A Comparative Study of YSZ Suspensions and Coatings
Abstract
1. Introduction
2. Experimental
2.1. Suspensions and Feed Material
2.2. Coatings Generation
3. Results and Discussion
3.1. Suspension Characteristics
3.1.1. Dried Suspensions
3.1.2. Particle Size Distributions
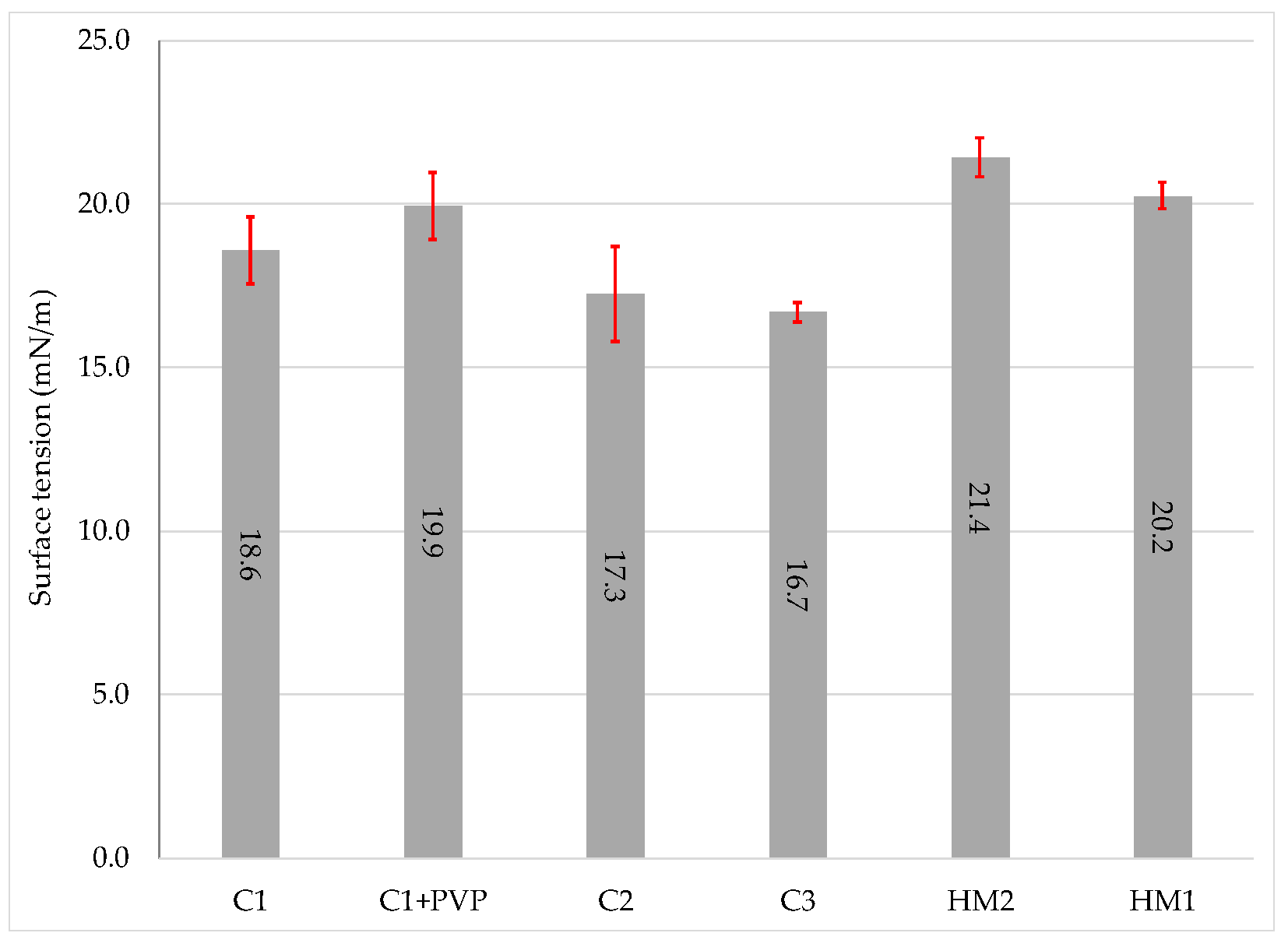
3.1.3. Surface Tensions
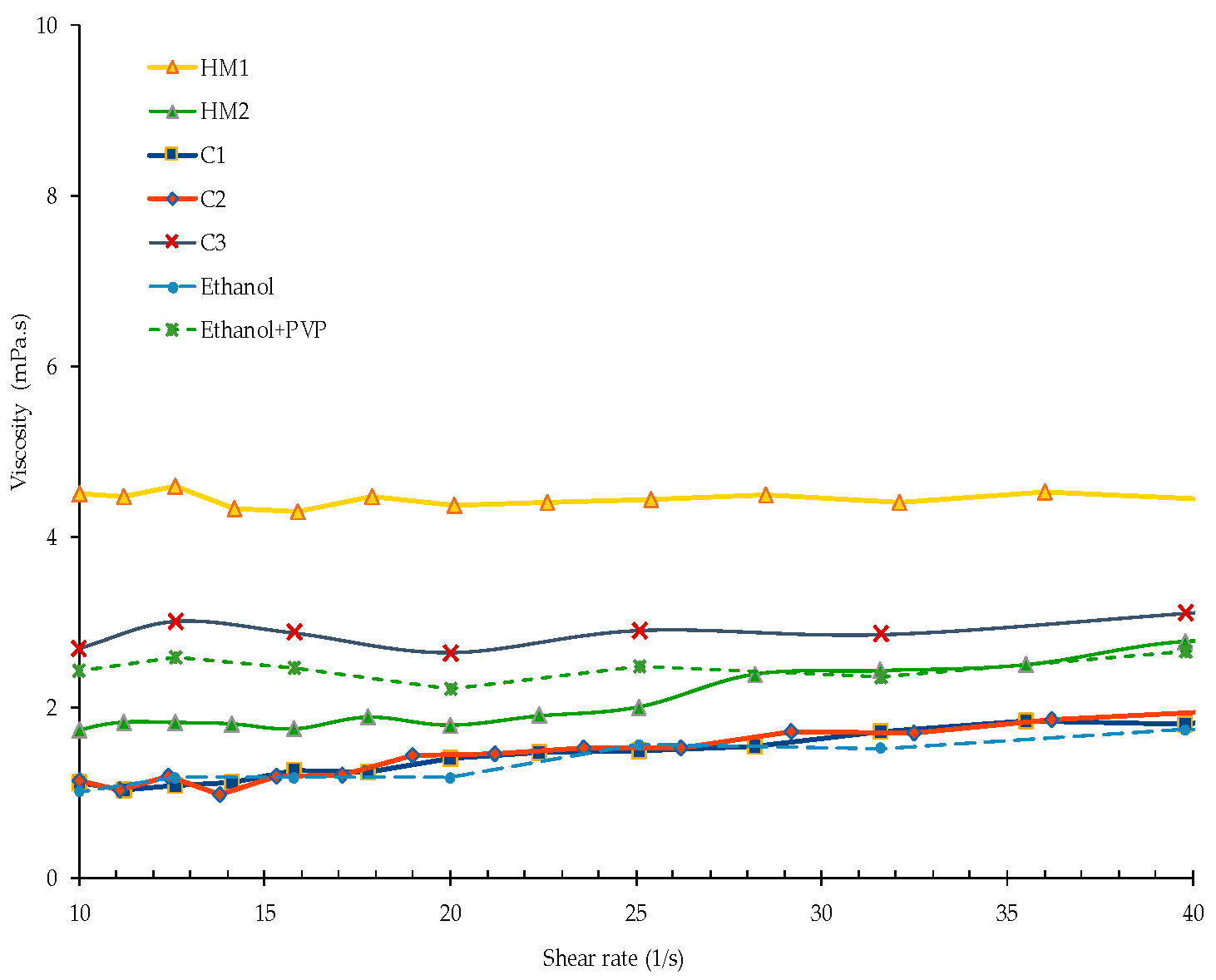
