Pulsed Laser Deposited Biocompatible Lithium-Doped Hydroxyapatite Coatings with Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. PLD Experimental Protocol
2.2. Characterization of Synthesized Structures
2.2.1. Atomic Force Microscopy
2.2.2. In Vitro Biological Tests
Biocompatibility Assays
Antimicrobial Assays
3. Results
3.1. AFM Analysis
3.2. Biocompatibility Assays
3.2.1. Cytotoxicity Test
3.2.2. Cellular Cycle Assay
3.3. Anti-Biofilm Activity
3.3.1. Anti-Staphylococcal Biofilm Activity of Obtained Coatings
3.3.2. Anti-Fungal Biofilm Activity of Obtained Coatings
4. Discussion
4.1. Biocompatibility
4.2. Possible Mechanisms Explaining the Antimicrobial Activity of Synthesized Structures
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing anti-biofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, L.M.; Cotter, P.D.; Hill, C.; Alvarez-Ordóñez, A. New weapons to fight old enemies: Novel strategies for the (bio) control of bacterial biofilms in the food industry. Front. Microbiol. 2016, 7, 400–405. [Google Scholar] [CrossRef]
- Del Pozo, J.L. Biofilm-related disease. Expert Rev. Anti-Infect. Ther. 2018, 16, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Petrachi, T.; Resca, E.; Piccinno, M.S.; Biagi, F.; Strusi, V.; Dominici, M.; Veronesi, E. An alternative approach to investigate biofilm in medical devices: A feasibility study. Int. J. Environ. Res. Public Health 2017, 14, 1587. [Google Scholar] [CrossRef] [PubMed]
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claessen, D.; Rozen, D.E.; Kuipers, O.P.; Søgaard-Andersen, L.; Van Wezel, G.P. Bacterial solutions to multicellularity: A tale of biofilms, filaments and fruiting bodies. Nat. Rev. Microbiol. 2014, 12, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.-S.; Michael, O. Molecular basis of in-vivo biofilm formation by bacterial pathogens. Chem. Biol. 2012, 19, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Lazar, V. Quorum sensing in biofilms—How to destroy the bacterial citadels or their cohesion/power? Anaerobe 2011, 17, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Olar, R.; Badea, M.; Marinescu, D.; Chifiriuc, M.C.; Bleotu, C.; Grecu, M.N.; Iorgulescu, E.E.; Lazar, V. N,N-dimethylbiguanide complexes displaying low cytotoxicity as potential large spectrum antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 3027–3034. [Google Scholar] [CrossRef] [PubMed]
- Limban, C.; Missir, A.V.; Nuta, D.C.; Caproiu, M.T.; Morusciag, L.; Chirita, C.; Cupii, A.; Gurgu, H. Advances in research of new 2-((4-ethylphenoxy)methyl)-N-(arylcarbamothioyl)benzamides. Farmacia 2015, 63, 376–380. [Google Scholar]
- Limban, C.; Missir, A.V.; Nuţă, D.C.; Căproiu, M.T.; Papacocea, M.T.; Chiriţă, C.O. Synthesis of some new 2-((4-chlorophenoxy)methyl)-N-(arylcarbamothioyl) benzamides as potential antifungal agents. Farmacia 2016, 64, 775–779. [Google Scholar]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S. Bad bugs, no drugs: No ESKAPE! An update from the infectious diseases society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Anghel, I.; Holban, A.M.; Andronescu, E.; Grumezescu, A.M.; Chifiriuc, M.C. Efficient surface functionalization of wound dressings by a phytoactivenanocoating refractory to Candida albicans biofilm development. Biointerphases 2013, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, M.C.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Cotar, A.I.; Grumezescu, V.; Bezirtzoglou, E.; Lazar, V.; Radulescu, R. Water dispersible magnetite nanoparticles influence the efficacy of antibiotics against planktonic and biofilm embedded enterococcus faecalis cells. Anaerobe 2013, 22, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphates: Occurrence properties biomineralization pathological calcification and biomimetic applications. Biomatter 2011, 1, 121–164. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Boi, M.; Bianchi, M. A review on ionic substitutions in hydroxyapatite thin films: Towards complete biomimetism. Coatings 2018, 8, 269. [Google Scholar] [CrossRef]
