Photocathodic Protection of Cobalt Doped ZnO Nanorod Arrays for 316 Stainless Steel and Q235 Carbon Steel in 3.5 wt.% NaCl Solution
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of ZnO Nanorod Arrays
2.2. Characterization
2.3. Electrode Fabrication and Photoelectrochemical Measurements
3. Results and Discussion
3.1. Morphology and Crystal Structure Analysis
3.2. Optical Properties Analysis
3.3. Photocathodic Protection Properties
3.4. Possible Photocathodic Protection Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Imokawa, T.; Fujisawa, R.; Suda, A.; Tsujikawa, S. Protection of 304 stainless steel with TiO2 coating. Corros. Eng. 1994, 43, 482–486. [Google Scholar] [CrossRef]
- Hu, J.; Guan, Z.C.; Liang, Y.; Zhou, J.Z.; Liu, Q.; Wang, H.P.; Zhang, H.; Du, R.G. Bi2S3 modified single crystalline rutile TiO2 nanorod array films for photoelectrochemical cathodic protection. Corros. Sci. 2017, 125, 59–67. [Google Scholar] [CrossRef]
- Guan, Z.C.; Wang, X.; Jin, P.; Tang, Y.Y.; Wang, H.P.; Song, G.L.; Du, R.G. Enhanced photoelectrochemical performances of ZnS-Bi2S3/TiO2/WO3 composite film for photocathodic protection. Corros. Sci. 2018, 143, 31–38. [Google Scholar] [CrossRef]