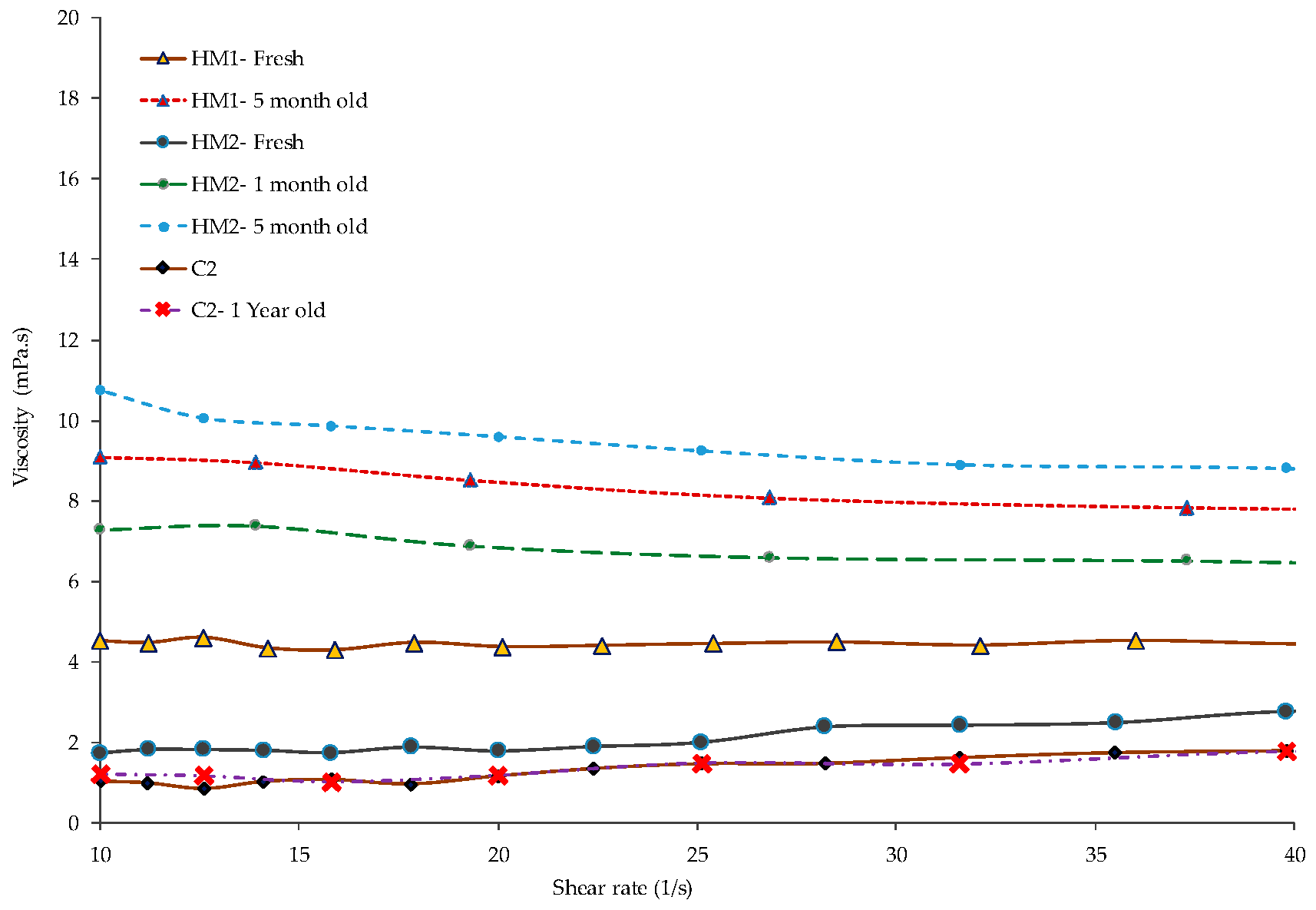
3.1.4. Viscosity
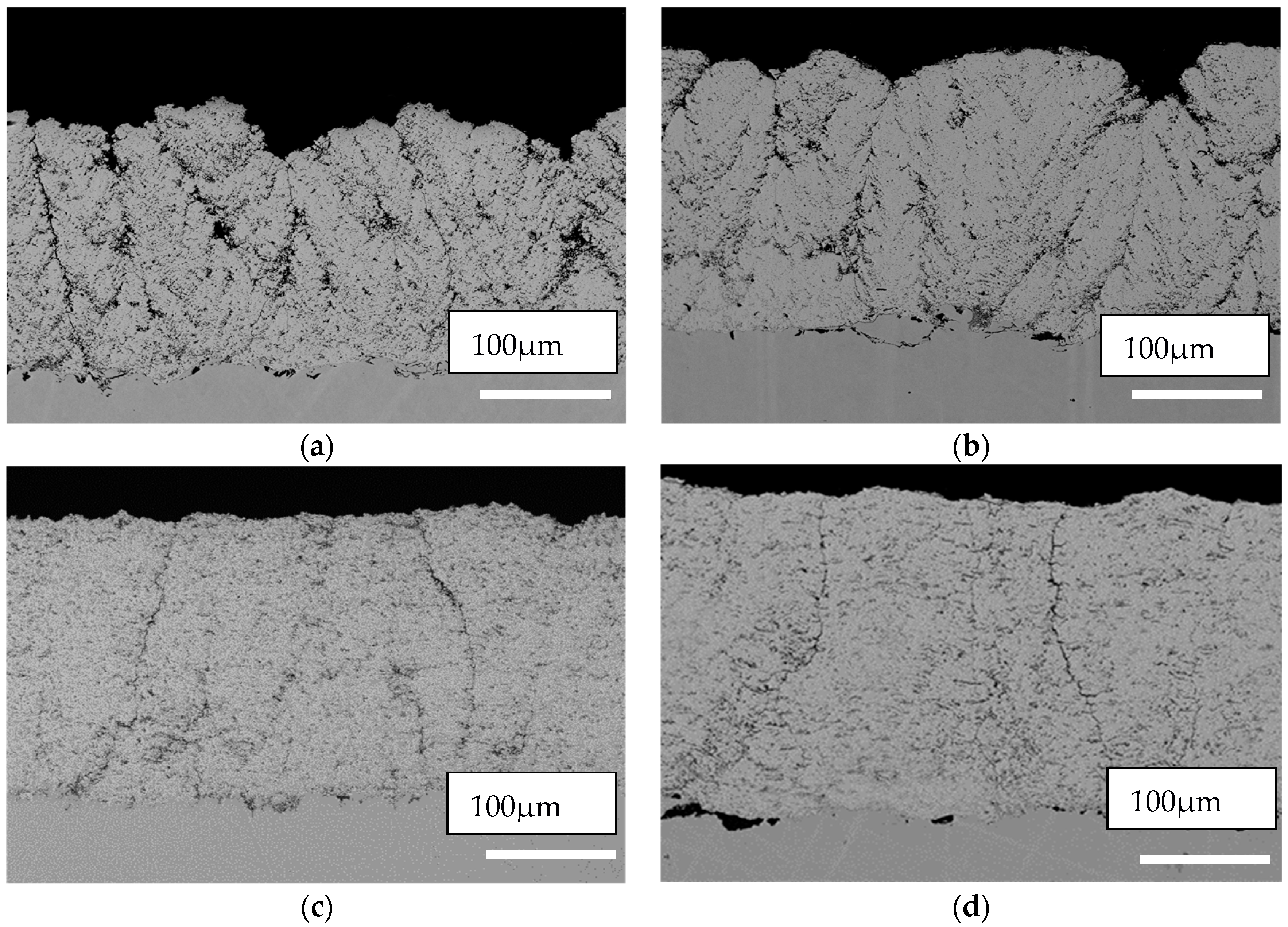
3.2. Coatings Microstructures
4. Conclusions
- While in the absence of surfactant, an increase in Dv50 that ranges 2–4 times of the original particle size is observed, the surfactant addition preserves the original particle size distribution.
