The Evaluation on Corrosion Resistance and Dross Formation of Zn–23 wt % Al–0.3 wt % Si–x wt % Mg Alloy
Abstract
:1. Introduction
2. Experimental
2.1. Material
2.2. Experimental Processes
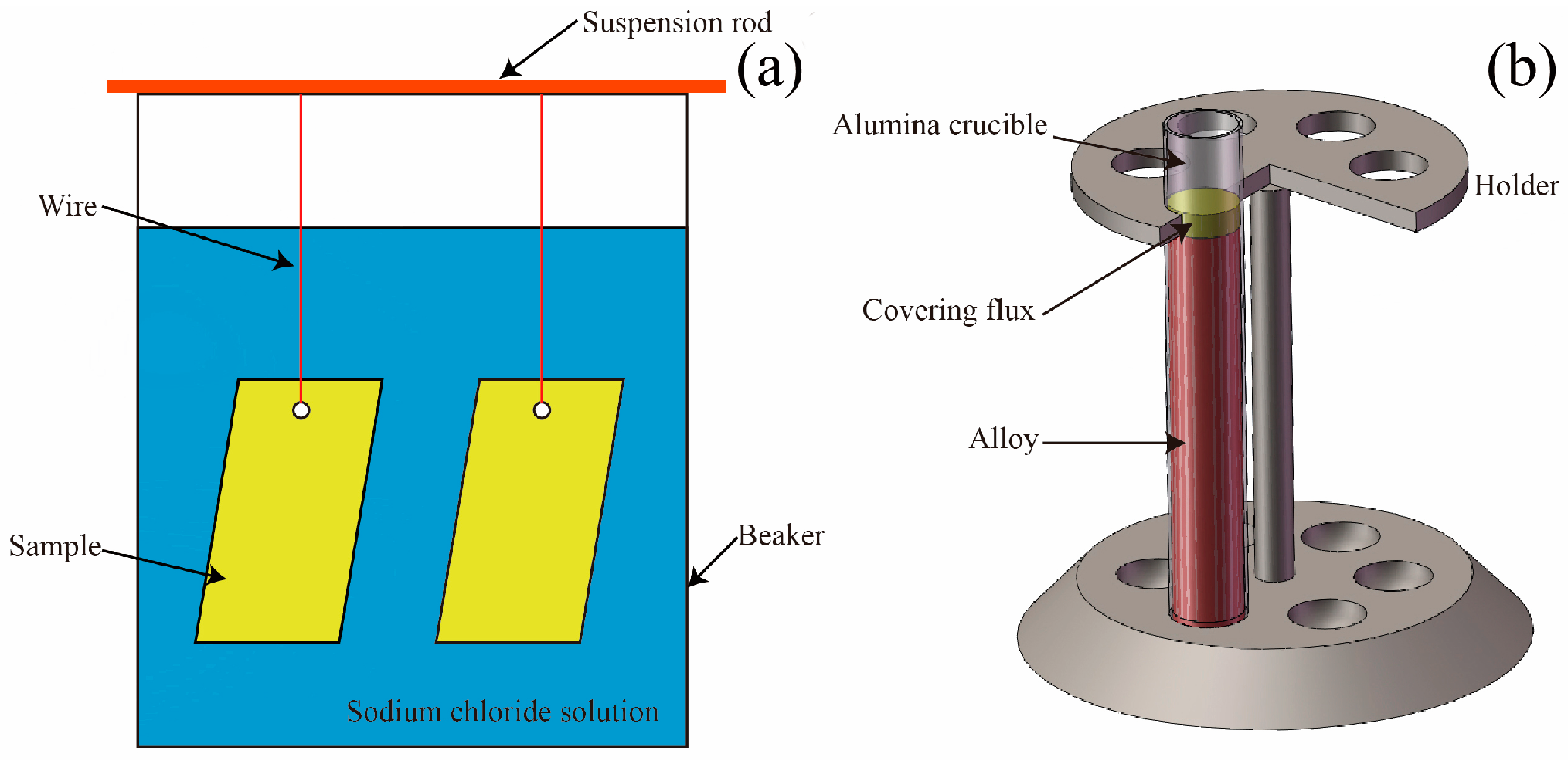
