Aluminum Coated Micro Glass Spheres to Increase the Infrared Reflectance
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.1.1. Micro Glass Spheres
2.1.2. PVD-Coated Reference Spheres
2.2. Liquid Phase Deposition (LPD)
2.2.1. Pre-Conditioning
2.2.2. LPD of Aluminum
2.2.3. Post-Treatment
Turbula® Mixer:
Magnetic Stir Bar Treatment:
Furnace Treatment:
2.3. Experimental Characterization Methods
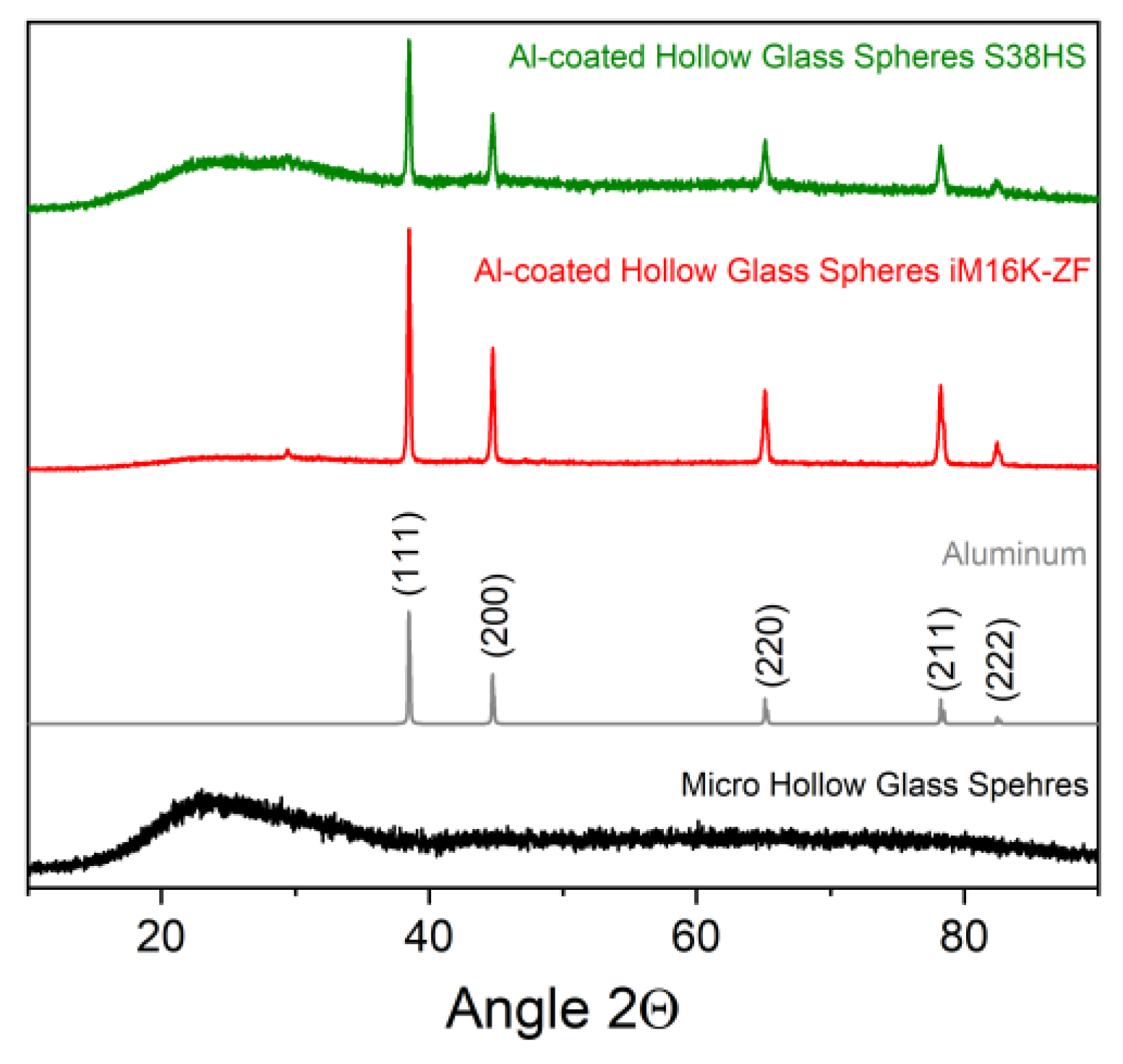
2.3.1. Qualitative Phase composition
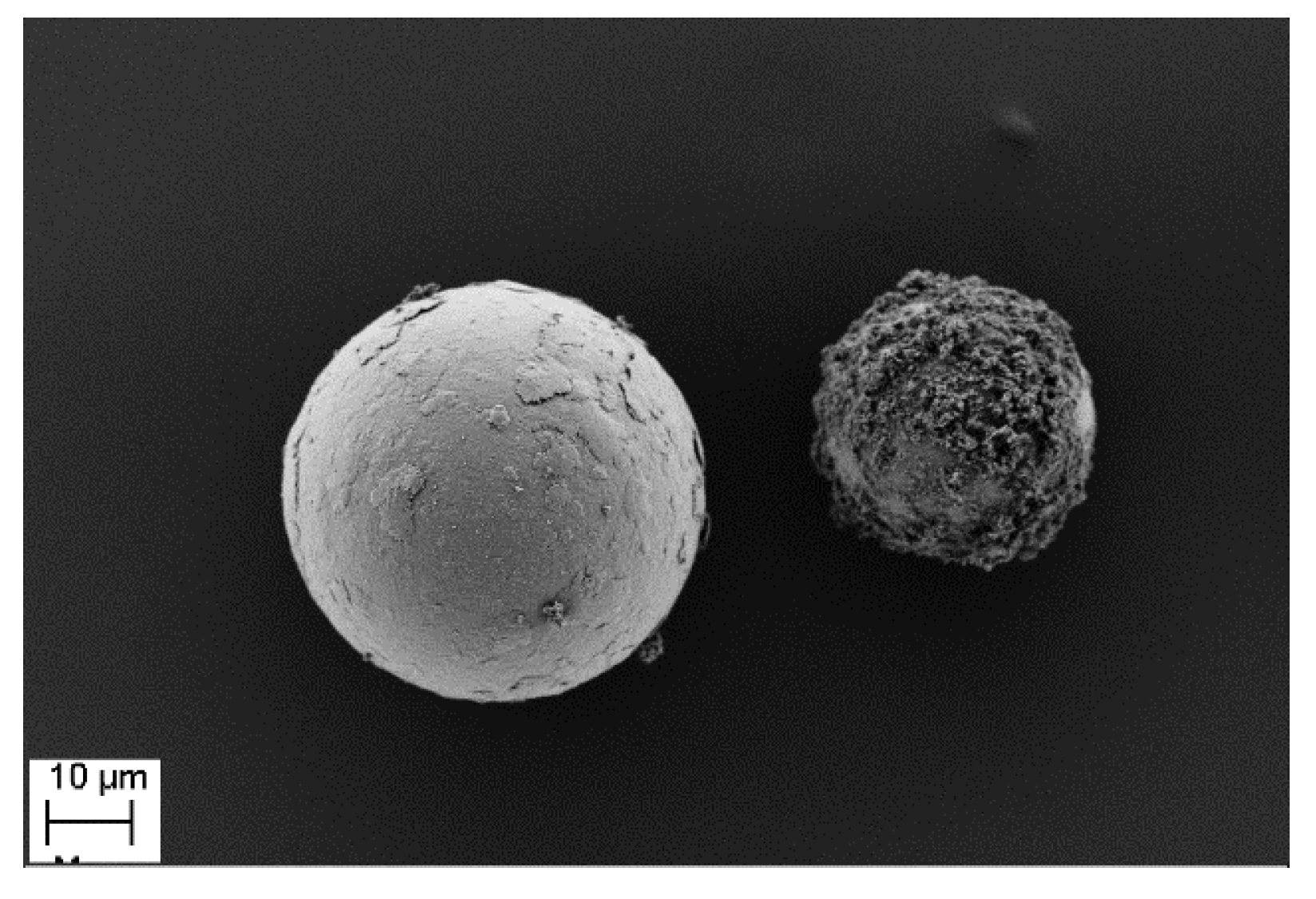
2.3.2. Morphology Characterization
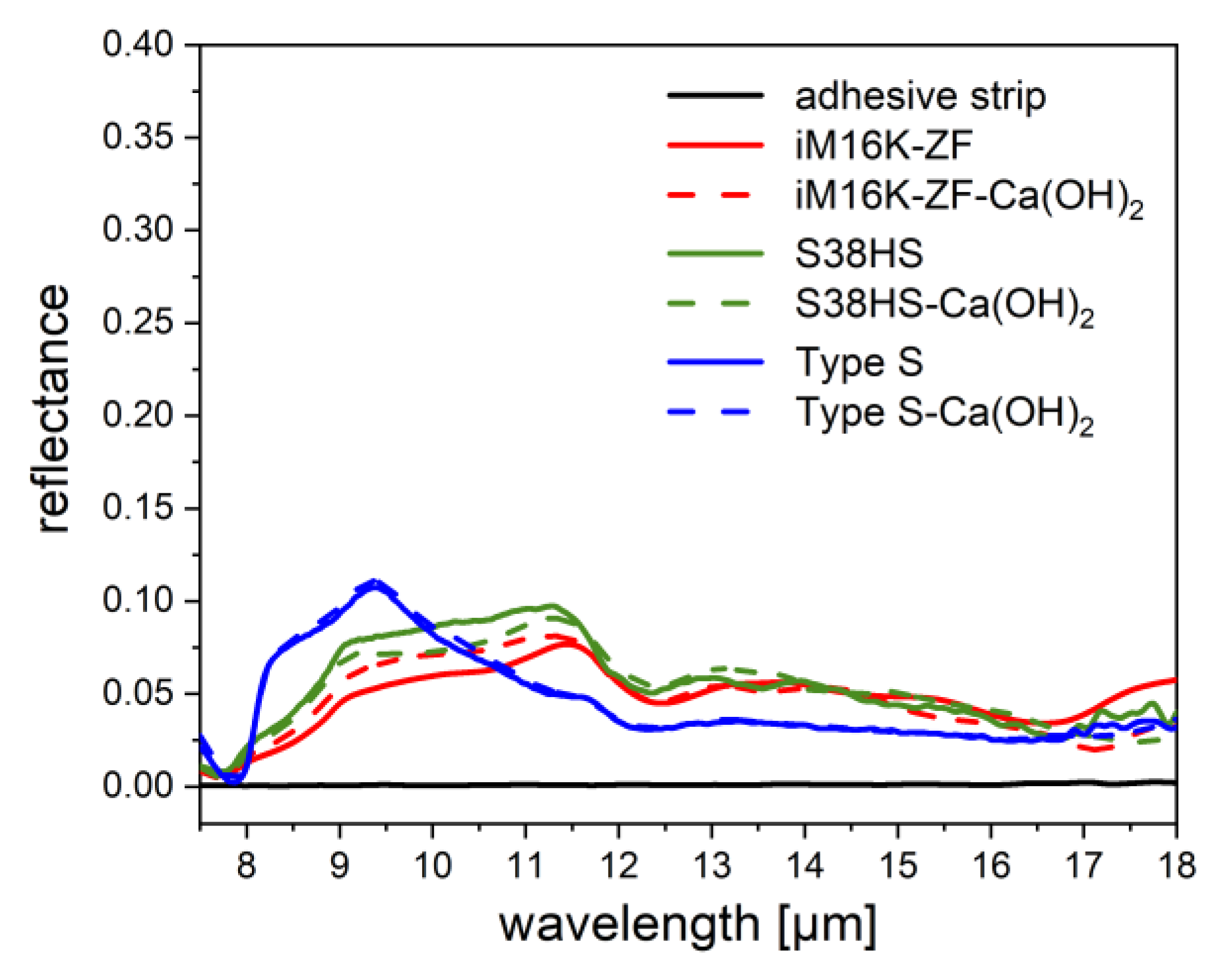
2.3.3. Reflection Measurement
3. Results and Discussion
3.1. Morphological Examination
3.1.1. Setup 1—Round Flask with Magnetic Stir Bar
3.1.2. Setup 2—Rotating Flask
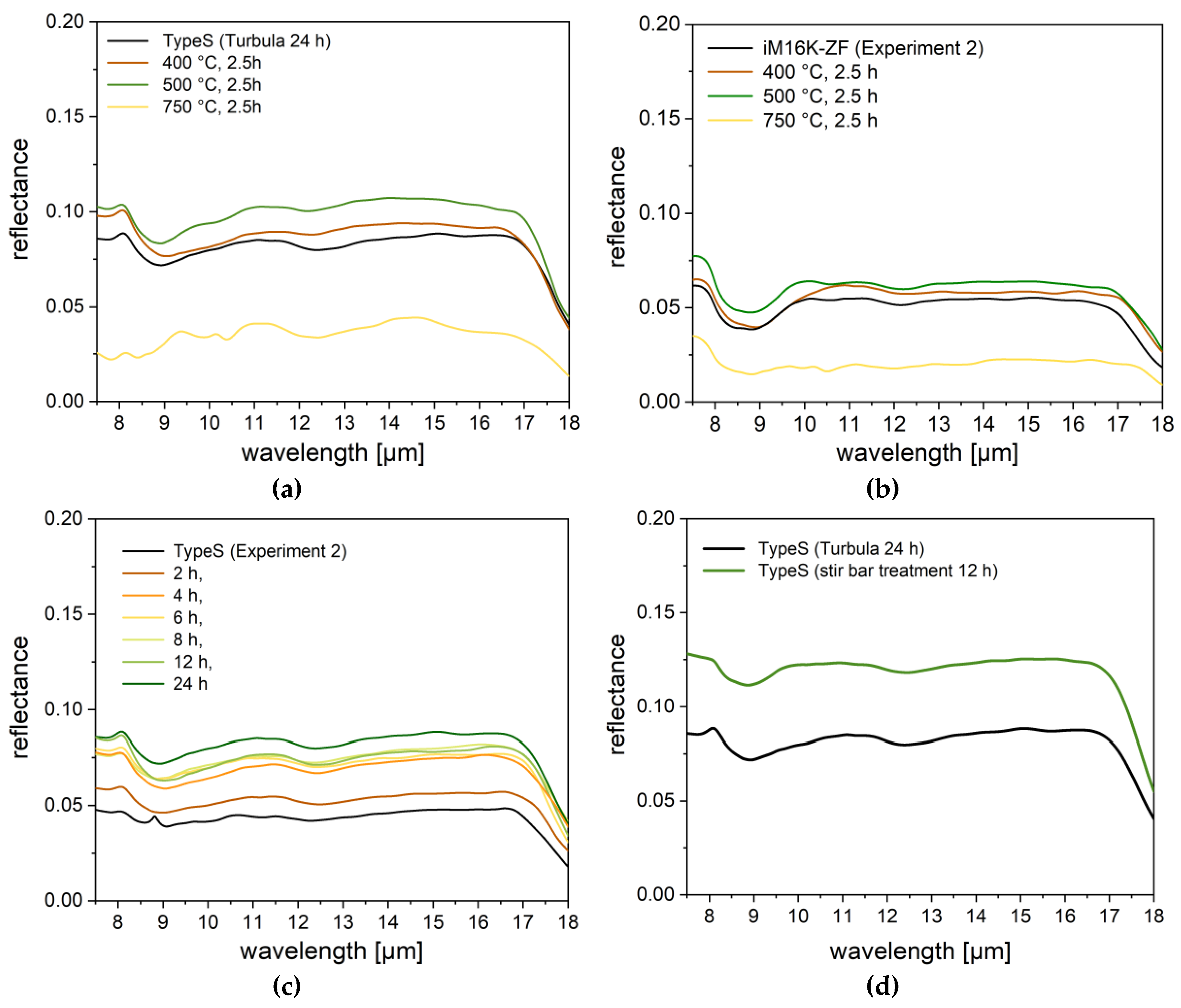
3.1.3. Post-Treatment
3.2. Reflection Measurements
3.2.1. PVD-Coated Spheres
3.2.2. LPD-Coated spheres—Setup 1
3.2.3. LPD-Coated spheres—Setup 2
3.2.4. Post-Treated Spheres
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wypch, G. Handbook of Fillers; Chem Tec Publishing: Toronto, ON, Canada, 2016; p. 4. ISBN 9781895198911. [Google Scholar]
- Kulshreshtha, A.K.; Vasile, C. Handbook of Polymer Blends and Composites; Rapra Tehnology Ltd.: Shrewsbury, UK, 2003; p. 4B. ISBN 9781859573044. [Google Scholar]
- He, D.; Jiang, B. The elastic modulus of filled polymer composites. J. Appl. Polym. Sci. 1993, 49, 617. [Google Scholar] [CrossRef]