- Freshly made suspensions (HM) leave more floccules during drying than the commercial ones. Meanwhile, they show higher viscosities than the commercial suspensions. These can play a role on their behavior also during in-flight drying and differentiate them from the commercial suspensions.
- Surface tension due to addition of up to 5 wt % solid of PVP does not show a large variation.
- The addition of surfactant even in small quantity clearly increases the viscosity. This influence on viscosity of the liquid carrier (for the present suspensions) is more than that of as high as 25 wt % solid content.
- Aging of the fresh HM suspensions also increases the viscosity for some time. Subsequently, it reduces the viscosity after a longer time, which is probably due to more time for better wetting.
- Radial injection (no atomization during injection- cross flow atomization in plasma plum) showed a similar coating structure for the two commercial suspensions with similar viscosities and surface tension values. However, denser structures were formed using the HM suspensions with higher viscosities and surface tensions.
- Axial injection (atomized injection- coaxial flow atomization) showed great difference in the microstructure of the coatings from two commercial suspensions, despite similar viscosities and surface tensions. However, that of HM shows greater similarity to the commercial suspension with similar particle shape and size.
- Commercial suspensions allow for the manipulation of microstructure, while using small amendment of the formula.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Markocsan, N.; Gupta, M.; Joshi, S.; Nylén, P.; Li, X.; Wigren, J. Liquid feedstock plasma spraying: An emerging process for advanced thermal barrier coatings. J. Therm. Spray Technol. 2017, 26, 1104–1114. [Google Scholar] [CrossRef]
- Ucasz, M.T. Thermal Barrier Coating Repair. U.S. Patent Application US20160281204A1, 29 September 2016. [Google Scholar]
- Gupta, M.; Dwivedi, G.; Nylén, P.; Vackel, A.; Sampath, S. An experimental study of microstructure-property relationships in thermal barrier coatings. J. Therm. Spray Technol. 2013, 22, 659–670. [Google Scholar] [CrossRef]