- Lambert, F.; Angélique, L.; Pierre, D.; Sophie, S.; Pierre, L.; Eric, R. Influence of space-filling materials in subantral bone augmentation: Blood clot vs. autogenous bone chips vs. bovine hydroxyapatite. Clin. Oral Implant. Res. 2011, 22, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, O.; Eray, M.E.; Sibel, D.; Serdar, S.; Simeon, A.; Faik, N.O. Composites of bovine hydroxyapatite (BHA) and ZnO. J. Mater. Sci. 2008, 43, 2536–2540. [Google Scholar] [CrossRef]
- Oktar, F.N.; Agathopoulos, S.; Ozyegin, L.S.; Gunduz, O.; Demirkol, N.; Bozkurt, Y.; Salman, S. Mechanical properties of bovine hydroxyapatite (BHA) composites doped with SiO2, MgO, Al2O3, and ZrO2. J. Mater. Sci. Mater. Med. 2007, 18, 2137–2143. [Google Scholar] [CrossRef]
- Duta, L.; Mihailescu, N.; Popescu, A.C.; Luculescu, C.R.; Mihailescu, I.N.; Cetin, G.; Gunduz, O.; Oktar, F.N.; Popa, A.C.; Kuncser, A.; et al. Comparative physical, chemical and biological assessment of simple and titanium-doped ovine dentine-derived hydroxyapatite coatings fabricated by pulsed laser deposition. Appl. Surf. Sci. 2017, 413, 129–139. [Google Scholar] [CrossRef]
- Popescu, A.C.; Florian, P.E.; Stan, G.E.; Popescu-Pelin, G.; Zgura, I.; Enculescu, M.; Oktar, F.N.; Trusca, R.; Sima, L.E.; Roseanu, A.; et al. Physical-chemical characterization and biological assessment of simple and lithium-doped biological-derived hydroxyapatite thin films for a new generation of metallic implants. Appl. Surf. Sci. 2018, 439, 724–735. [Google Scholar] [CrossRef]
- Duta, L.; Oktar, F.N.; Stan, G.E.; Popescu-Pelin, G.; Serban, N.; Luculescu, C.; Mihailescu, I.N. Novel doped hydroxyapatite thin films obtained by pulsed laser deposition. Appl. Surf. Sci. 2013, 265, 41–49. [Google Scholar] [CrossRef]
- Gunduz, O.; Ahmad, Z.; Ekren, N.; Agathopoulos, S.; Salman, S.; Oktar, F.N. Reinforcing of biologically derived apatite with commercial inert glass. J. Thermoplast. Compos. Mater. 2009, 22, 407–419. [Google Scholar] [CrossRef]
- Khandan, A.; Majid, A.; Neriman, O.; Hamid, G. Study of the bioactivity, wettability and hardness behaviour of the bovine hydroxyapatite-diopside bio-nanocomposite coating. J. Taiwan Inst. Chem. Eng. 2016, 60, 538–546. [Google Scholar] [CrossRef]
- Mihailescu, N.; Stan, G.E.; Duta, L.; Mariana, C.C.; Coralia, B.; Sopronyi, M.; Luculescu, C.; Oktar, F.N.; Mihailescu, I.N. Structural, compositional, mechanical characterization and biological assessment of bovine-derived hydroxyapatite coatings reinforced with MgF2 or MgO for implants functionalization. Mater. Sci. Eng. C 2016, 59, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, O.; Godec, C.; Ahmad, Z.; Gokce, H.; Yetmez, M.; Kalkandelen, C.; Sahin, Y.M.; Oktar, F.N. Preparation and evaluation of cerium oxide-bovine hydroxyapatite composites for biomedical engineering applications. J. Mech. Behav. Biomed. 2014, 35, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Boutinguiza, M.; Pou, J.; Comesaña, R.; Lusquiños, F.; de Carlos, A.; León, B. Biological hydroxyapatite obtained from fish bones. Mater. Sci. Eng. C 2012, 32, 478–486. [Google Scholar] [CrossRef]
- Eisenhart, S. EU Regulation 722. New EU Animal Tissue Regulations in Effect for Some Medical Devices; Emergo: Hong Kong, China, 2013; Available online: https://www.emergobyul.com/blog/2013/09/new-eu-animal-tissue-regulations-effect-some-medical-devices (accessed on 16 January 2019).
- ISO 22442-1 Medical Devices Utilizing Animal Tissues and Their Derivatives—Part 1: Application of Risk Management; International Organization for Standardization: Berlin, Germany, 2015.