- Lee, J.; Yee, A.F. Fracture of glass bead/epoxy composites: On micro-mechanical deformations. Polymer 2000, 41, 8363–8373. [Google Scholar] [CrossRef]
- Yung, K.C.; Zhu, B.L.; Yue, T.M.; Xie, C.S. Preparation and properties of hollow glass microsphere-filled epoxy-matrix composites. Compos. Sci. Technolol. 2009, 69, 260–264. [Google Scholar] [CrossRef]
- Xu, N.; Dai, J.; Zhu, Z.; Huang, X.; Wu, P. Synthesis and characterization of hollow glass–ceramics microspheres. Ceram. Int. 2011, 37, 2663–2667. [Google Scholar] [CrossRef]
- Li, B.; Yuan, J.; An, Z.; Zhang, J. Effect of microstructure and physical parameters of hollow glass microsphere on insulation performance. Mater. Lett. 2011, 65, 1992–1994. [Google Scholar] [CrossRef]
- Drobny, J.G. Handbook of Thermoplastic Elastomers; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, p. 25. ISBN 9780323221368. [Google Scholar]
- Weatherhead, R.G. FRP Technology Fibre Reinforced Resin Systems; Springer: Berlin/Heidelberg, Germany, 2012; Volume 1, p. 381. [Google Scholar] [CrossRef]
- Budov, V.V. Hollow Glass Microspheres. Use, Properties and Technology (Review). Glass Ceram. 1994, 51, 230–235. [Google Scholar] [CrossRef]
- Lehmann, S.; Schwinger, L.; Scharfe, B.; Gerdes, T.; Erhardt, M.; Riechert, C.; Fischer, H.-B.; Schmidt-Rodenkirchen, A.; Scharfe, F.; Wolff, F. Mikro-Hohlglaskugeln als Basis Energieeffizienter Dämmung von Gebäuden. In Proceedings of the HighTechMatbau Conference, Berlin, Germany, 4–5 December 2018; p. 21. [Google Scholar]