- Sun, W.X.; Cui, S.W.; Wei, N.; Chen, S.G.; Liu, Y.P.; Wang, D.A. Hierarchical WO3/TiO2 nanotube nanocomposites for efficientphotocathodic protection of 304 stainless steel under visible light. J. Alloy. Comp. 2018, 749, 741–749. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.T.; Zhang, L.; Hou, B.R. Preparation and photocathodic protection performance of CdSe/reduced graphene oxide/TiO2 composite. Corros. Sci. 2015, 94, 342–349. [Google Scholar] [CrossRef]
- Dash, P.; Manna, A.; Mishra, N.C.; Varma, S. Synthesis and characterization of aligned ZnO nanorods for visible light photocatalysis. Phys. E 2019, 107, 38–46. [Google Scholar] [CrossRef]
- Chen, Y.C.; Li, Y.J.; Hsu, Y.K. Enhanced performance of ZnO-based dye-sensitized solar cells by glucose treatment. J. Alloy. Comp. 2018, 748, 382–389. [Google Scholar] [CrossRef]
- Borysiewicz, M.A.; Wzorek, M.; Wojciechowski, T.; Wojtowicz, T.; Kaminńska, E.; Piotrowska, A. Photoluminescence of nanocoral ZnO films. J. Lumin. 2014, 147, 367–371. [Google Scholar] [CrossRef]