- Eaton, P.; West, P. Atomic Force Microscopy, 1st ed.; Oxford University Press: Oxford, UK, 2010; pp. 1–256. [Google Scholar]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [Green Version]
- ISO 10993-5 Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Berlin, Germany, 2009.
- Duta, L.; Ristoscu, C.; Stan, G.E.; Husanu, M.A.; Besleaga, C.; Chifiriuc, M.C.; Lazar, V.; Bleotu, C.; Miculescu, F.; Mihailescu, N.; et al. New bio-active, antimicrobial and adherent coatings of nanostructured carbon double-reinforced with silver and silicon by Matrix-Assisted Pulsed Laser Evaporation for medical applications. Appl. Surf. Sci. 2018, 441, 871–883. [Google Scholar] [CrossRef]
- Stan, G.E.; Popescu, A.C.; Mihailescu, I.N.; Marcov, D.A.; Mustata, R.C.; Sima, L.E.; Petrescu, S.M.; Ianculescu, A.; Trusca, R.; Morosanu, C.O. On the bioactivity of adherent Bioglass thin films synthesized by magnetron sputtering techniques. Thin Solid Films 2010, 518, 5955–5964. [Google Scholar] [CrossRef]
- Clover, J.; Gowen, M. Are MG-63 and HOS TE85 human osteosarcoma cell lines representative models of the osteoblastic phenotype? Bone 1994, 15, 585–591. [Google Scholar] [CrossRef]
- Skindersoe, M.E.; Krogfelt, K.A.; Blom, A.; Jiang, G.; Prestwich, G.D.; Mansell, J.P. Dual action of lysophosphatidate-functionalised titanium: Interactions with human (MG63) Osteoblasts and methicillin resistant staphylococcus aureus. PLoS ONE 2015, 10, e0143509. [Google Scholar] [CrossRef] [PubMed]
- Mitik-Dineva, N.; Wang, J.; Truong, V.K.; Stoddart, P.; Malherbe, F.; Crawford, R.J.; Ivanova, E.P. Escherichia coli, pseudomonas aeruginosa, and staphylococcus aureus attachment patterns on glass surfaces with nanoscale roughness. Curr. Microbiol. 2009, 58, 268–273. [Google Scholar]
- Chua, P.-H.; Neoh, K.-G.; Kang, E.-T.; Wang, W. Surface functionalization of titanium with hyaluronic acid/chitosan polyelectrolyte multilayers and RGD for promoting osteoblast functions and inhibiting bacterial adhesion. Biomaterials 2008, 29, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Paulone, S.; Ardizzoni, A.; Tavanti, A.; Piccinelli, S.; Rizzato, C.; Lupetti, A.; Colombari, B.; Pericolini, E.; Polonelli, L.; Magliani, W.; et al. The synthetic killer peptide KP impairs Candida albicans biofilm in vitro. PLoS ONE 2017, 13, e0181278. [Google Scholar] [CrossRef] [PubMed]
- Tsui, C.; Kong, E.F.; Jabra-Rizk, M.A. Pathogenesis of Candida albicans biofilm. Pathog. Dis. 2016, 74, ftw018. [Google Scholar] [CrossRef] [PubMed]