- Kemsley, J.N. What’s that stuff? Road Markings-Pigments, polymers, and reflective spheres help keep you safe on the road. Chem. Eng. News 2010, 88(36), 67. [Google Scholar]
- Jin, H.; Xu, D.; Li, J.; Wang, K.; Bi, Z.; Xu, G.; Xu, X. Highly Solar-Reflective Litchis-Like Core-Shell HGM/TiO2 Microspheres Synthesized by Controllable Heterogeneous Precipitation Method. NANO Brief Rep. Rev. 2017, 12, 1750080. [Google Scholar] [CrossRef]
- Willert-Porada, M. (Ed.) Second Progress Report Forglas; Subproject I.2; Universität Bayreuth: Bayreuth, Germany, 2011; pp. 51–74. [Google Scholar]
- Mohelnikova, J. Materials for reflective coatings of window glass applications. Constr. Build. Mater. 2009, 23, 1993–1998. [Google Scholar] [CrossRef]
- Cozza, E.S.; Alloisio, M.; Comite, A.; di Tanna, G.; Vicini, S. NIR-reflecting properties of new paints for energy-efficient buildings. Sol. Energy 2015, 116, 108–116. [Google Scholar] [CrossRef]
- Zhang, H. Silver plating on hollow glass microsphere and coating finishing of PET/cotton fabric. J. Ind. Text. 2012, 42, 283–296. [Google Scholar] [CrossRef]
- Xu, X.; Luo, X.; Zhuang, H.; Li, W.; Zhang, B. Electroless silver coating on fine copper powder and its effects on oxidation resistance. Mater. Lett. 2003, 57, 3987–3991. [Google Scholar] [CrossRef]