- VanEvery, K.; Krane, M.J.M.; Trice, R.W. Parametric study of suspension plasma spray processing parameters on coating microstructures manufactured from nanoscale yttria-stabilized zirconia. Surf. Coat. Technol. 2012, 206, 2464–2473. [Google Scholar] [CrossRef]
- Meillot, E.; Vert, R.; Caruyer, C.; Damiani, D.; Vardelle, M. Manufacturing nanostructured YSZ coatings by suspension plasma spraying (SPS): Effect of injection parameters. J. Phys. D 2011, 44, 194008. [Google Scholar] [CrossRef]
- VanEvery, K.; Krane, M.J.M.; Trice, R.W.; Wang, H.; Porter, W.; Besser, M.; Sordelet, D.; Ilavsky, J.; Almer, J. Column formation in suspension plasma-sprayed coatings and resultant thermal properties. J. Therm. Spray Technol. 2011, 20, 817–828. [Google Scholar] [CrossRef]
- Pourang, K.; Moreau, C.; Dolatabadi, A. Effect of substrate and its shape on in-flight particle characteristics in suspension plasma spraying. J. Therm. Spray Technol. 2016, 25, 44–54. [Google Scholar] [CrossRef]
- Oberste Berghaus, J.; Bouaricha, S.; Legoux, J.-G.; Moreau, C. Injection conditions and in-flight particle states in suspension plasma spraying of alumina and zirconia nano-ceramics. In Proceedings of the International Thermal Spray Conference, Basel, Switzerland, 2–4 May 2005; pp. 512–518. [Google Scholar]
- Fauchais, P.; Rat, V.; Coudert, J.-F.; Etchart-Salas, R.; Montavon, G. Operating parameters for suspension and solution plasma-spray coatings. Surf. Coat. Tech. 2008, 202, 4309–4317. [Google Scholar] [CrossRef]
- Fauchais, P.; Montavon, G.; Lima, R.S.; Marple, B.R. Engineering a new class of thermal spray nano-based microstructures from agglomerated nanostructured particles, suspensions and solutions: An invited review. J. Phys. D 2011, 44, 093001. [Google Scholar] [CrossRef]
- Fauchais, P.; Vardelle, M.; Vardelle, A.; Goutier, S. What do we know, what are the current limitations of suspension plasma spraying? J. Therm. Spray Technol. 2015, 24, 1120–1129. [Google Scholar] [CrossRef]
- Fazilleau, J.; Delbos, C.; Rat, V.; Coudert, J.F.; Fauchais, P.; Pateyron, B. Phenomena involved in suspension plasma spraying part 1: Suspension injection and behavior. Plasma Chem. Plasma Process. 2006, 26, 371–391. [Google Scholar] [CrossRef]
