Surface Segregation of Amphiphilic PDMS-Based Films Containing Terpolymers with Siloxane, Fluorinated and Ethoxylated Side Chains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Procedure for the Preparation of Terpolymers
2.3. Preparation of Films
2.4. Characterization
3. Results and Discussion
3.1. Synthesis of Terpolymers
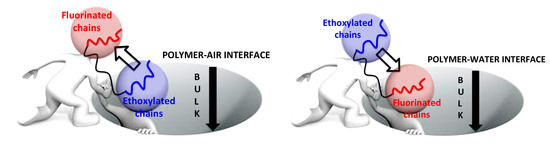
3.2. Surface Segregation of the Amphiphilic Surface-Active Terpolymer
3.3. Surface Composition after Immersion in Water
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Narrainen, A.P.; Hutchings, L.R.; Ansari, I.; Thompson, R.L.; Clarke, N. Multi-End-Functionalized polymers: Additives to modify polymer properties at surfaces and interfaces. Macromolecules 2007, 40, 1969–1980. [Google Scholar] [CrossRef]
- Lee, H.; Archer, L.A. Functionalizing polymer surfaces by surface migration of copolymer additives: Role of additive molecular weight. Polymer 2002, 43, 2721–2728. [Google Scholar] [CrossRef]
- Martinelli, E.; Galli, G.; Cwikel, D.; Murmur, A. Wettability and surface tension of amphiphilic polymer films: time-dependent measurements of the most stable contact angle. Macromol. Chem. Phys. 2012, 213, 1448–1456. [Google Scholar] [CrossRef]
- Yasani, B.R.; Martinelli, E.; Galli, G.; Glisenti, A.; Mieszkin, S.; Callow, M.E.; Callow, J.A. A comparison between different fouling-release elastomer coatings containing surface-active polymers. Biofouling 2014, 30, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Archer, L.A. Functionalizing polymer surfaces by field-induced migration of copolymer additives. 1. Role of surface energy gradients. Macromolecules 2001, 34, 4572–4579. [Google Scholar] [CrossRef]
- Martinelli, E.; Fantoni, C.; Galli, G.; Gallot, B.; Glisenti, A. Low surface energy properties of smectic fluorinated block copolymer/SEBS blends. Mol. Cryst. Liq. Cryst. 2009, 500, 51–62. [Google Scholar] [CrossRef]
- Inutsuka, M.; Yamada, N.L.; Ito, K.; Yokoyama, H. High density polymer brush spontaneously formed by the segregation of amphiphilic diblock copolymers to the polymer/water interface. ACS Macro Lett. 2013, 2, 265–268. [Google Scholar] [CrossRef]
- Martinelli, E.; Hill, S.D.; Finlay, J.A.; Callow, M.E.; Callow, J.A.; Glisenti, A.; Galli, G. Amphiphilic modified-styrene copolymer films: antifouling/fouling release properties against the green alga Ulva linza. Prog. Org. Coat. 2016, 90, 235–242. [Google Scholar] [CrossRef]
- Mielczarski, J.; Mielczarski, E.; Galli, G.; Morelli, A.; Martinelli, E.; Chiellini, E. The surface-segregated nanostructure of fluorinated copolymer-poly(dimethylsiloxane) blend films. Langmuir 2010, 26, 2871–2876. [Google Scholar] [CrossRef] [PubMed]
- Sorgi, C.; Martinelli, E.; Galli, G.; Pucci, A. Julolidine-labelled fluorinated block copolymers for the development of two-layer films with highly sensitive vapochromic response. Sci. China Chem. 2018, 61, 947–956. [Google Scholar] [CrossRef]