- Morales, A.; Durán, A. Sol-Gel Protection of Front Surface Silver and Aluminum Mirrors. J. Sol-Gel Sci. Technol. 1997, 8, 451–457. [Google Scholar] [CrossRef]
- Lugolole, R.; Obwoya, S.K. The Effect of Thickness of Aluminium Films on Optical Reflectance. J. Ceram. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Eder, A.; Schmid, G.H.S.; Mahr, H.; Eisenmenger-Sittner, C. Aspects of thin film deposition on granulates by physical vapor deposition. Eur. Phys. J. 2016, 70, 247. [Google Scholar] [CrossRef]
- Schmid, G.H.S.; Eisenmenger-Sittner, C. A method for uniformly coating powdery substrates by magnetron sputtering. Surf. Coat. Technol. 2013, 236, 353–360. [Google Scholar] [CrossRef]
- Park, J.-H. Chemical Vapor Deposition. In Surface Engineering Series Volume 2; ASM International: Almere, The Netherlands, 2001; pp. 1–3. ISBN 0-87170-731-4. [Google Scholar]
- Lee, H.M.; Choi, S.-Y.; Kim, K.T.; Yun, J.-Y.; Jung, D.S.; Park, S.B.; Park, J. A Novel Solution-Stamping Process for Preparation of a Highly Conductive Aluminum Thin Film. Adv. Mater. 2011, 23, 5524–5528. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J. The Physical of Atmopsheres, 3rd ed.; Cambridge University Press: Cambridge, UK, 2002; xv +320p, ISBN 0-521-80456-6. [Google Scholar]