- Boumezoued, A.; Guergouri, K.; Barille, R.; Rechem, D.; Zaabat, M.; Rasheed, M. ZnO nanopowders doped with bismuth oxide, from synthesis to electrical application. J. Alloy. Comp. 2019, 791, 550–558. [Google Scholar] [CrossRef]
- Agnieszka, K.R.; Teofil, J. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar]
- Huang, J.; Yin, Z.G.; Zheng, Q.D. Applications of ZnO in organic and hybrid solar cells. Energy Environ. Sci. 2011, 4, 3861–3877. [Google Scholar] [CrossRef]
- Weintraub, B.; Zhou, Z.Z.; Li, Y.H.; Deng, Y.L. Solution synthesis of one-dimensional ZnO nanomaterials and their applications. Nanoscale 2010, 2, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
- Dhamodharan, P.; Manoharan, C.; Bououdina, M.; Venkadachalapathy, R.; Ramalingam, S. Al-doped ZnO thin films grown onto ITO substrates as photoanode in dye sensitized solar cell. Solar Energy 2017, 141, 127–144. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, R.; Singh, R.K. Progress on transition metal-doped ZnO nanoparticles and its application. Ind. Eng. Chem. Res. 2019, 58, 17130–17163. [Google Scholar] [CrossRef]