- Raffa, P.; Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Polymeric surfactants: Synthesis, properties, and links to applications. Chem. Rev. 2015, 115, 8504–8563. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.; Martinelli, E. Amphiphilic polymer platforms: Surface engineering of films for marine antibiofouling. Macromol. Rapid Commun. 2017, 38. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Aibani, N.; Callan, J.F.; Callan, B. Recent advances in amphiphilic polymers for simultaneous delivery of hydrophobic and hydrophilic drugs. Ther. Deliv. 2016, 7, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Nesvadba, P. Radical polymerization in industry. In The Encyclopedia of Radicals in Chemistry, Biology and Materials; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Li, L.; Raghupathi, K.; Song, C.; Prasada, P.; Thayumanavan, S. Self-assembly of random copolymers. Chem. Commun. 2014, 50, 13417–13432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, K.; Terashima, T.; Sugita, T.; Takenaka, M.; Sawamoto, M. Amphiphilic random copolymers with hydrophobic/hydrogen-bonding urea pendants: Self-folding polymers in aqueous and organic media. Macromolecules 2016, 49, 7917–7927. [Google Scholar] [CrossRef]
- Terashima, T.; Sugita, T.; Fukae, K.; Sawamoto, M. Synthesis and single-chain folding of amphiphilic random copolymers in water. Macromolecules 2014, 47, 589–600. [Google Scholar] [CrossRef]
- Koda, Y.; Terashima, T.; Sawamoto, M. Multimode self-folding polymers via reversible and thermoresponsive self-assembly of amphiphilic/fluorous random copolymers. Macromolecules 2016, 49, 4534–4543. [Google Scholar] [CrossRef]
- Guazzelli, E.; Masotti, E.; Biver, T.; Pucci, A.; Martinelli, E.; Galli, G. The self-assembly over nano- to submicro-length scales in water of a fluorescent julolidine-labeled amphiphilic random terpolymer. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 797–804. [Google Scholar] [CrossRef]
- Martinelli, E.; Guazzelli, E.; Galli, G.; Telling, M.T.F.; Dal Poggetto, G.; Immirzi, B.; Domenici, F.; Paradossi, G. Prolate and temperature-responsive self-assemblies of amphiphilic random copolymers with perfluoroalkyl and polyoxyethylene side chains in solution. Macromol. Chem. Phys. 2018, 219, 1800210. [Google Scholar] [CrossRef]
- Galli, G.; Barsi, D.; Martinelli, E.; Glisenti, A.; Finlay, J.A.; Callow, M.E.; Callow, J.A. Copolymer films containing amphiphilic side chains of well-defined fluoroalkyl-segment length with biofouling-release potential. RSC Adv. 2016, 6, 67127–67135. [Google Scholar] [CrossRef]
- Rufin, M.A.; Ngo, B.K.D.; Barry, M.E.; Page, V.M.; Hawkins, M.L.; Stafslien, S.J.; Grunlan, M.A. Antifouling silicones based on surface-modifying additive amphiphiles. Green Mater. 2017, 5, 4–13. [Google Scholar] [CrossRef]
- Stafslien, S.J.; Christianson, D.; Daniels, J.; VanderWal, L.; Chernykha, A.; Chisholm, B.J. Combinatorial materials research applied to the development of new surface coatings XVI: Fouling-release properties of amphiphilic polysiloxane coatings. Biofouling 2015, 31, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Del Moro, I.; Galli, G.; Barbaglia, M.; Bibbiani, C.; Mennillo, E.; Oliva, M.; Pretti, C.; Antonioli, D.; Laus, M. Photopolymerized network polysiloxane films with dangling hydrophilic/hydrophobic chains for the biofouling release of invasive marine serpulid Ficopomatus enigmaticus. ACS Appl. Mater. Interfaces 2015, 7, 8293–8301. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Pretti, C.; Oliva, M.; Glisenti, A.; Galli, G. Sol-gel polysiloxane films containing different surface-active trialkoxysilanes for the release of the marine foulant Ficopomatus enigmaticus. Polymer 2018, 145, 426–433. [Google Scholar] [CrossRef]
