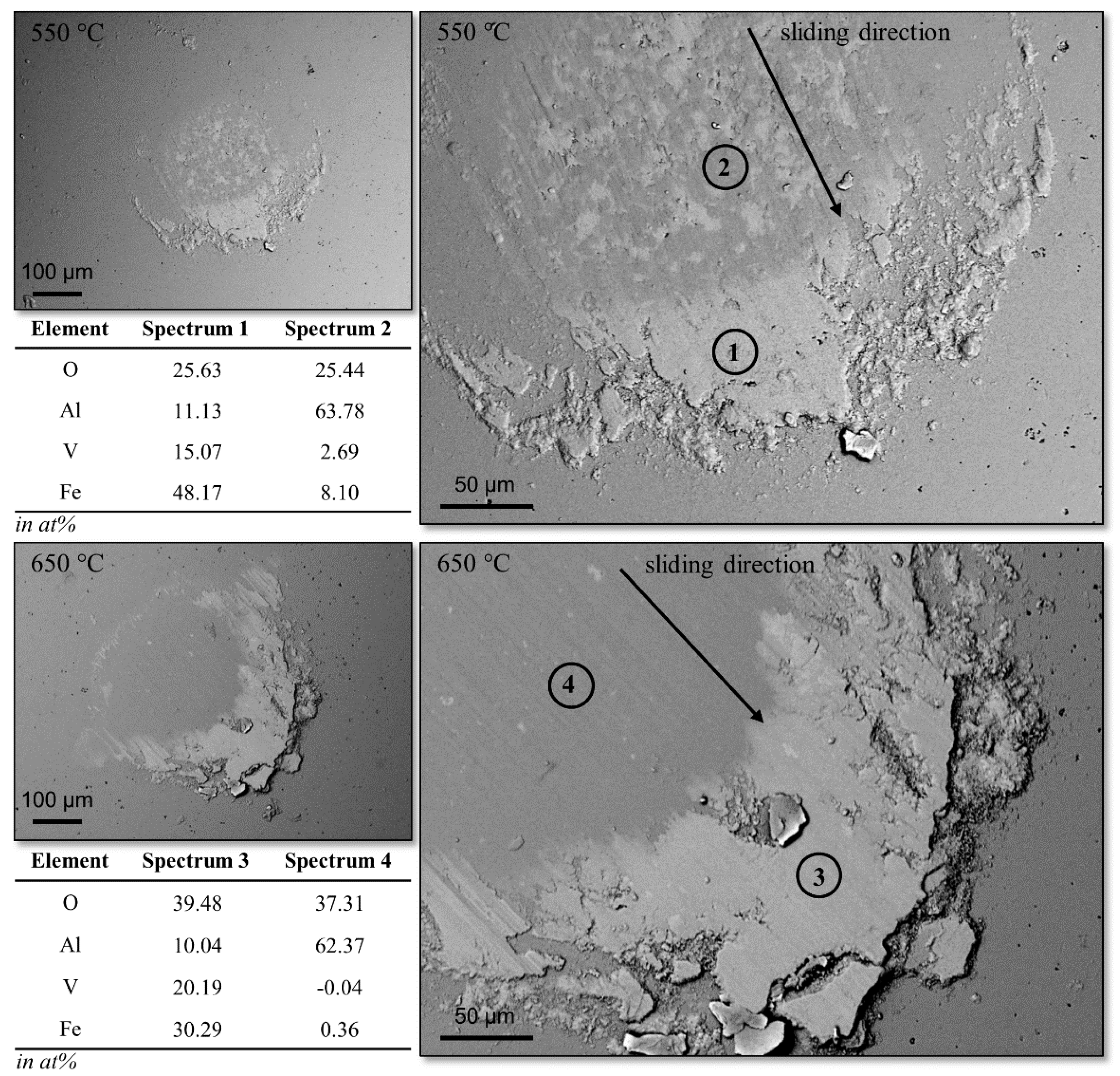
3.1. Tribological Investigation
3.2. XANES Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woydt, M.; Skopp, A.; Dörfel, I.; Witke, K. Wear Engineering Oxides/Antiwear Oxides©. Tribol. Trans. 1999, 42, 21–31. [Google Scholar] [CrossRef]
- Franz, R.; Mitterer, C. Vanadium containing self-adaptive low-friction hard coatings for high-temperature applications: A review. Surf. Coat. Technol. 2013, 228, 1–13. [Google Scholar] [CrossRef]
- Brugnara, R.H. Hochtemperaturaktive HPPMS-Verschleißschutzschichten durch Bildung reibmindernder Magnéli-Phasen im System (Cr,Al,X)N. Ph.D. Thesis, Technische Hochschule Aachen, Aachen, Germany, January 2016. (In German). [Google Scholar]
- Stegemann, B.; Klemm, M.; Horn, S.; Woydt, M. Switching adhesion forces bycrossing the metal-insulator transition in Magneli-type vanadium oxide crystals. Beilstein J. Nanotechnol. 2011, 2, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Reeswinkel, T.; Music, D.; Schneider, J.M. Ab initio calculations of the structure and mechanical properties of vanadium oxides. J. Phys. Condens. Matter 2009, 21, 145404. [Google Scholar] [PubMed]
- Lamsal, C.; Ravindra, N.M. Optical properties of vanadium oxides-an analysis. J. Mater. Sci. 2013, 48, 6341–6351. [Google Scholar] [CrossRef]
- Hryha, E.; Rutqvist, E.; Nyborg, L. Stoichiometric vanadium oxides studied by XPS. Surf. Interface Anal. 2012, 44, 1022–1025. [Google Scholar] [CrossRef]
- Kharton, V. Solid State Electrochemistry I: Fundamentals, Materials and their Applications; John Wiley & Sons: Weinheim, Germany, 2009. [Google Scholar]
- Woydt, M.; Skopp, A.; Dörfel, I.; Witke, K. Wear engineering oxides/anti-wear oxides. Wear 1998, 218, 84–95. [Google Scholar]
- Lugscheider, E.; Bärwulf, S.; Barimani, C. Properties of tungsten and vanadium oxides deposited by MSIP-PVD process for self-lubricating applications. Surf. Coat. Technol. 1999, 120–121, 458–464. [Google Scholar] [CrossRef]
- Lugscheider, E.; Knotek, O.; Bobzin, K.; Bärwulf, S. Tribological properties, phase generation and high temperature phase stability of tungsten- and vanadium-oxides deposited by reactive MSIP-PVD process for innovative lubrication applications. Surf. Coat. Technol. 2000, 133–134, 362–368. [Google Scholar] [CrossRef]