- Yang, M.; Yin, G.F.; Huang, Z.B.; Liao, X.M.; Kang, Y.Q.; Yao, Y.D. Well-aligned ZnO rod arrays grown on glass substrate from aqueous solution. Appl. Surf. Sci. 2008, 254, 2917–2921. [Google Scholar] [CrossRef]
- Pandiyan, R.; Micheli, V.; Ristic, D.; Bartali, R.; Pepponi, G.; Barozzi, M.; Gottardi, G.; Ferrari, M.; Laidani, N. Structural and near-infra red luminescence properties of Nd-doped TiO2 films deposited by RF sputtering. J. Mater. Chem. 2012, 22, 22424–22432. [Google Scholar] [CrossRef]
- Wu, J.J.; Liu, S.C. Low-temperature growth of well-aligned ZnO nanorods by chemical vapor deposition. Adv. Mater. 2002, 14, 215–218. [Google Scholar] [CrossRef]
- Kim, C.G.; Sung, K.; Chung, T.M.; Jung, D.Y.; Kim, Y. Monodispersed ZnO nanoparticles from a single molecular precursor. Chem. Commun. 2003, 16, 2068–2069. [Google Scholar] [CrossRef]
- Zhou, M.J.; Zeng, Z.O.; Zhong, L. Photogenerated cathode protection properties of nano-sized TiO2/WO3 coating. Corros. Sci. 2009, 51, 1386–1391. [Google Scholar] [CrossRef]