2.2.1. Immersion Test
2.2.2. Dross Generation Test
2.3. Experimental Analysis Equipment
3. Results and Discussion
3.1. Microstructure of the Casting Sample
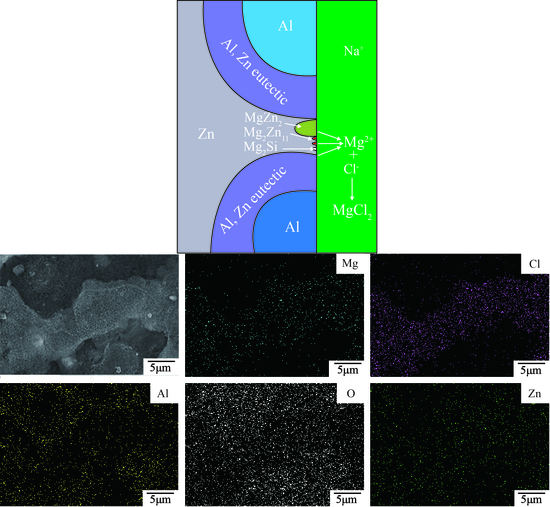
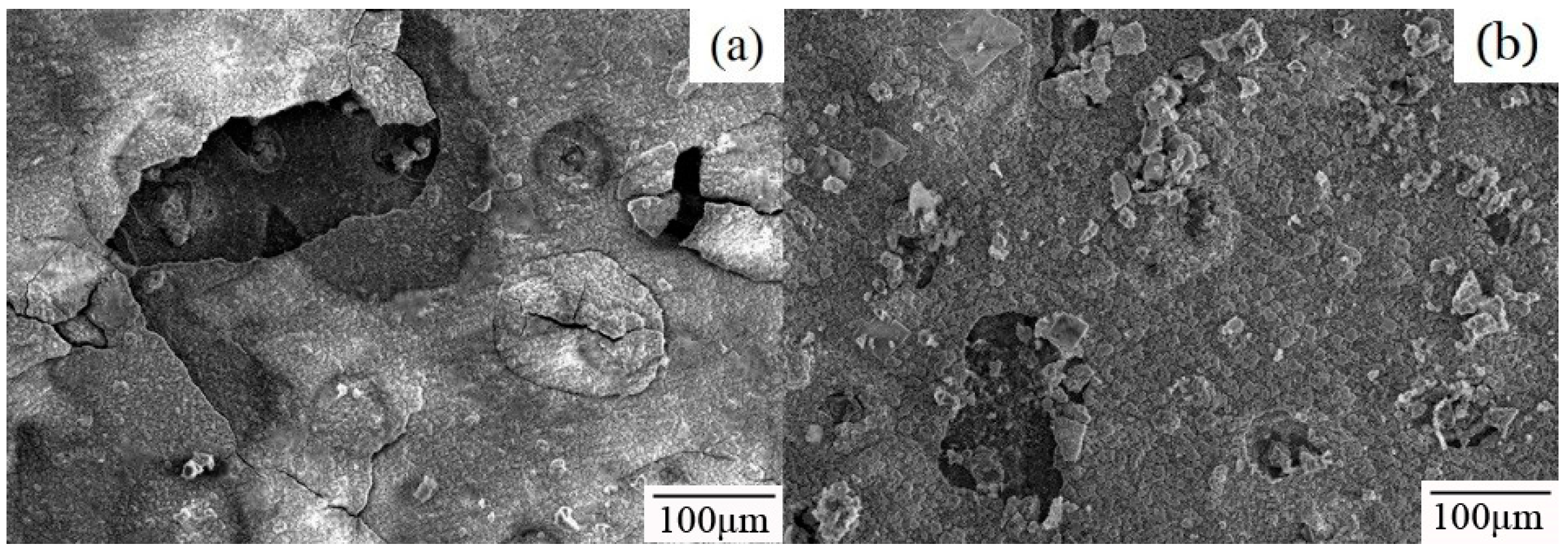
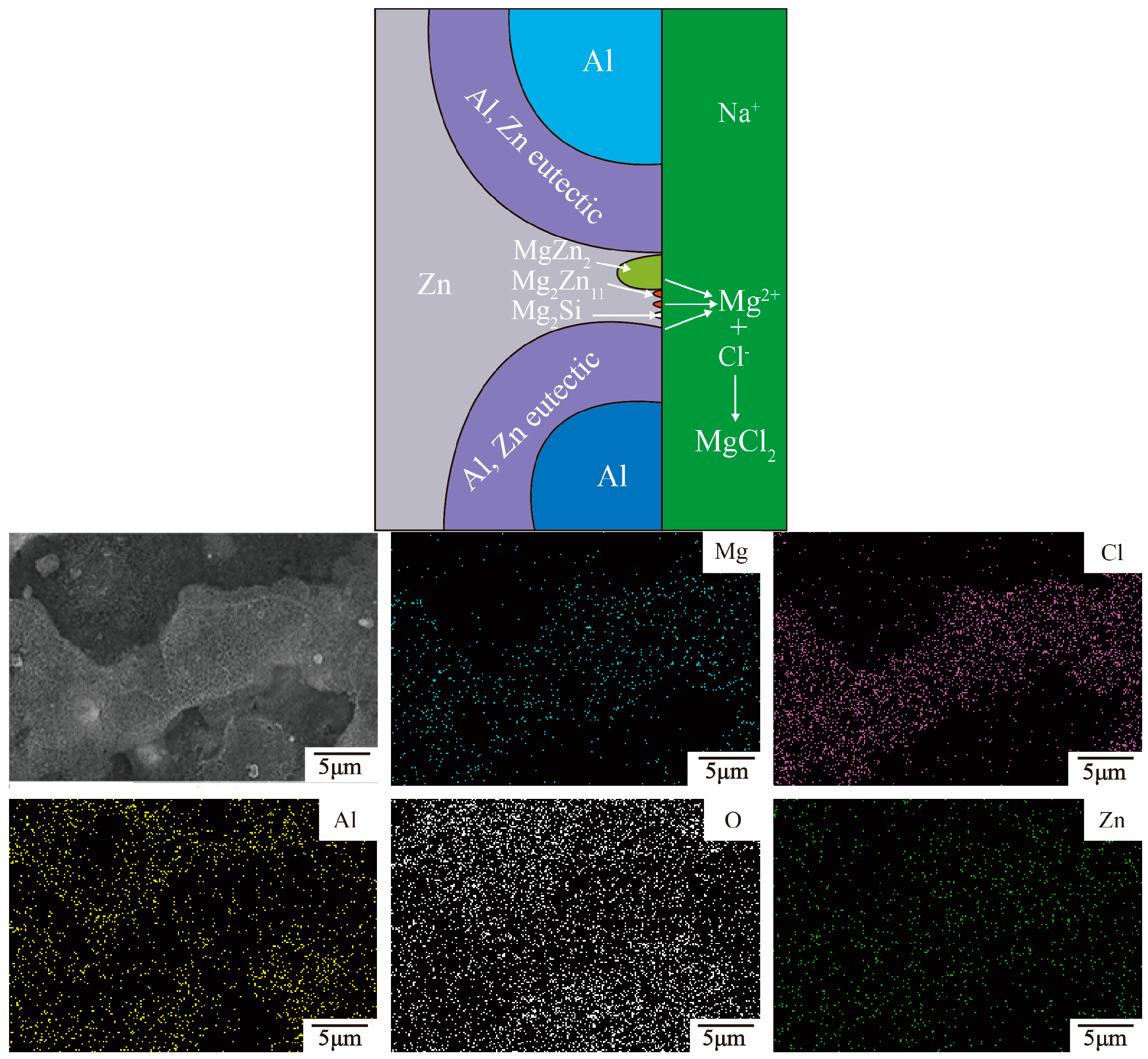
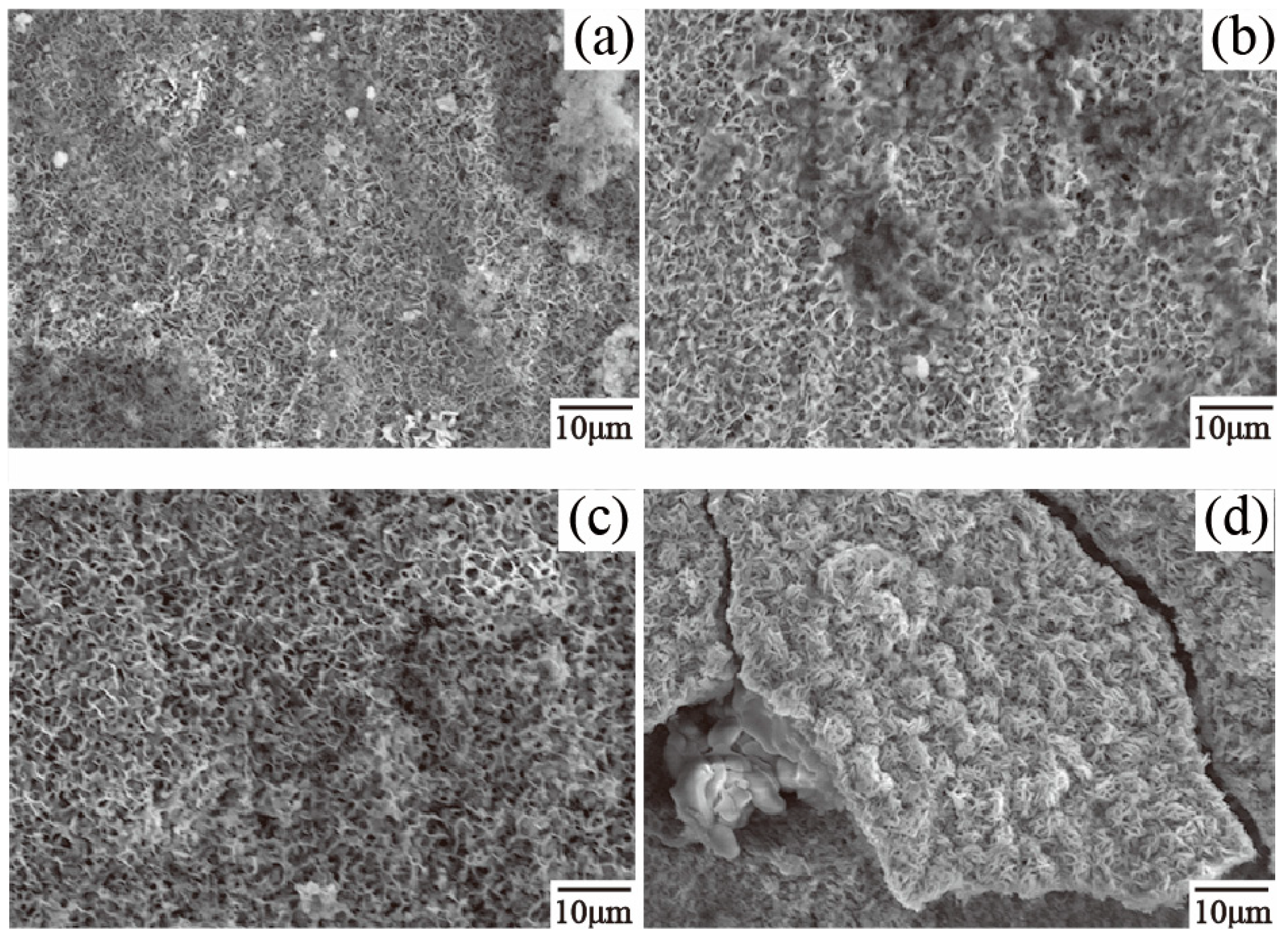
3.2. Microstructure and Phase Composition of Immersion Samples
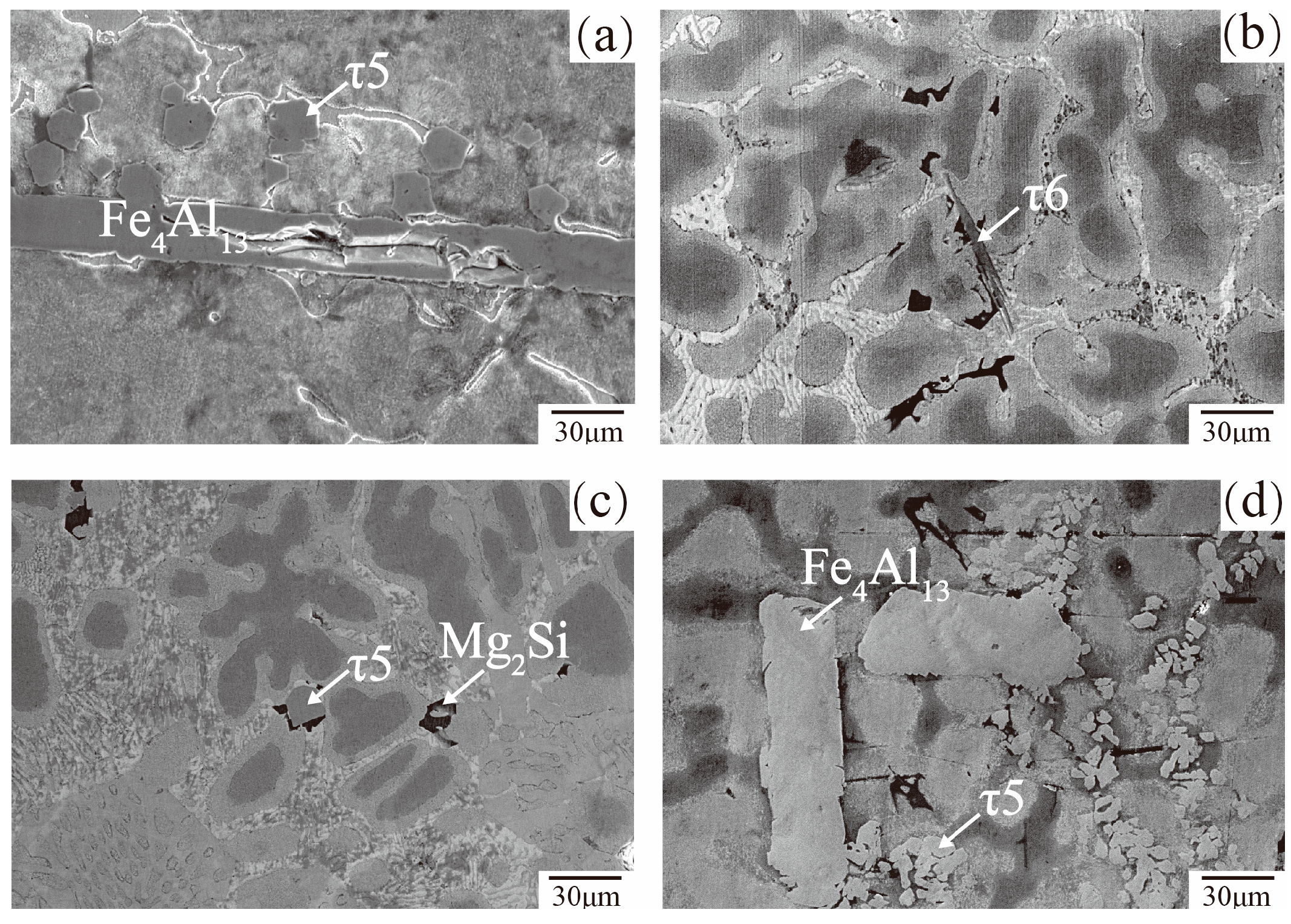