- Groza, A.; Ciobanu, C.S.; Popa, C.L.; Iconaru, S.L.; Chapon, P.; Luculescu, C.; Ganciu, M.; Predoi, D. Structural properties and antifungal activity against Candida albicans biofilm of different composite layers based on Ag/Zn doped Hydroxyapatite-polydimethylsiloxanes. Polymers 2016, 8, 131. [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic substitutions in hydroxyapatite: Current status of the derived biofunctional effects and their in vitro interrogation methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef]
- Cleare, A.; Pariante, C.M.; Young, A.H.; Anderson, I.M.; Christmas, D.; Cowen, P.J.; Dickens, C.; Ferrier, I.N.; Geddes, J.; Gilbody, S.; et al. Evidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 2008 British Association for Psychopharmacology guidelines. J. Psychopharmacol. 2015, 29, 459–525. [Google Scholar] [CrossRef]
- Cohen, O.; Rais, T.; Lepkifker, E.; Vered, I. Lithium carbonate therapy is not a risk factor for osteoporosis. Horm. Metab. Res. 1998, 30, 594–597. [Google Scholar] [CrossRef]
- Nordenstrom, J.; Elvius, M.; Bagedahl-Strindlund, M.; Zhao, B.; Torring, O. Biochemical hyperparathyroidism and bone-mineral status in patients treated long-term with lithium. Metabolism 1994, 43, 1563–1567. [Google Scholar] [CrossRef]
- Satija, N.K.; Sharma, D.; Afrin, F.; Tripathi, R.P.; Gangenahalli, G. High throughput transcriptome profiling of lithium stimulated human mesenchymal stem cells reveals priming towards osteoblastic lineage. PLoS ONE 2013, 8, e55769. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A.; Omrani, G.R.; Nasab, M.M. Lithium’s effect on bone mineral density. Bone 2009, 44, 331–334. [Google Scholar] [CrossRef]
- Yang, M.L.; Li, J.J.; So, K.F.; Chen, J.Y.H.; Cheng, W.S.; Wu, J.; Wang, Z.M.; Gao, F.; Young, W. Efficacy and safety of lithium carbonate treatment of chronic spinal cord injuries: A double-blind, randomized, placebo-controlled clinical trial. Spinal Cord 2012, 50, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Gitlin, M. Lithium side effects and toxicity: Prevalence and management strategies. Int. J. Bipolar Disord. 2016, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Ott, M.; Stegmayr, B.; Salander, R.E.; Werneke, U. Lithium intoxication: Incidence, clinical course and renal function—A population-based retrospective cohort study. J. Psychopharmacol. 2016, 30, 1008–1019. [Google Scholar] [CrossRef]
- Rybakowski, J.K. Challenging the negative perception of lithium and optimizing its long-term administration. Front. Mol. Neurosci. 2018, 11, 349. [Google Scholar] [CrossRef]