- Zhang, T.T.; Liu, Y.P.; Liang, J.; Wang, D.A. Enhancement of photoelectrochemical and photocathodic protection properties of TiO2 nanotube arrays by simple surface UV treatment. Appl. Surf. Sci. 2017, 394, 440–445. [Google Scholar] [CrossRef]
- Sahnesarayi, M.K.; Sarpoolaky, H.; Rastegari, S. Effect of heat treatment temperature on the performance of nano-TiO2 coating in protecting 316L stainless steel against corrosion under UV illumination and dark conditions. Surf. Coat. Technol. 2014, 258, 861–870. [Google Scholar] [CrossRef]
- Wu, D.W.; Huang, Z.B.; Yin, G.F.; Yao, Y.D.; Liao, X.M.; Han, D.; Huang, X.; Gu, J.W. Preparation, structure and properties of Mn-doped ZnO rod arrays. CrystEngComm 2010, 12, 192–198. [Google Scholar] [CrossRef]

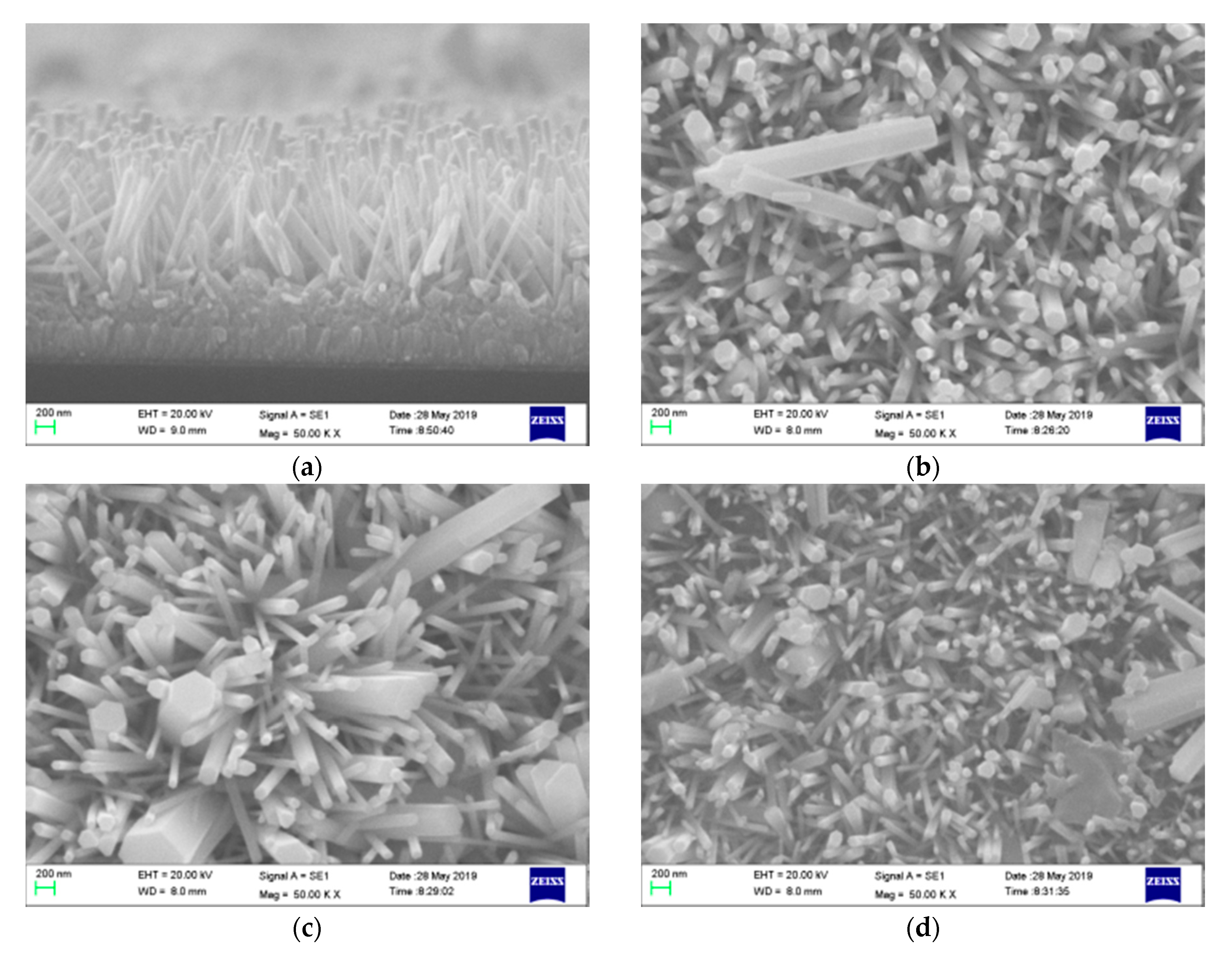




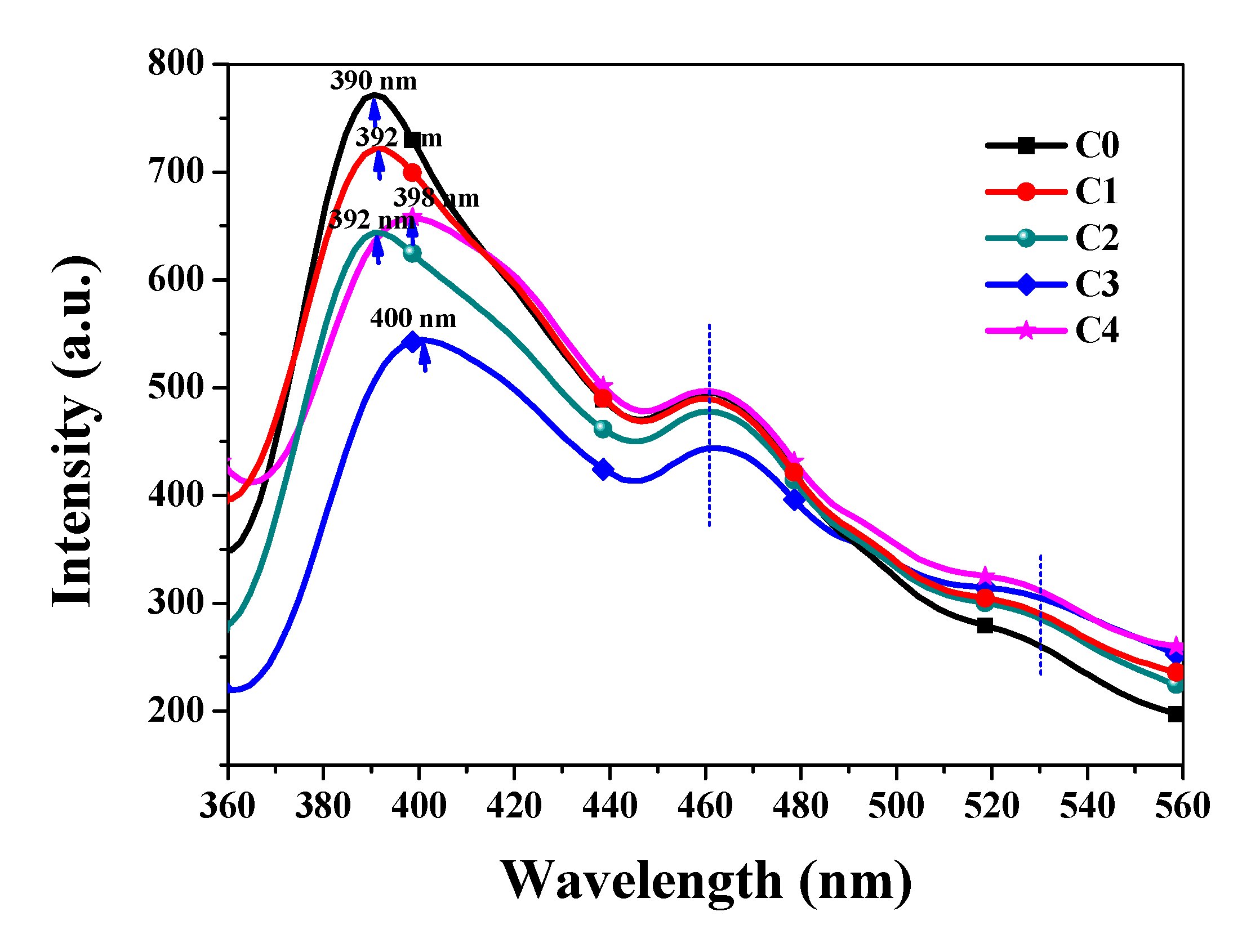

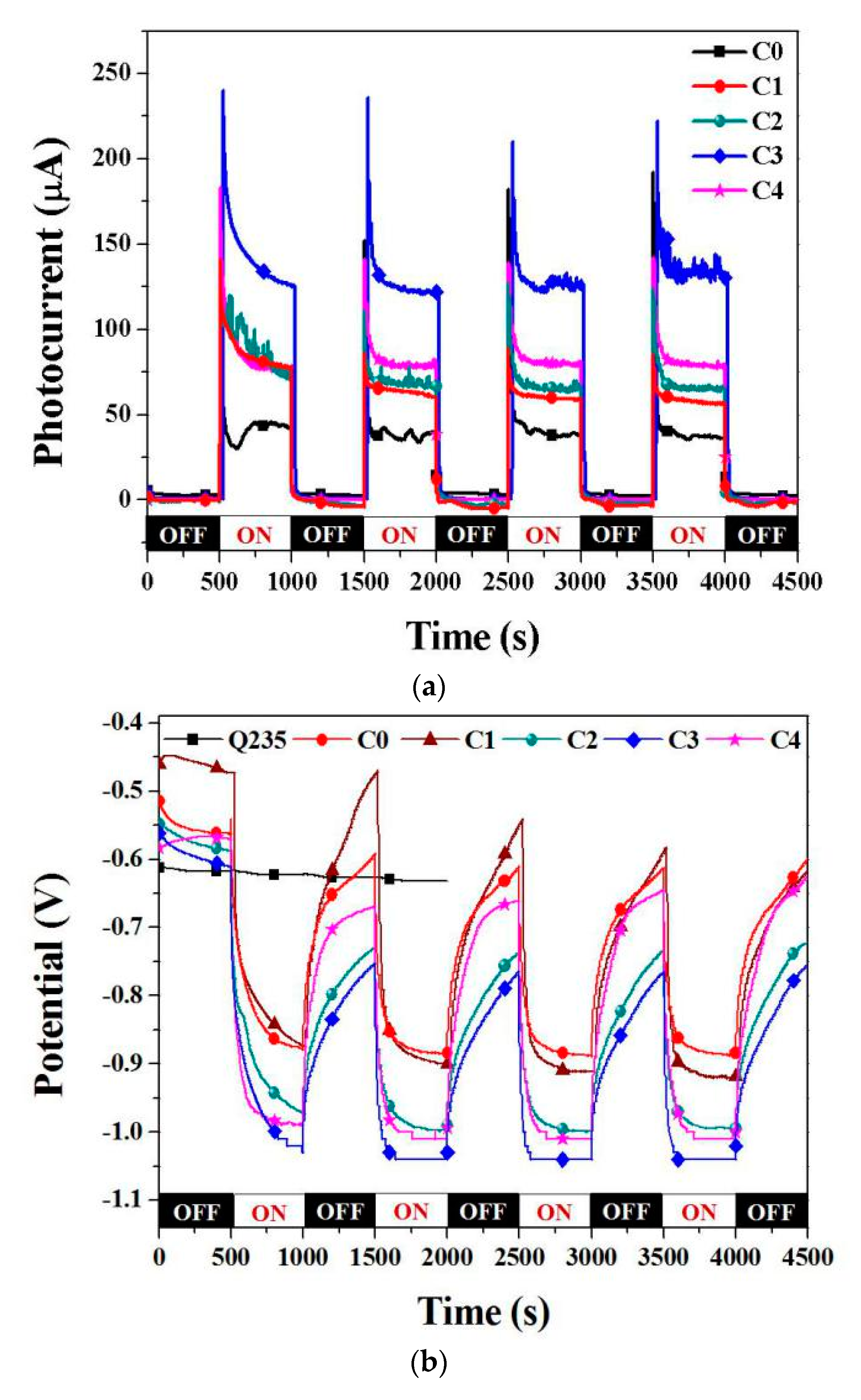
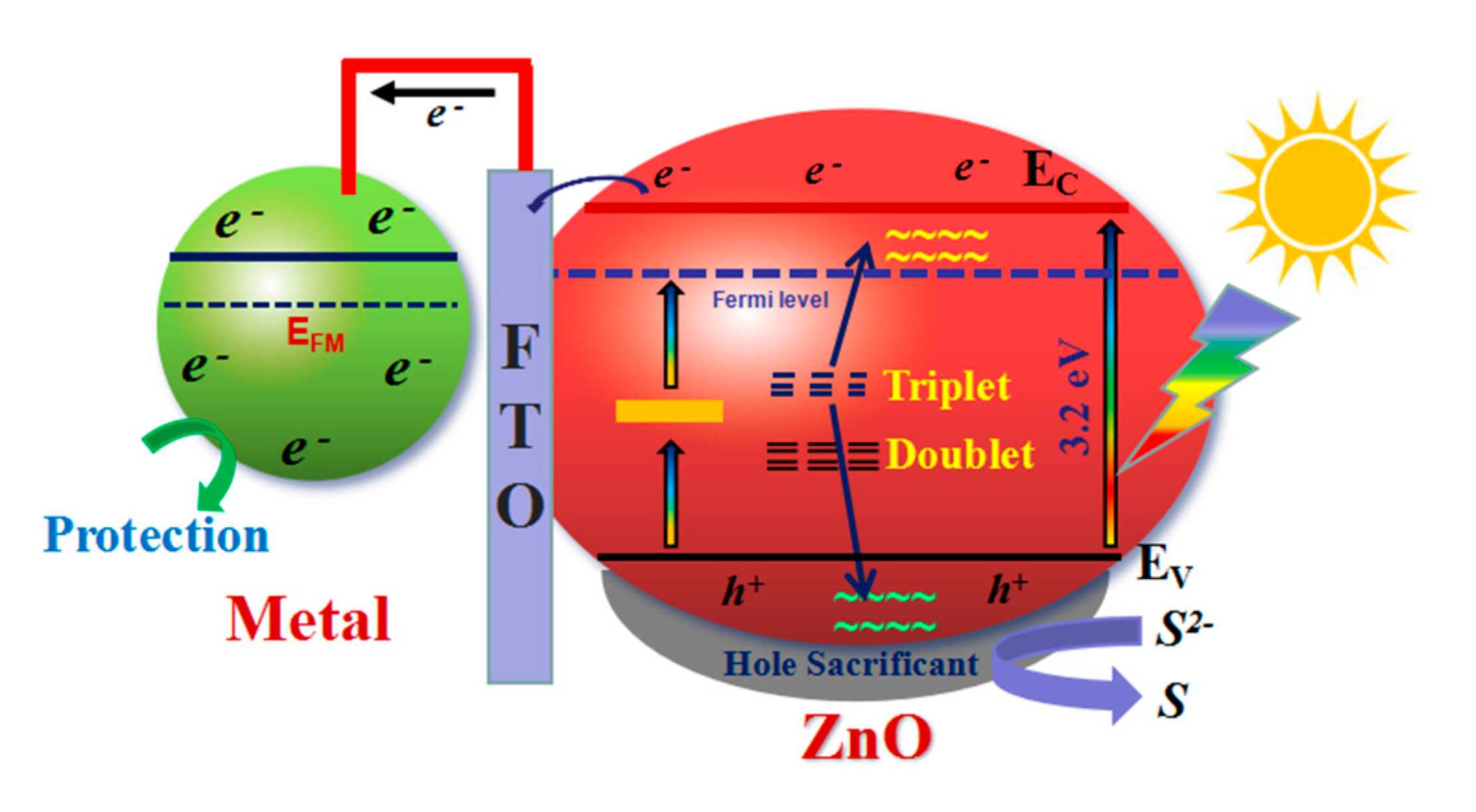
| Type | C | Mn | Si | S | P | Ni | Cr | Mo |
|---|---|---|---|---|---|---|---|---|
| 316 SS | 0.08 | 1.80 | 0.90 | 0.029 | 0.045 | 14.00 | 17.00 | 2.00 |
| Q235 | 0.19 | 0.59 | 0.30 | 0.05 | 0.44 | – | – | – |
| Sample | C1 | C2 | C3 | C4 |