- Noguer, A.C.; Olsen, S.M.; Hvilsted, S.; Kiil, S. Diffusion of surface-active amphiphiles in silicone-based fouling-release coatings. Prog. Org. Coat. 2017, 106, 77–86. [Google Scholar] [CrossRef]
- Martinelli, E.; Gunes, D.; Wenning, B.M.; Ober, C.K.; Finlay, J.A.; Callow, M.E.; Callow, J.A.; Di Fino, A.; Clare, A.S.; Galli, G. Effects of surface-active block copolymers with oxyethylene and fluoroalkyl side chains on the antifouling performance of silicone-based film. Biofouling 2016, 32, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.L.; Wenning, B.; Rizis, G.; Calabrese, D.R.; Finlay, J.A.; Franco, S.C.; Zuckermann, R.N.; Clare, A.S.; Kramer, E.J.; Ober, C.K.; et al. Role of backbone chemistry and monomer sequence in amphiphilic oligopeptide- and oligopeptoid-functionalized PDMS- and PEO-based block copolymers for marine antifouling and fouling release coatings. Macromolecules 2017, 50, 2656–2667. [Google Scholar] [CrossRef]
- Weinman, C.J.; Finlay, J.A.; Park, D.; Paik, M.Y.; Krishnan, S.; Sundaram, H.S.; Dimitriou, M.; Sohn, K.E.; Callow, M.E.; Callow, J.A.; et al. ABC triblock surface active block copolymer with grafted ethoxylated fluoroalkyl amphiphilic side chains for marine antifouling/fouling-release applications. Langmuir 2009, 25, 12266–12274. [Google Scholar] [CrossRef] [PubMed]
- Gombotz, W.R.; Guanghui, W.; Horbett, T.A.; Hoffman, A.S. Protein adsorption to poly(ethylene oxide) surfaces. J. Biomed. Mater. Res. 1991, 25, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Weinman, C.J.; Ober, C.K. Advances in polymers for anti-biofouling surfaces. J. Mater. Chem. 2008, 18, 3405–3413. [Google Scholar] [CrossRef]
- Brady, R.F. Foul-release coatings for warships. Def. Sci. J. 2005, 55, 75–81. [Google Scholar] [CrossRef]
- Lejars, M.; Marigaillan, A.; Bressy, C. Foul release coatings: A nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Wang, N.; Ober, C.K.; Finlay, J.A.; Callow, M.E.; Callow, J.A.; Hexemer, A.; Sohn, K.E.; Kramer, E.J.; Fischer, D.A. Comparison of the fouling release properties of hydrophobic fluorinated and hydrophilic PEGylated block copolymer surfaces: Attachment strength of the diatom Navicula and the green alga Ulva. Biomacromolecules 2006, 7, 1449–1462. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.; Martinelli, E.; Chiellini, E.; Ober, C.K.; Glisenti, A. Low surface energy characteristics of mesophase forming ABC, ACB triblock copolymers with fluorinated B blocks. Mol. Cryst. Liq. Cryst. 2005, 441, 211–226. [Google Scholar] [CrossRef]
- Martinelli, E.; Galli, G.; Krishnan, S.; Paik, M.Y.; Ober, C.K.; Fischer, D.A. New poly(dimethylsiloxane)/poly(perfluorooctylethyl acrylate) block copolymers: Structure and order across multiple length scales in thin films. J. Mater. Chem. 2011, 21, 15357–15368. [Google Scholar] [CrossRef]
- Wenning, B.M.; Martinelli, E.; Mieszkin, S.; Finlay, J.A.; Fischer, D.; Callow, J.A.; Callow, M.E.; Leonardi, A.K.; Ober, C.K.; Galli, G. Model amphiphilic block copolymers with tailored molecular weight and composition in PDMS-based films to limit soft biofouling. ACS Appl. Mater. Interfaces 2017, 9, 16505–16516. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Glisenti, A.; Gallot, B.; Galli, G. Surface properties of mesophase-forming fluorinated bicycloacrylate/polysiloxane methacrylate copolymers. Macromol. Chem. Phys. 2009, 210, 1746–1753. [Google Scholar] [CrossRef]