- Post, R.M. The new news about lithium: An underutilized treatment in the United States. Neuropsychopharmacology 2018, 43, 1174–1179. [Google Scholar] [CrossRef]
- Mayer, I.; Berger, U.; Markitziu, A.; Gidalia, I. The uptake of lithium ions by synthetic carbonated hydroxyapatite. Calcif. Tissue Int. 1986, 38, 293–295. [Google Scholar] [CrossRef]
- Koutsoukos, P.G.; Nancollas, G.H. The effect of lithium on the precipitation of hydroxyapatite from aqueous solutions. Colloids Surf. 1986, 17, 361–370. [Google Scholar] [CrossRef]
- Shainberg, A.P.M.; Valério, P.; Zonari, A.; Oktar, F.N.; Ozyegin, L.S.; Graça, M.P.F.; Leite, M.F.; Goes, A.M. Attachment and proliferation of osteoblasts on lithium-hydroxyapatite composites. Adv. Mater. Sci. Eng. 2012, 2012, 650574. [Google Scholar] [CrossRef]
- Gough, J.; Notingher, I.; Hench, L.L. Osteoblast attachment and mineralized nodule formation on rough and smooth 45S5 bioactive glass monoliths. J. Biomed. Mater. Res. Part A 2004, 68, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, X.; Gu, Z.; Qin, H.; Li, L.; Liu, J.; Yu, X. In vitro study on the degradation of lithium-doped hydroxyapatite for bone tissue engineering scaffold. Mater. Sci. Eng. C 2016, 66, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Meunier, P.J.; Roux, C.; Seeman, E.; Ortolani, S.; Badurski, J.E.; Spector, T.D.; Cannata, J.; Balogh, A.; Lemmel, E.M.; Pors-Nielsen, S.; et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N. Engl. J. Med. 2004, 350, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Wexler, E.M.; Geschwind, D.H.; Palmer, T.D. Lithium regulates adult hippocampal progenitor development through canonical Wnt pathway activation. Mol. Psychiatry 2008, 13, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Khorami, M.; Hesaraki, S.; Behnamghader, A.; Nazarian, H.; Shahrabi, S. In vitro bioactivity and biocompatibility of lithium substituted 45S5 bioglass. Mater. Sci. Eng. C 2011, 31, 1584–1592. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Yu, X.; Xu, Y.; Feng, T.; Ren, D. In vitro study in stimulating the secretion of angiogenic growth factors of strontium-doped calcium polyphosphate for bone tissue engineering. J. Mater. Sci. Mater. Med. 2011, 22, 683–692. [Google Scholar] [CrossRef]
- Tang, G.; Xu, J.; Chen, R.; Qian, Y.; Shen, G. Lithium delivery enhances bone growth during midpalatal expansion. J. Dent. Res. 2011, 90, 336–340. [Google Scholar] [CrossRef]
- Oh, S.H.; Choi, S.Y.; Choi, S.H.; Lee, Y.K.; Kim, K.N. The influence of lithium fluoride on in vitro biocompatibility and bioactivity of calcium aluminate-PMMA composite cement. J. Mater. Sci. Mater. Med. 2004, 15, 25–33. [Google Scholar] [CrossRef]