- Handscomb, C.S.; Kraft, M.; Bayly, A.E. A new model for the drying of droplets containing suspended solids after shell formation. Chem. Eng. Sci. 2009, 64, 228–246. [Google Scholar] [CrossRef]
- Chen, D.; Jordan, E.H.; Gell, M. Effect of solution concentration on splat formation and coating microstructure using the solution precursor plasma spray process. Surf. Coat. Technol. 2008, 202, 2132–2138. [Google Scholar] [CrossRef]
- Jabbari, F.; Jadidi, M.; Wuthrich, R.; Dolatabadi, A. A numerical study of suspension injection in plasma-spraying process. J. Therm. Spray Technol. 2014, 23, 3–13. [Google Scholar] [CrossRef]
- Jadidi, M.; Mousavi, M.; Moghtadernejad, S.; Dolatabadi, A. A three-dimensional analysis of the suspension plasma spray impinging on a flat substrate. J. Therm. Spray Technol. 2015, 24, 11–23. [Google Scholar] [CrossRef]
- Lasheras, J.C.; Hopfinger, E.J. Liquid jet instability and atomization in a coaxial gas stream. Ann. Rev. Fluid Mech. 2000, 32, 275–308. [Google Scholar] [CrossRef]
- Berry, J.D.; Neeson, M.J.; Dagastine, R.R.; Chan, D.Y.C.; Tabor, R.F. Measurement of surface and interfacial tension using pendant drop tensiometry. J. Colloid Interface Sci. 2015, 454, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Preecha, P.; Wanless, E.J.; Arquero, O.-A.; Franks, G.V. The effect of ionic surfactant adsorption on the rheology of ceramic glaze suspensions. J. Am. Ceram. Soc. 2005, 88, 540–546. [Google Scholar]
- Sato, T.; Kohnosu, S. Effect of surfactant on rheological properties of aqueous titanium dioxide suspensions. J. Colloid Interface Sci. 1992, 152, 543–547. [Google Scholar] [CrossRef]
- Galindo-Rosales, F.; Rubio-Hernández, F.J.; Velázquez-Navarro, J.F. Shear-thickening behavior of Aerosil® R816 nanoparticles suspensions in polar organic liquids. Rheologica Acta 2009, 48, 699–708. [Google Scholar] [CrossRef]
- Ganvira, A.; Filomena Calinas, R.; Markocsan, N.; Curry, N.; Joshi, S. Experimental visualization of microstructure evolution during suspension plasma spraying of thermal barrier coatings. J. Eur. Ceram. Soc. 2019, 39, 470–481. [Google Scholar] [CrossRef]


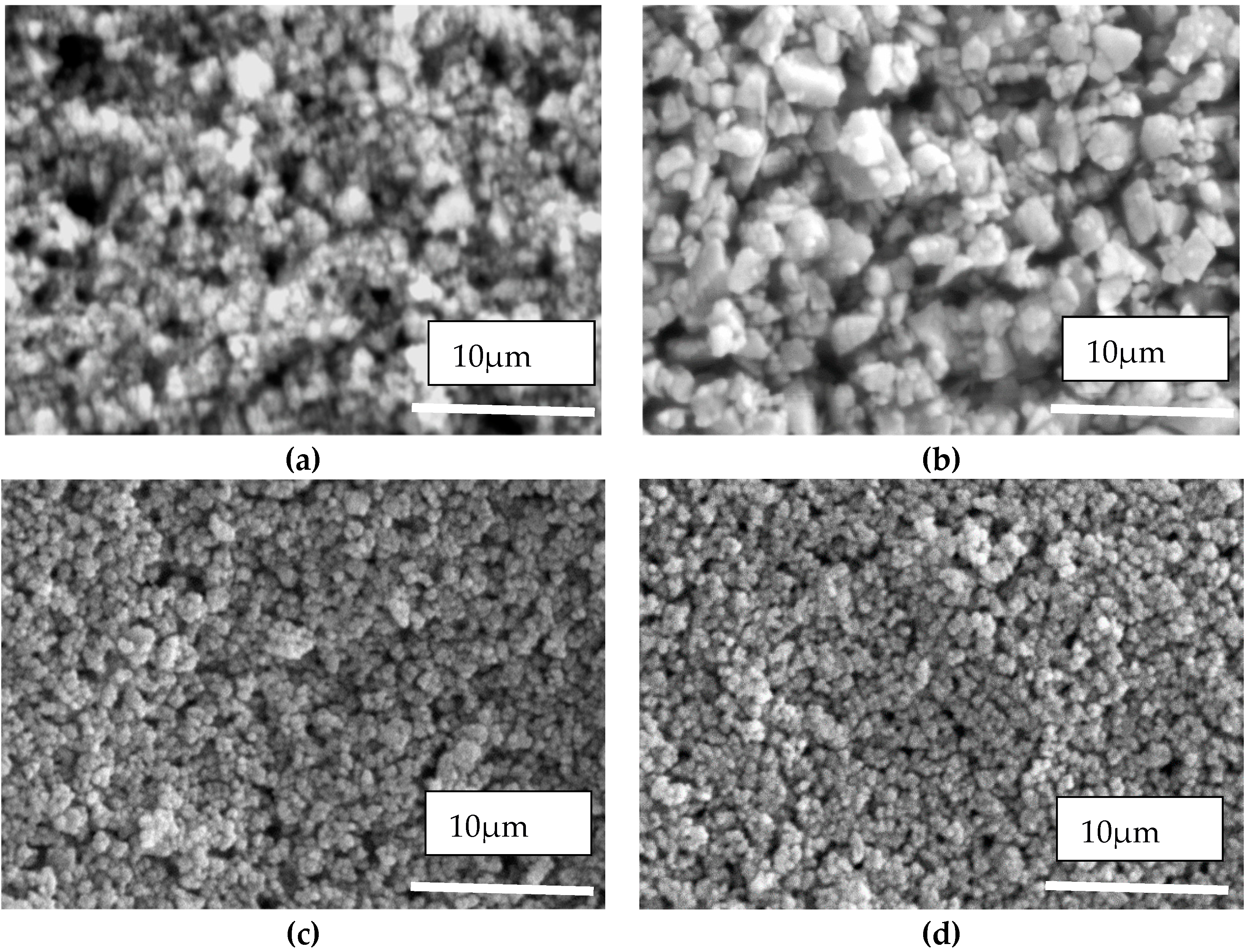



| Supplier | Suspension Name | Formulation |
|---|---|---|
| Innovnano | C1 | 25 wt %YSZ in ethanol |
| Treibacher | C2 | 25 wt %YSZ in ethanol |