- Sundaram, H.S.; Cho, Y.; Dimitriou, M.D.; Weinman, C.J.; Finlay, J.A.; Cone, G.; Callow, M.E.; Callow, J.A.; Kramer, E.J.; Ober, C.K. Fluorine-free mixed amphiphilic polymers based on PDMS and PEG side chains for fouling release applications. Biofouling 2011, 27, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, P.; Stafslien, S.; Daniels, J.; Webster, D.C. High throughput combinatorial characterization of thermosetting siloxane-urethane coatings having spontaneously formed microtopographical surfaces. J. Coat. Technol. Res. 2007, 4, 131–138. [Google Scholar] [CrossRef]
- Bodkhe, R.B.; Stafslien, S.J.; Cilz, N.; Daniels, J.; Thompson, S.E.M.; Callow, M.E.; Callow, J.A.; Webster, D.C. Polyurethanes with amphiphilic surfaces made using telechelic functional PDMS having orthogonal acid functional groups. Prog. Org. Coat. 2012, 75, 38–48. [Google Scholar] [CrossRef]
- Martinelli, E.; Guazzelli, E.; Galli, G. Recent advances in designed non-toxic polysiloxane coatings to combat marine biofouling. In Marine Coatings and Membranes; Mittal, V., Ed.; Central West Publishing: Orange, NSW, Australia, 2019. [Google Scholar]
- Shirley, D.A. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy, Physical Electronics; Perkin-Elmer Corp.: Eden Prairie, MN, USA, 1992. [Google Scholar]
- McIntyre, N.S.; Chan, T.C. Practical Surface Analysis 1; Briggs, D., Seah, M.P., Eds.; Wiley: Chichester, UK, 1990; p. 485. [Google Scholar]
- Marabotti, I.; Morelli, A.; Orsini, L.M.; Martinelli, E.; Galli, G.; Chiellini, E.; Lien, E.M.; Pettitt, M.E.; Callow, M.E.; Callow, J.A.; et al. Fluorinated/siloxane copolymer blends for fouling release: Chemical characterisation and biological evaluation with algae and barnacles. Biofouling 2009, 25, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Gudipati, C.S.; Greenleaf, C.M.; Johnson, J.A.; Pryoncpan, P.; Wooley, K.L. Hyperbranched fluoropolymer and linear poly(ethylene glycol) based amphiphilic crosslinked networks as efficient antifouling coatings: An insight into the surface compositions, topographies, and morphologies. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 6193–6208. [Google Scholar] [CrossRef]
- Krishnan, S.; Ayothi, R.; Hexemer, A.; Finlay, J.A.; Sohn, K.E.; Perry, R.; Ober, C.K.; Kramer, E.J.; Callow, M.E.; Callow, J.A.; et al. Anti-biofouling properties of comblike block copolymers with amphiphilic side chains. Langmuir 2006, 22, 5075–5086. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Paik, M.Y.; Ober, C.K.; Martinelli, E.; Galli, G.; Sohn, K.E.; Kramer, E.J.; Fischer, D.A. NEXAFS depth profiling of surface segregation in block copolymer thin films. Macromolecules 2010, 43, 4733–4743. [Google Scholar] [CrossRef]
- Martinelli, E.; Pelusio, G.; Yasani, B.R.; Glisenti, A.; Galli, G. Surface chemistry of amphiphilic polysiloxane/triethyleneglycol-modified poly(pentafluorostyrene) block copolymer films before and after water immersion. Macromol. Chem. Phys. 2015, 216, 2086–2094. [Google Scholar] [CrossRef]





| Terpolymer | Composition a) (mol%) | Mnb) (g/mol) | Mw/Mnb) | Tg,Sic) (°C) | Tg,EGc) (°C) | Tm,Sic) (°C) | Tm,EGc) (°C) |
|---|---|---|---|---|---|---|---|
| p(Sib-F-EGb) | 26/45/29 | 21000 | 2.22 | –129 | - | –53 | 21 |
| p(Sib-F-EGa) | 22/52/26 | 21000 | 2.06 | –129 | - | –54 | - |
| p(Sia-F-EGa) | 19/58/23 | 9000 | 2.94 | –124 | –52 | - | - |
| Film | φ (°) | C (%) | O (%) | F (%) | Si (%) | Cexp/Ctheor | Oexp/Otheor | Fexp/Ftheor | Siexp/Sitheor |