- Miguez-Pacheco, V.; Büttner, T.; Maçon, A.L.B.; Jones, J.R.; Fey, T.; de Ligny, D.; Greil, P.; Chevalier, J.; Malchere, A.; Boccaccini, A.R. Development and characterization of lithium-releasing silicate bioactive glasses and their scaffolds for bone repair. J. Non-Cryst. Solids 2016, 432, 65–72. [Google Scholar] [CrossRef]
- Keong, L.C.; Halim, A.S. In vitro models in biocompatibility assessment for biomedical-grade chitosan derivatives in wound management. Int. J. Mol. Sci. 2009, 10, 1300–1313. [Google Scholar] [CrossRef] [PubMed]
- Buskermolen, J.K.; Reijnders, C.M.A.; Spiekstra, S.W.; Steinberg, T.; Kleverlaan, C.J.; Feilzer, A.J.; Bakker, A.D.; Gibbs, S. Development of a full-thickness human gingiva equivalent constructed from immortalized keratinocytes and fibroblasts. Tissue Eng. Part C Methods 2016, 22, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Schalock, P.C.; Crawford, G.; Nedorost, S.; Scheinman, P.L.; Atwater, A.R.; Mowad, C.; Brod, B.; Ehrlich, A.; Watsky, K.L.; Sasseville, D.; et al. Patch testing for evaluation of hypersensitivity to implanted metal devices: A perspective from the american contact dermatitis society. Dermatitis 2016, 27, 241–247. [Google Scholar] [CrossRef] [PubMed]
- An, Y.H.; Friedman, R.J. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. (Appl. Biomater.) 1998, 43, 338–348. [Google Scholar] [CrossRef]
- Lieb, J. Lithium and antidepressants: Inhibiting eicosanoids, stimulating immunity, and defeating microorganisms. Med. Hypotheses 2002, 59, 429–432. [Google Scholar] [CrossRef]
- Fabrizi, C.; Pompili, E.; Somma, F.; De Vito, S.; Ciraci, V.; Artico, M.; Lenzi, P.; Fornai, F.; Fumagalli, L. Lithium limits trimethyltin-induced cytotoxicity and proinflammatory response in microglia without affecting the concurrent autophagy impairment. J. Appl. Toxicol. 2017, 37, 207–213. [Google Scholar] [CrossRef]
- Moghanian, A.; Firoozi, S.; Tahriri, M.; Sedghi, A. A comparative study on the in vitro formation of hydroxyapatite, cytotoxicity and antibacterial activity of 58S bioactive glass substituted by Li and Sr. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 349–360. [Google Scholar] [CrossRef]
- Douglas, L.J. Candida biofilms and their role in infection. Trends Microbiol. 2003, 11, 30–36. [Google Scholar] [CrossRef]
- Sadik, O.; Gheorghe, I.; Chifiriuc, M.C. Virulence and pathogenicity aspects in Candida albicans infections. Rev. Biol. Biomed. Sci. 2018, 1, 11–16. [Google Scholar] [CrossRef]