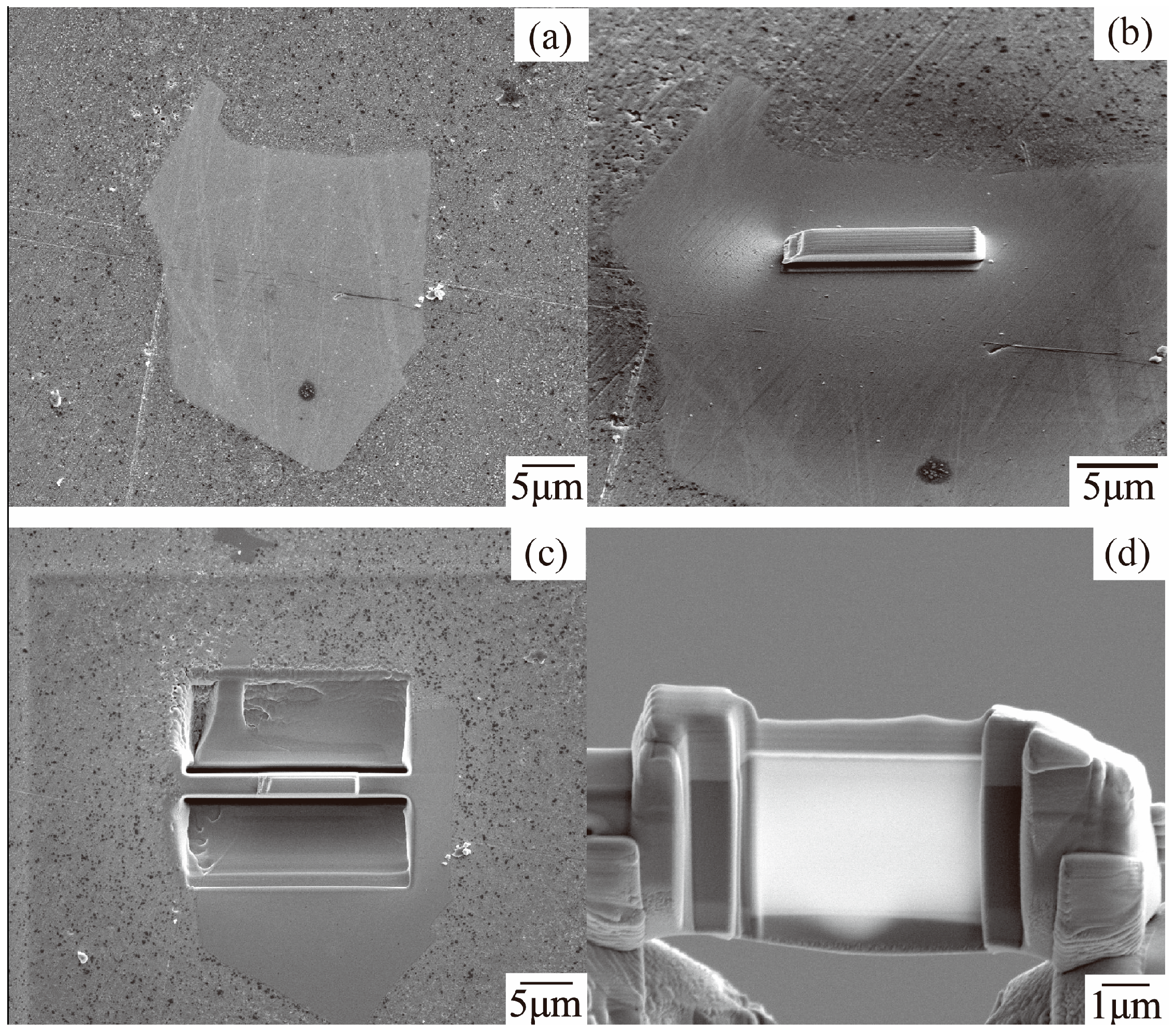
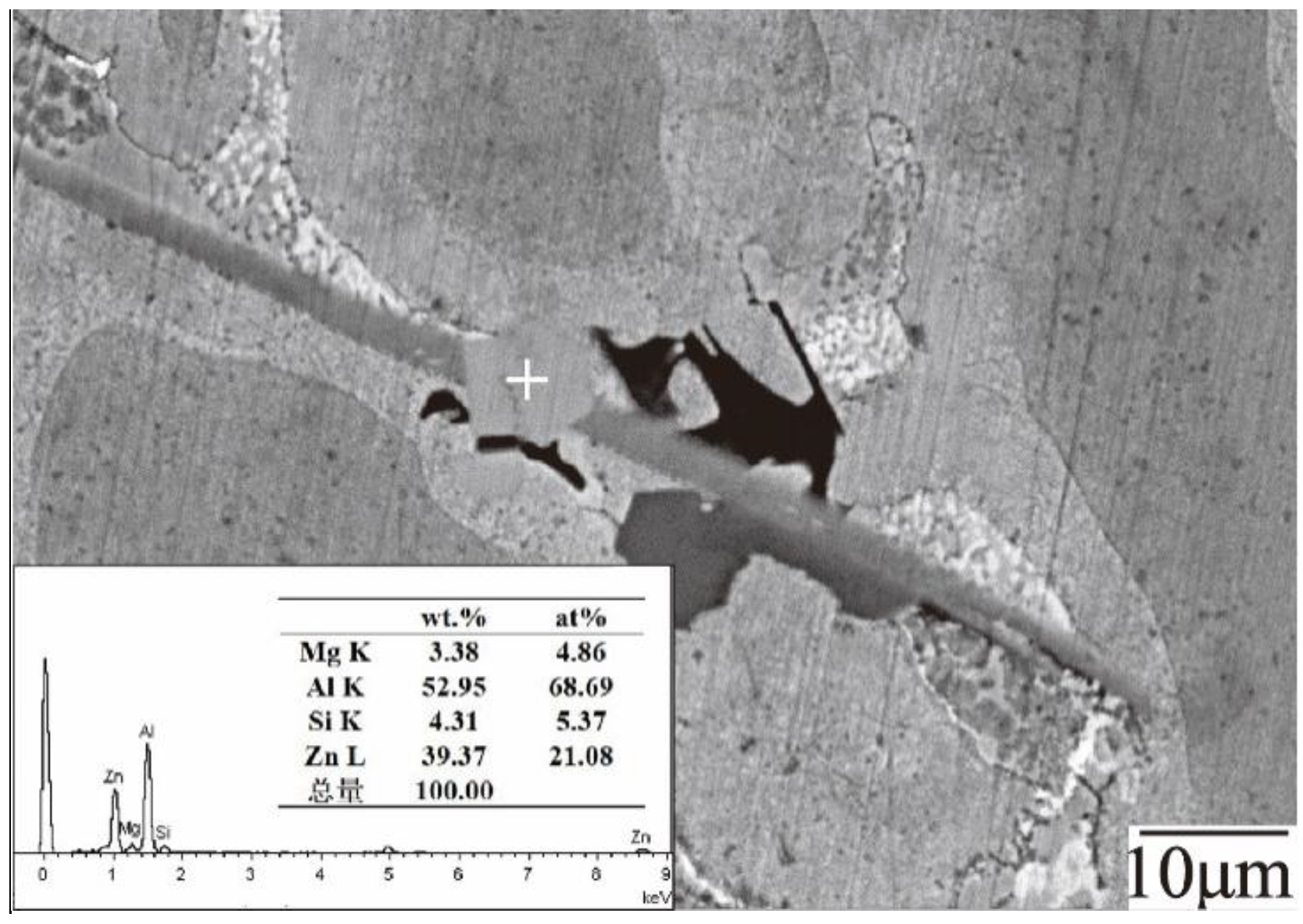
3.3. Microstructure and Phase Composition of Dross
4. Conclusions
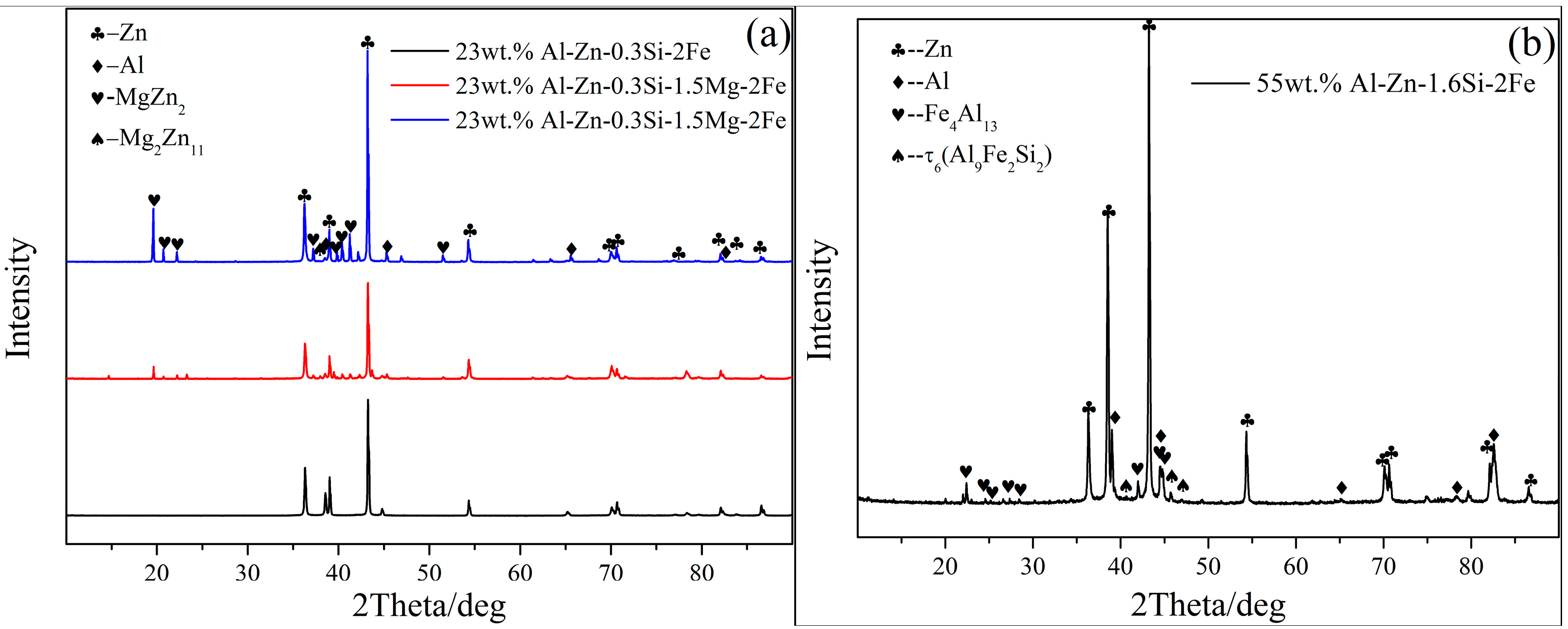
- The 23AZS and 55AZS alloys consist of Al, Zn, and Si phases, and the 23AZS–1.5Mg and 23AZS–3Mg alloys consist of Al, Zn, Mg2Si, MgZn2, and Mg2Zn11 phases.
- In general, the corrosive resistance of different alloys are equal in the initial stage, while the corrosive resistance of 23AZS–xMg (x = 0, 1.5, 3) is higher than 55AZS in the latter stage, and the 23AZS–1.5Mg alloy shows the optimal property in this investigation.
- The corrosion rate of 55AZS at the beginning is high, the rate decreased after 30 days of immersion, and the corrosion rate decreased due to corrosion inhibition by the formation of Zn0.67Al0.33(OH)2 (CO3)0.165·xH2O.