- Gulbiński, W.; Suszko, T.; Sienicki, W.; Warcholiński, B. Tribological properties of silver- and copper-doped transition metal oxide coatings. Wear 2003, 254, 129–135. [Google Scholar] [CrossRef]
- Vernardou, D.; Louloudakis, D.; Spanakis, E.; Katsarakis, N.; Koudoumas, E. Electrochemical properties of vanadium oxide coatings grown by hydrothermal synthesis on FTO substrates. New J. Chem. 2014, 38, 1959–1964. [Google Scholar] [CrossRef]
- Louloudakis, D.; Vernardou, D.; Spanakis, E.; Katsarakis, N.; Koudoumas, E. Electrochemical properties of vanadium oxide coatings grown by APCVD on glass substrates. Surf. Coat. Technol. 2013, 230, 186–189. [Google Scholar] [CrossRef]
- Qui, Y.; Zhang, S.; Lee, J.W.; Li, B.; Wang, Y.; Zhao, D.; Sun, D. Towards hard yet self-lubricious CrAlSiN coatings. J. Alloy. Comp. 2015, 618, 132–138. [Google Scholar]
- Fernandes, F.; Loureiro, A.; Polcar, T.; Cavaleiro, A. The effect of increasing V content on the structure, mechanical properties and oxidation resistance of Ti-Si-V-N films deposited by DC reactive magnetron sputtering. Appl. Sur. Sci. 2014, 289, 114–123. [Google Scholar] [CrossRef]
- Bobzin, K.; Bagcivan, N.; Ewering, M.; Brugnara, R.H.; Theiss, S. DC-MSIP/HPPMS (Cr,Al,V)N and (Cr,Al,W)N thin films for high-temperature friction reduction. Surf. Coat. Technol. 2011, 205, 2887–2892. [Google Scholar] [CrossRef]
- Franz, R.; Neidhardt, J.; Mitterer, C.; Schaffer, B.; Hutter, H.; Kaindl, R.; Sartory, B.; Tessadri, R.; Lechthaler, M.; Polcik, P. Oxidation and diffusion processes during annealing of AlCrVN hard coatings. J. Vac. Sci. Technol. A 2008, 26, 302–308. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Chiu, W.T.; Hung, J.P. Mechanical properties and high temperature oxidation of CrAlSiN/TiVN hard coatings synthesized by cathodic arc evaporation. Surf. Coat. Technol. 2016, 303 Pt A, 18–24. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, S.; Lee, J.W.; Li, B.; Wang, Y.; Zhao, D. Self-lubricating CrAlN/VN multilayer coatings at room temperature. Appl. Surf. Sci. 2013, 279, 189–196. [Google Scholar] [CrossRef]