|---|---|---|---|---|
| Co concentration (%) | <1 | 1.2 | 2.1 | 1.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Chen, G.; Li, W.; Wu, D. Photocathodic Protection of Cobalt Doped ZnO Nanorod Arrays for 316 Stainless Steel and Q235 Carbon Steel in 3.5 wt.% NaCl Solution. Coatings 2019, 9, 803. https://doi.org/10.3390/coatings9120803
Zhang X, Chen G, Li W, Wu D. Photocathodic Protection of Cobalt Doped ZnO Nanorod Arrays for 316 Stainless Steel and Q235 Carbon Steel in 3.5 wt.% NaCl Solution. Coatings. 2019; 9(12):803. https://doi.org/10.3390/coatings9120803
Chicago/Turabian StyleZhang, Xiong, Guanghui Chen, Weihua Li, and Dianwu Wu. 2019. "Photocathodic Protection of Cobalt Doped ZnO Nanorod Arrays for 316 Stainless Steel and Q235 Carbon Steel in 3.5 wt.% NaCl Solution" Coatings 9, no. 12: 803. https://doi.org/10.3390/coatings9120803
APA StyleZhang, X., Chen, G., Li, W., & Wu, D. (2019). Photocathodic Protection of Cobalt Doped ZnO Nanorod Arrays for 316 Stainless Steel and Q235 Carbon Steel in 3.5 wt.% NaCl Solution. Coatings, 9(12), 803. https://doi.org/10.3390/coatings9120803