- Brower, F.M.; Matzek, N.E.; Reigler, P.F.; Rinn, H.W.; Roberts, C.B.; Schmidt, D.L.; Snover, J.A.; Terada, K. Preparation and Properties of Aluminum Hydride. J. Am. Chem. Soc. 1976, 98, 2450. [Google Scholar] [CrossRef]
- Vanyukhin, K.D.; Zakharchenko, R.V.; Kargin, N.I.; Pashkov, M.V. Study of structure and surface morphology of two-layer contact Ti/Al metallization. Mod. Electron. Mater. 2016, 2, 54–59. [Google Scholar] [CrossRef]
- Das, N.; Islam, S. Design and Analysis of Nano-Structures Gratings for Conversion Efficiency Improvement in GaAs Solar Cells. Energies 2016, 9, 690. [Google Scholar] [CrossRef]
- Moushumy, N.; Das, N.; Alameh, K.; Lee, Y.T. Design and development of silver nanoparticles to reduce the reflection loss of solar cells. In Proceedings of the 8th International Conference on High-capacity Optical Networks and Emerging Technologies, Riyadh, Saudi Arabia, 19–21 December 2011. [Google Scholar] [CrossRef]
- Sharma, M.; Das, N.; Helwig, A.; Ahfock, T. Impact of Incident Light angle on the Conversion Efficiency of Nano-structured GaAs Solar Cells. In Proceedings of the Australasian Universities Power Engineering Conference (AUPEC), Melbourne, Australia, 19–22 November 2017. [Google Scholar] [CrossRef]













| iM16K-ZF * | S38HS | Type S | |
|---|---|---|---|
| Supplier | 3M/Dyneon | SiLi | |
| Composition | soda-lime-borosilicate glass | soda lime glass | |
| Sphere type | hollow | solid | |
| Density [g/cm3] | 0.46 | 0.38 | 2.50 |
| D 50 [µm] ** | 20 | 45 | 40–70 |