- Luo, Q. Temperature dependent friction and wear of magnetron sputtered coating TiAlN/VN. Wear 2011, 271, 2058–2066. [Google Scholar] [CrossRef] [Green Version]
- Qui, Y.; Zhang, S.; Li, B.; Wang, Y.; Lee, J.W.; Li, F.; Zhao, D. Improvement of tribological performance of CrN coating via multilayering with VN. Surf. Coat. Technol. 2013, 231, 357–363. [Google Scholar]
- Park, J.K.; Baik, Y.J. Increase of hardness and oxidation resistance of VN coating by nanoscale multilayered structurization with AlN. Mater. Lett. 2008, 62, 2528–2530. [Google Scholar] [CrossRef]
- Tillmann, W.; Kokalj, D.; Stangier, D.; Paulus, M.; Sternemann, C.; Tolan, M. Investigation on the oxidation behavior of AlCrVxN thin films by means of synchrotron radiation and influence on the high temperature friction. Appl. Surf. Sci. 2018, 427, 511–521. [Google Scholar] [CrossRef]
- Wriedt, H.A. The O–V (Oxygen-Vanadium) system. Bull. Alloy Phase Diagr. 1989, 10, 271–277. [Google Scholar] [CrossRef]
- Kutschej, K.; Mayrhofer, P.H.; Kathrein, M.; Polcik, P.; Mitterer, C. A new low-friction concept for Ti1−xAlxN based coatings in high-temperature applications. Surf. Coat. Technol. 2004, 188–189, 358–363. [Google Scholar] [CrossRef]
- Deng, Y.; Yu, S.F.; Yan, N.; Xing, S.L.; Huang, L.B. Effect of vanadium and niobium on abrasive behaviour of arc sprayed 4Cr13 coatings. Appl. Mech. Mater. 2013, 395, 712–717. [Google Scholar] [CrossRef]
- PL 40933 EN 07 PPG SmartArc® Gun Parts List (EN). Available online: https://www.oerlikon.com/metco/en/products-services/coating-equipment/thermal-spray/spray-guns/spray-guns-arc/ppg/ (accessed on 27 December 2018).
- Tillmann, W.; Hagen, L.; Kokalj, D.; Paulus, M.; Tolan, M. A study on the tribological behavior of vanadium-doped arc sprayed coatings. J. Therm. Spray Technol. 2017, 26, 503–516. [Google Scholar] [CrossRef]
- Federation of European Producers of Abrasives. FEPA Grains Standards. Available online: https://www.fepa-abrasives.com/abrasive-products/grains (accessed on 27 December 2018).