- Kart, D.; Tavernier, S.; Van Acker, H.; Nelis, H.J.; Coenye, T. Activity of disinfectants against multispecies biofilms formed by Staphylococcus aureus. Candida albicans and Pseudomonas aeruginosa. Biofouling 2014, 30, 377–383. [Google Scholar] [CrossRef]
- Jenkinson, H.F.; Lamont, R.J. Oral microbial communities in sickness and in health. Trend. Microbiol. 2005, 13, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Harriott, M.M.; Noverr, M.C. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: Effects on antimicrobial resistance. Antimicrob. Agents Chemother. 2009, 53, 3914–3922. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, N.L.; Gravina, N.; Ruso, J.M.; Marco-Brown, J.L.; Sieben, J.M.; Messina, P.V. Albumin-mediated deposition of bone-like apatite onto nano-sized surfaces: Effect of surface reactivity and interfacial hydration. J. Colloid Interface Sci. 2017, 494, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Giraldez, M.J.; Resua, C.G.; Lira, M.; Oliveira, M.E.C.R.; Magariños, B.; Toranzo, A.E.; Yebra-Pimentel, E. Contact lens hydrophobicity and roughness effects on bacterial adhesion. Optom. Vis. Sci. 2010, 87, E426–E431. [Google Scholar] [CrossRef]
- Tamburic, S.D.; Vuleta, G.M.; Ognjanovic, J.M. In vitro release of calcium and hydroxyl ions from two types of calcium hydroxide preparations. Int. Endod. J. 1993, 26, 125–130. [Google Scholar] [CrossRef]
- Absolom, D.R.; Lamberti, F.V.; Policova, Z.; Zingg, W.; van Oss, C.J.; Neumann, A.W. Surface thermodynamics of bacterial adhesion. Appl. Environ. Microb. 1983, 46, 90–97. [Google Scholar]
- Anselme, K.; Davidson, P.; Popa, A.; Giazzon, M.; Liley, M.; Ploux, L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef]
- Ionescu, A.; Wutscher, E.; Brambilla, E.; Schneider-Feyrer, S.; Giessibl, F.J.; Hahnel, S. Influence of surface properties of resin-based composites on in vitro streptococcus mutans biofilm development. Eur. J. Oral Sci. 2012, 120, 458–465. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M.; Papaioannou, W.; Van Eldere, J.; van Steenberghe, D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: Short-term observations. Int. J. Oral Maxillofac. Implant. 1996, 11, 169–178. [Google Scholar]
- Hansson, K.N.; Hansson, S. Skewness and kurtosis: Important parameters in the characterization of dental implant surface roughness—A computer simulation. ISRN Mater. Sci. 2011, 2011, 305312. [Google Scholar] [CrossRef]
- Cheng, T.; Chen, Y.; Nie, X. Insertion torques influenced by bone density and surface roughness of HA–TiO2 coatings. Thin Solid Films 2013, 549, 123–130. [Google Scholar] [CrossRef]