|---|---|---|---|---|---|---|---|---|---|
| p(Sib-F-EGa) | theor. | 51.4 | 22.0 | 8.4 | 18.2 | ||||
| 70 | 42.2 | 13.4 | 37.7 | 6.7 | 0.8 | 0.6 | 4.5 | 0.4 | |
| 20 | 45.2 | 20.3 | 20.5 | 14.0 | 0.9 | 0.9 | 2.4 | 0.8 | |
| p(Sib-F-EGb) | theor. | 54.0 | 24.2 | 5.5 | 16.3 | ||||
| 70 | 44.7 | 17.1 | 29.9 | 8.3 | 0.8 | 0.7 | 5.4 | 0.5 | |
| 20 | 47.0 | 22.3 | 17.3 | 13.4 | 0.9 | 0.9 | 3.1 | 0.8 | |
| p(Sia-F-EGa) | theor. | 52.3 | 16.6 | 23.9 | 7.2 | ||||
| 70 | 43.5 | 10.0 | 44.1 | 2.4 | 0.8 | 0.6 | 1.8 | 0.3 | |
| 20 | 45.7 | 14.3 | 34.8 | 5.2 | 0.9 | 0.9 | 1.5 | 0.7 | |
| p(Sib-F-EGa)4 | theor. | 50.0 | 24.9 | ~0.4 | 24.7 | ||||
| 70 | 41.1 | 11.2 | 43.0 | 4.7 | 0.8 | 0.4 | 107.5 | 0.2 | |
| 20 | 45.9 | 18.8 | 23.3 | 12.0 | 0.9 | 0.8 | 58.3 | 0.5 | |
| p(Sib-F-EGb)4 | theor. | 50.2 | 25.0 | ~0.2 | 24.6 | ||||
| 70 | 42.8 | 15.8 | 34.2 | 7.2 | 0.9 | 0.6 | 171.0 | 0.3 | |
| 20 | 48.0 | 22.7 | 16.8 | 12.5 | 1.0 | 0.9 | 84.0 | 0.5 | |
| p(Sia-F-EGa)4 | theor. | 50.1 | 24.6 | 1.1 | 24.2 | ||||
| 70 | 42.6 | 10.1 | 45.4 | 1.9 | 0.8 | 0.4 | 41.2 | 0.1 | |
| 20 | 46.3 | 12.3 | 37.9 | 3.5 | 0.9 | 0.5 | 34.4 | 0.1 |
| Film | φ (°) | Peak (i) (%) | Peak (ii) (%) | Peak (iii) (%) | Peak (iv) (%) | Peak (v) (%) |
|---|---|---|---|---|---|---|
| p(Sib-F-EGa)4a,b) | 70 | 29.1 | 32.3 | 4.8 | 27.2 | 6.6 |
| 20 | 61.0 | 24.3 | 3.5 | 11.2 | - | |
| p(Sib-F-EGb)4a,b) | 70 | 44.5 | 32.0 | 4.4 | 14.4 | 4.7 |
| 20 | 50.6 | 38.3 | 2.6 | 8.5 | - | |
| p(Sia-F-EGa)4a,b) | 70 | 14.6 | 38.0 | 6.0 | 34.7 | 6.7 |
| 20 | 35.0 | 27.6 | 9.1 | 23.3 | 5.0 |
| Film | φ (°) | C (%) | O (%) | F (%) | Si (%) | Cexp/Ctheor | Oexp/Otheor | Fexp/Ftheor | Siexp/Sitheor |
|---|---|---|---|---|---|---|---|---|---|
| p(Sib-F-EGa)4 | theor. | 50.0 | 24.9 | ~0.4 | 24.7 | ||||
| 70 | 43.4 | 14.7 | 34.3 | 7.6 | 0.9 | 0.6 | 85.7 | 0.3 | |
| 20 | 47.8 | 20.0 | 19.9 | 12.3 | 1.0 | 0.8 | 49.7 | 0.5 | |
| p(Sib-F-EGb)4 | theor. | 50.2 | 25.0 | ~0.2 | 24.6 | ||||
| 70 | 45.0 | 21.7 | 21.6 | 11.7 | 0.9 | 0.9 | 108.0 | 0.5 | |
| 20 | 48.9 | 24.6 | 11.2 | 15.3 | 1.0 | 1.0 | 56.0 | 0.6 | |
| p(Sia-F-EGa)4 | theor. | 50.1 | 24.6 | 1.1 | 24.2 | ||||
| 70 | 40.3 | 10.2 | 47.3 | 2.2 | 0.8 | 0.4 | 43.0 | 0.1 | |
| 20 | 43.5 | 12.6 | 40.5 | 3.4 | 0.9 | 0.5 | 36.8 | 0.1 |
| Film | Peak (i) (%) | Peak (ii) (%) | Peak (iii) (%) | Peak (iv) (%) | Peak (v) (%) |
|---|---|---|---|---|---|
| p(Sib-F-EGa)4 | 33.7 | 30.4 | 4.6 | 24.6 | 6.7 |
| p(Sib-F-EGb)4 | 66.7 | 27.1 | - | 6.2 | - |
| p(Sia-F-EGa)4 | 13.2 | 38.3 | 4.2 | 37.3 | 7.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinelli, E.; Guazzelli, E.; Glisenti, A.; Galli, G. Surface Segregation of Amphiphilic PDMS-Based Films Containing Terpolymers with Siloxane, Fluorinated and Ethoxylated Side Chains. Coatings 2019, 9, 153. https://doi.org/10.3390/coatings9030153
Martinelli E, Guazzelli E, Glisenti A, Galli G. Surface Segregation of Amphiphilic PDMS-Based Films Containing Terpolymers with Siloxane, Fluorinated and Ethoxylated Side Chains. Coatings. 2019; 9(3):153. https://doi.org/10.3390/coatings9030153
Chicago/Turabian StyleMartinelli, Elisa, Elisa Guazzelli, Antonella Glisenti, and Giancarlo Galli. 2019. "Surface Segregation of Amphiphilic PDMS-Based Films Containing Terpolymers with Siloxane, Fluorinated and Ethoxylated Side Chains" Coatings 9, no. 3: 153. https://doi.org/10.3390/coatings9030153