- Newbery, A.P.; Grant, P.S.; Neiser, R.A. The velocity and temperature of steel droplets during electric arc spraying. Surf. Coat. Technol. 2005, 195, 91–101. [Google Scholar] [CrossRef]
- Guo, W.; Wu, Y.; Zhang, J.; Yuan, W. Effect of the long-term heat treatment on the cyclic oxidation behavior of Fe-based amorphous/nanocrystalline coatings prepared by high-velocity arc spray process. Surf. Coat. Technol. 2016, 307, 392–398. [Google Scholar] [CrossRef]
- Cheng, J.; Zhao, S.; Liu, D.; Feng, Y.; Liang, X. Microstructure and fracture toughness of the FePSiB-based amorphous/nanocrystalline coatings. Mater. Sci. Eng. A 2017, 696, 341–347. [Google Scholar] [CrossRef]
- Solé, V.A.; Papillon, E.; Cotte, M.; Walter, P.; Susini, J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta B 2007, 62, 63–68. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Poumellec, B.; Marucco, J.F.; Touzelin, B. X-ray-absorption near-edge structure of titanium and vanadium in (titanium,vanadium) dioxide rutile solid solutions. Phys. Rev. B 1987, 35, 2284–2294. [Google Scholar] [CrossRef]
- Perfilyev, V.; Moshkovich, A.; Lapsker, I.; Laikhtman, A.; Rapoport, L. The effect of vanadium content and temperature on stick–slip phenomena under friction of CrV(x)N coatings. Wear 2013, 307, 44–51. [Google Scholar] [CrossRef]
- Hutchings, I.; Shipway, P. Wear by hard particles. In Tribology, 2nd ed.; Hutchings, I., Shipway, P., Eds.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 165–236. [Google Scholar]
- Wong, J.; Lytle, F.W.; Messmer, R.P.; Maylotte, D.H. K−edge absorption spectra of selected vanadium compounds. Phys. Rev. B 1984, 30, 5596–5610. [Google Scholar] [CrossRef]
- Chaurand, P.; Rose, J.; Briois, V.; Salome, M.; Proux, O.; Nassif, V.; Olivi, L.; Susini, J.; Hazemann, J.L.; Bottero, J.Y. New methodological approach for the vanadium K-edge X-ray absorption near-edge structure interpretation: Application to the speciation of vanadium in oxide phases from steel slag. J. Phys. Chem. B 2007, 111, 5101–5110. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.A.; Wandzilak, A.; Maganas, D.; Wurster, N.I.C.; Hugenbruch, S.; Kowalska, J.K.; Pollock, C.J.; Lima, F.A.; Finkelstein, K.D.; DeBeer, S. Experimental and theoretical correlations between vanadium K-edge X-ray absorption and Kβ emission spectra. J. Biol. Inorg. Chem. 2016, 21, 793–805. [Google Scholar] [CrossRef]
- Tanaka, T.; Yamashita, H.; Tsuchitani, R.; Funabiki, T.; Yoshida, S. X-ray absorption (EXAFS/XANES) study of supported vanadium oxide catalysts. Structure of surface vanadium oxide species on silica and (γ-alumina at a low level of vanadium loading. J. Chem. Soc. Faraday Trans. 1 1988, 84, 2987–2999. [Google Scholar] [CrossRef]
- Krause, B.; Darma, S.; Kaufholz, M.; Mangold, S.; Doyle, S.; Ulrich, S.; Leiste, H.; Stuber, M.; Baumbach, T. Composition-dependent structure of polycrystalline magnetron-sputtered V-Al-C-N hard coatings studied by XRD, XPS XANES and EXAFS. J. Appl. Crystallogr. 2013, 46, 1064–1075. [Google Scholar] [CrossRef]
- Giuli, G.; Paris, E.; Mungall, J.; Romano, C.; Dingwell, D. V oxidation state and coordination number in silicate glasses by XAS. Am. Mineral. 2004, 89, 1640–1646. [Google Scholar] [CrossRef]
- Sutton, S.R.; Karner, J.; Papike, J.; Delaney, J.S.; Shearer, C.; Newville, M.; Eng, P.; Rivers, M.; Dyar, M.D. Vanadium K edge XANES of synthetic and natural basaltic glasses and application to microscale oxygen barometry. Geochim. Cosmochim. Acta 2005, 69, 2333–2348. [Google Scholar] [CrossRef]
- Passerini, S.; Smyrl, W.H.; Berrettoni, M.; Tossici, R.; Rosolen, M.; Marassi, R.; Decker, F. XAS and electrochemical characterization of lithium intercalated V2O5 xerogels. Solid State Ion. 1996, 90, 5–14. [Google Scholar] [CrossRef]
- Wilke, M.; Farges, F.; Petit, P.E.; Brown, G.E.J.; Martin, F. Oxidation state and coordination of Fe in minerals: An Fe K-XANES spectroscopic study. Am. Mineral. 2001, 86, 714–730. [Google Scholar] [CrossRef]
- Wilke, M.; Partzsch, G.M.; Bernhardt, R.; Lattard, D. Determination of the iron oxidation state in basaltic glasses using XANES at the K-edge. Chem. Geol. 2004, 213, 71–87. [Google Scholar] [CrossRef]