- Han, L.; Wang, M.; Sun, H.; Li, P.; Wang, K.; Ren, F.; Lu, X. Porous titanium scaffolds with self-assembled micro/nano hierarchical structure for dual functions of bone regeneration and anti-infection. J. Biomed. Mater. Res. A 2017, 105, 3482–3492. [Google Scholar] [CrossRef] [PubMed]
- Karr, J.C.; Lauretta, J. In vitro activity of calcium sulfate and hydroxyapatite antifungal disks loaded with amphotericin B or voriconazole in consideration for adjunctive osteomyelitis management. J. Am. Podiatr. Med. Assoc. 2015, 105, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.Q.; AlJohny, B.O.; Wainwright, M. Antibacterial effects of pure metals on clinically important bacteria growing in planktonic cultures and biofilms. Afr. J. Microbiol. Res. 2014, 8, 1080–1088. [Google Scholar]
- Harrison, J.C.; Zyla, T.R.; Bardes, E.S.; Lew, D.J. Stress-specific activation mechanisms for the “cell integrity” MAPK pathway. J. Biol. Chem. 2004, 279, 2616–2622. [Google Scholar]







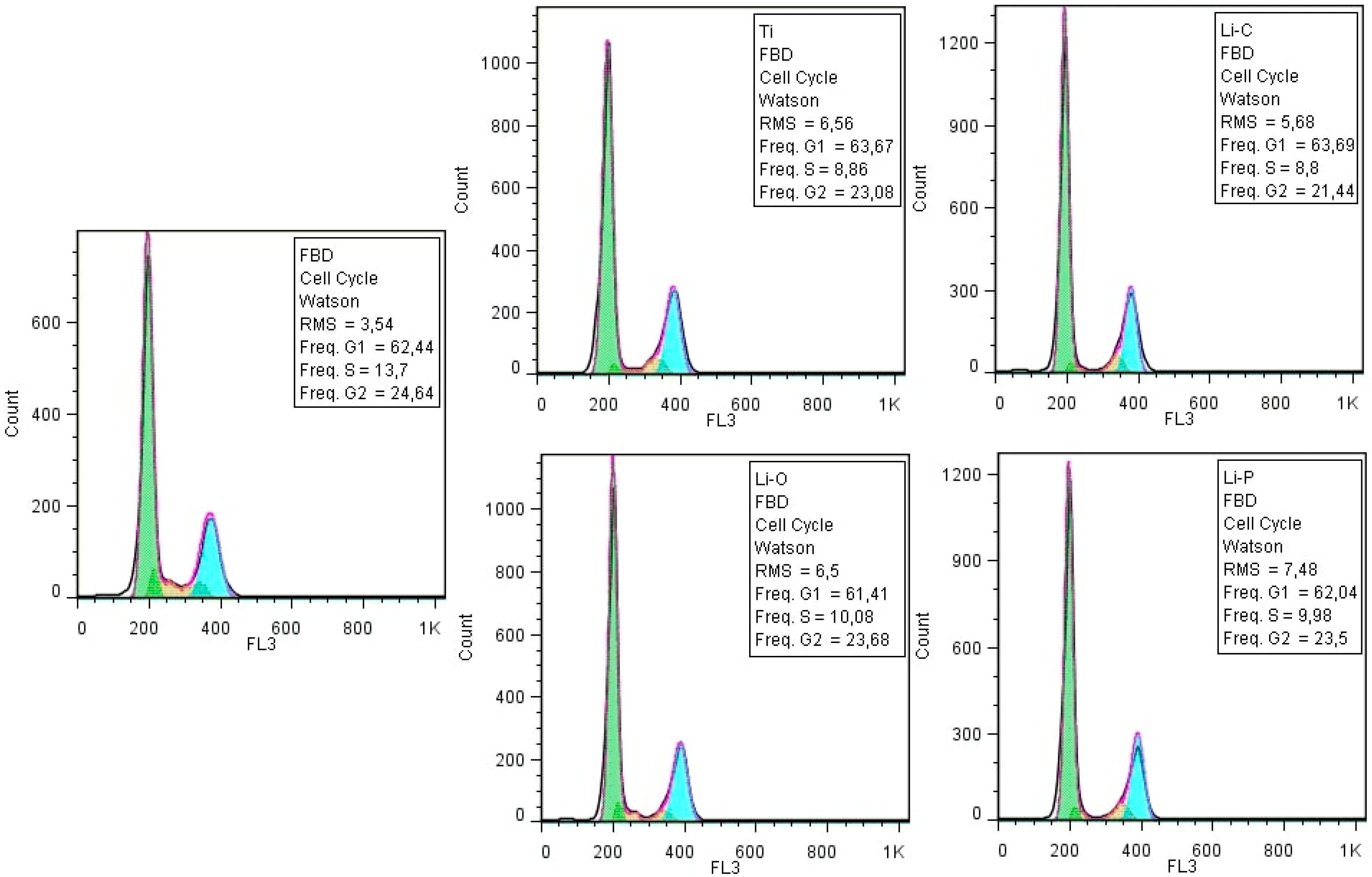
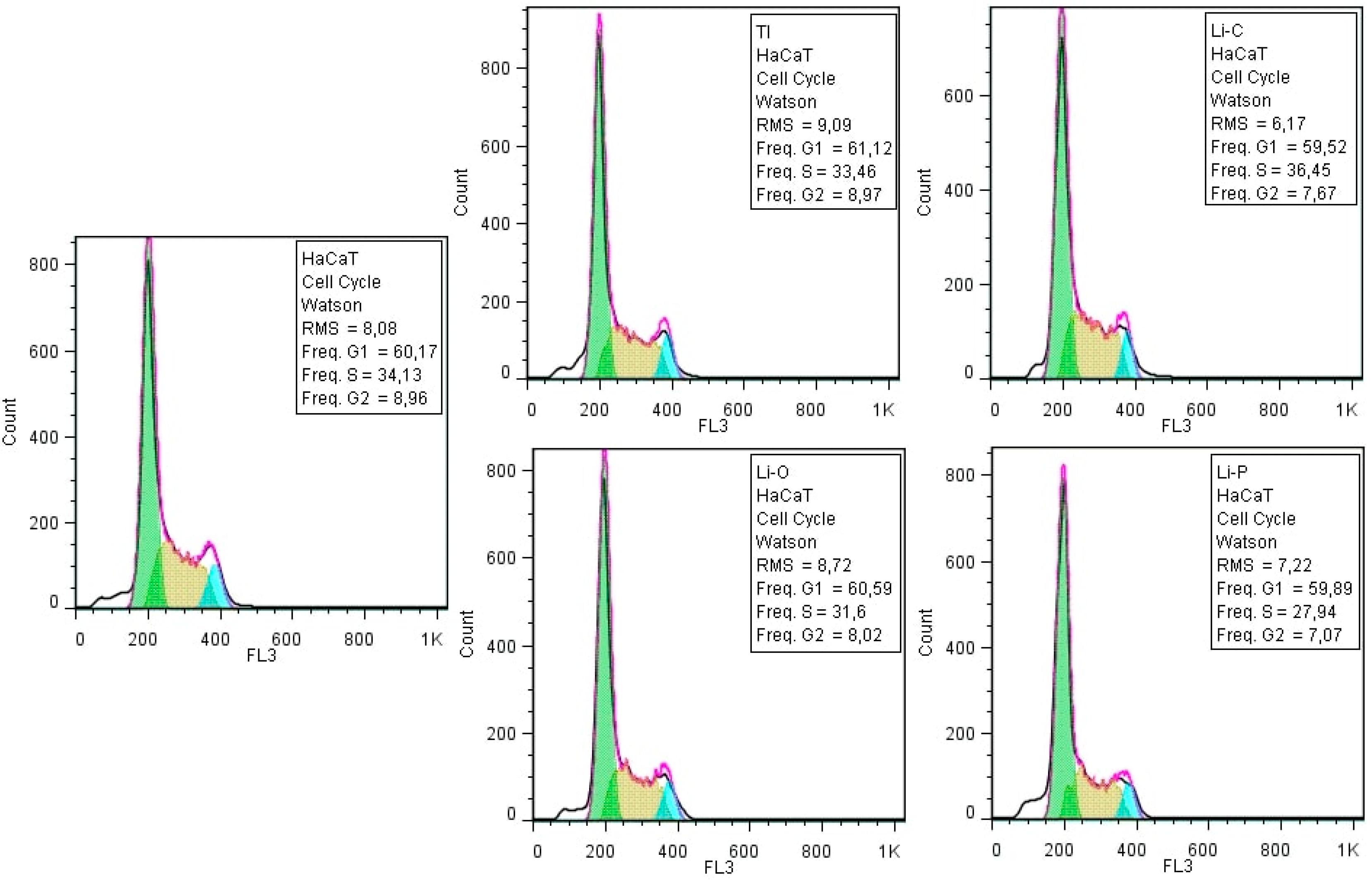





| Sample Code | Sample Description |
|---|---|
| Ti | Bare Ti control |
| Si | Simple Si control |
| Li-0 | Simple BHA film deposited on Ti |
| Li-C | BHA:Li2CO3 film deposited on Ti |
| Li-P | BHA:Li3PO4 film deposited on Ti |
| Sample Code | Amplitude Parameter | Scanning Area (0.5 × 0.5 µm2) |
|---|---|---|
| Si | Ra (nm) | 0.10 |
| Rsk | 0.09 | |
| Rku | 3.03 | |
| Li-0 | Ra (nm) | 5.86 |
| Rsk | 0.46 | |
| Rku | 3.11 | |
| Li-C | Ra (nm) | 10.26 |
| Rsk | 0.56 | |
| Rku | 3.49 | |
| Li-P | Ra (nm) | 4.32 |
| Rsk | 0.17 | |
| Rku | 5.58 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duta, L.; Chifiriuc, M.C.; Popescu-Pelin, G.; Bleotu, C.; Gradisteanu, G.; Anastasescu, M.; Achim, A.; Popescu, A. Pulsed Laser Deposited Biocompatible Lithium-Doped Hydroxyapatite Coatings with Antimicrobial Activity. Coatings 2019, 9, 54. https://doi.org/10.3390/coatings9010054
Duta L, Chifiriuc MC, Popescu-Pelin G, Bleotu C, Gradisteanu G, Anastasescu M, Achim A, Popescu A. Pulsed Laser Deposited Biocompatible Lithium-Doped Hydroxyapatite Coatings with Antimicrobial Activity. Coatings. 2019; 9(1):54. https://doi.org/10.3390/coatings9010054
Chicago/Turabian StyleDuta, Liviu, Mariana Carmen Chifiriuc, Gianina Popescu-Pelin, Coralia Bleotu, Gratiela (Pircalabioru) Gradisteanu, Mihai Anastasescu, Alexandru Achim, and Andrei Popescu. 2019. "Pulsed Laser Deposited Biocompatible Lithium-Doped Hydroxyapatite Coatings with Antimicrobial Activity" Coatings 9, no. 1: 54. https://doi.org/10.3390/coatings9010054