| Compressive strength [MPa] | 110 | 38.5 | 250–300 *** |
| Anti-agglomeration agent | No | Yes | unspecified |
| iM16K-ZF | S38HS | Type S | |
|---|---|---|---|
| Coating volume [l] | 1 | 1 | 0.1 |
| Coating time [h] | 4, 8, 12 | 8, 16, 21 | 1, 2, 4 |
| Corresponding layer thickness [nm] | 4, 12, 19 | 12, 29, 34 | 8, 19, 36 |
| Setup | Experiment | Type of Spheres | Amount of MGS | Reaction Time [min] | Pre-Conditioning | Post-Treatment |
|---|---|---|---|---|---|---|
| 1 (with stir bar) | 1 A | SMGS | 10 g | 120 240 300 | yes | no |
| 1B | MHGS | 6.5 mL | 240 | yes | no | |
| 2 (rotating flask) | 2 A | SMGS | 6.5 mL | 240 | yes | yes |
| 2 B | MHGS | 6.5 mL | yes |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwinger, L.; Lehmann, S.; Zielbauer, L.; Scharfe, B.; Gerdes, T. Aluminum Coated Micro Glass Spheres to Increase the Infrared Reflectance. Coatings 2019, 9, 187. https://doi.org/10.3390/coatings9030187
Schwinger L, Lehmann S, Zielbauer L, Scharfe B, Gerdes T. Aluminum Coated Micro Glass Spheres to Increase the Infrared Reflectance. Coatings. 2019; 9(3):187. https://doi.org/10.3390/coatings9030187
Chicago/Turabian StyleSchwinger, Laura, Sebastian Lehmann, Lukas Zielbauer, Benedikt Scharfe, and Thorsten Gerdes. 2019. "Aluminum Coated Micro Glass Spheres to Increase the Infrared Reflectance" Coatings 9, no. 3: 187. https://doi.org/10.3390/coatings9030187