| Treibacher-amended | C3 | 25 wt %YSZ in ethanol + 5 solid wt % PVP |
| Homemade 1 | HM1 | 25 wt % YSZ + 5 solid wt % PVP in ethanol |
| Homemade 2 | HM2 | 25 wt %YSZ in ethanol (similar to commercial formula) |
| Sample Number | Suspension Name | Plasma Gas (L/min) | Gas Pressure (psi) | Current (A) | Feed Rate (mL/min) (Set = 20) | Robot Speed (m/s) | Spray Distance (mm) | Injector Diameter (µm) | Number of Passes |
|---|---|---|---|---|---|---|---|---|---|
| 1 | C1 | Ar + He (25,25) | 60 | 600 | 19–20 | 1 | 30 | 150 | 69 |
| 2 | C2 | 21–23 | 80 | ||||||
| 3 | C3 | 20–23 | 80 | ||||||
| 4 | HM1 | 20–24 | 80 | ||||||
| 5 | HM2 | 22–24 | 80 |
| Sample # | Suspension Name | Atomizing Gas (N2) (L/min) | Plasma Gases (L/min) | Total Gas (l/min) | Current (Amp) | Feed Rate (mL/min) (Set = 40) | Robot Speed (m/s) | Spray Sistance (mm) | Number of Pass |
|---|---|---|---|---|---|---|---|---|---|
| 1 | C1 | 15 | Ar/N2/H2 (75/10/15) | 245 | 190 | 32–48 | 1 | 50 | 40 |
| 2 | C2 | 30 | |||||||
| 3 | C3 | 30 | |||||||
| 4 | HM2 | 30 |
| Suspension Name | Original Powder Size (µm) | Particle Size Distribution in the Suspension (µm) (Volume-Based) | ||
|---|---|---|---|---|
| D10 | D50 | D90 | ||
| C1 | 0.4–0.5 (D50) | 1.08 | 2.14 | 4.07 |
| C2 | 0.15–0.4, 0.4–0.8, 0.9–1.7 (D10, D50, D90) | 0.85 | 1.63 | 2.97 |
| C3 | Same as C2 | 0.24 | 0.51 | 1.06 |
| HM1 | 0.4 (D50) | 0.15 | 0.4 | 0.80 |
| HM2 | 0.4 (D50) | 0.45 | 1.00 | 1.88 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasi, F.; Alebrahim, E.; Dolatabadi, A.; Moreau, C. A Comparative Study of YSZ Suspensions and Coatings. Coatings 2019, 9, 188. https://doi.org/10.3390/coatings9030188
Tarasi F, Alebrahim E, Dolatabadi A, Moreau C. A Comparative Study of YSZ Suspensions and Coatings. Coatings. 2019; 9(3):188. https://doi.org/10.3390/coatings9030188
Chicago/Turabian StyleTarasi, Fariba, Elnaz Alebrahim, Ali Dolatabadi, and Christian Moreau. 2019. "A Comparative Study of YSZ Suspensions and Coatings" Coatings 9, no. 3: 188. https://doi.org/10.3390/coatings9030188
APA StyleTarasi, F., Alebrahim, E., Dolatabadi, A., & Moreau, C. (2019). A Comparative Study of YSZ Suspensions and Coatings. Coatings, 9(3), 188. https://doi.org/10.3390/coatings9030188