- Petit, P.-E.; Farges, F.; Wilke, M.; Solé, V.A. Determination of the iron oxidation state in Earth materials using XANES pre-edge information. J. Synchrotron Radiat. 2001, 8, 952–954. [Google Scholar] [CrossRef] [Green Version]
- Farges, F. Ab initio and experimental pre-edge investigations of the Mn K-edge XANES in oxide-type materials. Phys. Rev. B 2005, 71, 155109. [Google Scholar] [CrossRef]
- Rehr, J.J.; Albers, R.C. Theoretical approaches to x-ray absorption fine structure. Rev. Mod. Phys. 2000, 72, 621–654. [Google Scholar] [CrossRef]




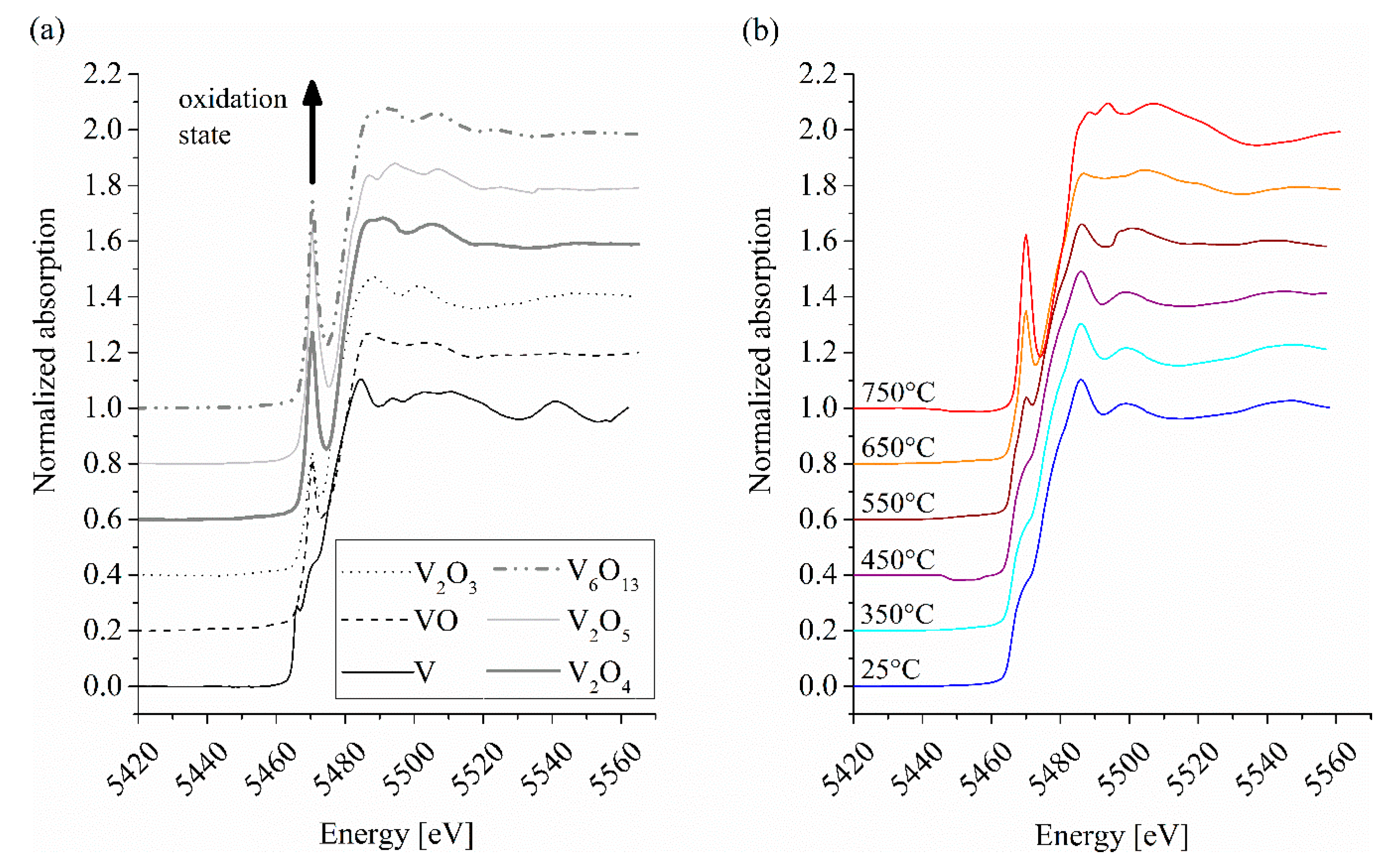

| Phase | Oxidation-State | Pre-Edge | Main-Edge Position (eV) | |
|---|---|---|---|---|
| Normalized Intensity | Position (eV) | |||
| V | 0 | 0.29 | 1 | 9.5 |
| VO | 2 | 0.62 | 5.5 | 8.7 |
| V2O3 | 3 | 0.44 | 5.5 | 10.8 |
| V2O4 | 4 | 0.67 | 5.5 | 13.2 |
| V6O13 | 4.3 | 0.75 | 5.5 | 14.4 |
| V2O5 | 5 | 0.85 | 5.5 | 13.8 |
| Coating Temperature (°C) | Pre-Edge | Main-Edge Position (eV) | |
|---|---|---|---|
| Normalized Intensity | Position (eV) | ||
| 25 | 0.39 * | 5 * | 9.5 |
| 350 | 0.39 * | 5 * | 9.5 |
| 450 | 0.43 * | 5 * | 9.5 |
| 550 | 0.47 | 5 | 9.8 |
| 650 | 0.57 | 5 | 11.6 |
| 750 | 0.74 | 5 | 12.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tillmann, W.; Hagen, L.; Kokalj, D.; Paulus, M.; Tolan, M. Temperature-Induced Formation of Lubricous Oxides in Vanadium Containing Iron-Based Arc Sprayed Coatings. Coatings 2019, 9, 18. https://doi.org/10.3390/coatings9010018
Tillmann W, Hagen L, Kokalj D, Paulus M, Tolan M. Temperature-Induced Formation of Lubricous Oxides in Vanadium Containing Iron-Based Arc Sprayed Coatings. Coatings. 2019; 9(1):18. https://doi.org/10.3390/coatings9010018
Chicago/Turabian StyleTillmann, Wolfgang, Leif Hagen, David Kokalj, Michael Paulus, and Metin Tolan. 2019. "Temperature-Induced Formation of Lubricous Oxides in Vanadium Containing Iron-Based Arc Sprayed Coatings" Coatings 9, no. 1: 18. https://doi.org/10.3390/coatings9010018