- The bottom dross of 55AZS and 23AZS alloys mainly contain τ6, Fe4Al13 and τ5 phases, the solubility of Fe calculated by Pandat will increase with the increase of Al content. Thus, the amount of dross phase (Fe4Al13 and τ6) in the 23AZS coating is less than that of 55AZS alloy, which is consistent with our experimental results.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seré, P.R.; Zapponi, M.; Ci, E.; Ar Di, S. Comparative corrosion behaviour of 55Aluminium-zinc alloy and zinc hot-dip coatings deposited on low carbon steel substrates. Corros. Sci. 1998, 40, 1711–1723. [Google Scholar] [CrossRef]
- Palma, E.; Puente, J.M.; Morcillo, M. The atmospheric corrosion mechanism of 55% Al–Zn coating on steel. Corros. Sci. 1998, 40, 61–68. [Google Scholar] [CrossRef]
- Rosalbino, F.; Angelini, E.; Maccio, D.; Saccone, A.; Delfino, S. Influence of rare earths addition on the corrosion behaviour of Zn–5% Al (Galfan) alloy in neutral aerated sodium sulphate solution. Electrochim. Acta 2007, 52, 7107–7114. [Google Scholar] [CrossRef]
- Edavan, R.P.; Kopinski, R. Corrosion resistance of painted zinc alloy coated steels. Corros. Sci. 2009, 51, 2429–2442. [Google Scholar] [CrossRef]
- Shawki, S.; Hamid, Z.A. Effect of aluminium content on the coating structure and dross formation in the hot-dip galvanizing process. Surf. Interface Anal. 2003, 35, 943–947. [Google Scholar] [CrossRef]
- Dong, A.P.; Shu, D.; Wang, J.; Cai, X.C.; Sun, B.D.; Cui, J.; Shen, J.G.; Ren, Y.S.; Yin, X.D. Continuous separation of Fe–Al–Zn dross phase from hot dip galvanised melt using alternating magnetic field. Mater. Sci. Technol. 2008, 24, 40–44. [Google Scholar] [CrossRef]
- Luo, Q.; Jin, F.; Li, Q.; Zhang, J.Y.; Chou, K.C. The mechanism of dross formation during hot-dip Al–Zn alloy coating process. J. Manuf. Sci. Prod. 2013, 13, 85–89. [Google Scholar] [CrossRef]
- Su, X.P.; Lai, X.F.; Wang, J.H.; Peng, H.P.; Tu, H.; Wu, C.J.; Liu, Y. A Device and Method for Removing Zinc Slag in Continuous Hot Dip Galvanized Znic-Aluminum. Chinese Patent CN 103184344A, 2013. [Google Scholar]
- Dong, A.P.; Shu, D.; Wang, J.; Sun, B.D.; Shen, J.G.; Ren, Y.S.; Lu, Y.L.; Yin, X.D. Separation behaviour of zinc dross from hot dip galvanising melts of different aluminium concentrations by alternating magnetic field. Ironmak. Steelmak. 2009, 36, 316–320. [Google Scholar] [CrossRef]
- Dong, A.P.; Shu, D.; Wang, J.; Sun, B.D. Identification of Fe–Al–Zn dross phase in galvanised zinc bath and its separation by method of alternating magnetic field. Ironmak. Steelmak. 2008, 35, 633–637. [Google Scholar] [CrossRef]
- OU-Yang, M.H.; Li, Z.; Wang, X.M.; Yin, F.C.; Wang, J.H.; Su, X.P. Control of bottom dross in 55% Al–Zn bath. Mater. Sci. Eng. Power Metall. 2008, 13, 139–143. [Google Scholar]
- Nakano, J.; Purdy, G.R.; Malakhov, D.V. Thermodynamic Aspects of Dross Generation. In Proceedings of the 7th International Conference on Zinc and Zinc Alloy Coated Steel Sheet, Osaka, Japan, 20 November 2007; pp. 135–140. [Google Scholar]
- Xu, J.; Gu, Q.F.; Li, Q.; Lu, H.S. Influence of Ti and La additions on the formation of intermetallic compounds in the Al–Zn–Si bath. Met. Mater. Trans. A 2016, 47, 6542–6554. [Google Scholar] [CrossRef]
- Osamura, K.; Okuda, H.; Ochiai, S. Isothermal phase decomposition diagram in Al–Zn binary alloys. Scr. Metall. 1985, 19, 1379–1384. [Google Scholar] [CrossRef]
- Oolijven, W.J.; Ranjan, M.; Joshi, A. Update on the Status of the Development of a high-Al alloy for General Galvanizing. In Proceedings of the 21st Inter General Galvanizing Conference, Naples, Italy, 1 January 2007; pp. 43–56. [Google Scholar]
- Joshi, A.V. Development of a Novel Si-Modified Zn–Al Eutectoid Alloy for Hot-Dip Batch Galvanizing. Master’s Thesis, Dissertation, University of Cincinnati, Cincinnati, OH, USA, 3 April 2006. [Google Scholar]
- Li, Z.F. Research on new Hot-Dipped Zinc-Aluminum Coating and Process. Master’s Thesis, Dissertation, Xiangtan University, Xiangtan, China, 16 May 2012. (In Chinese). [Google Scholar]
- Tsujimura, T.; Komatsu, A.; Andoh, A. Influence of Mg Content in Coating Layer and Coating Structure on Corrosion Resistance of Hot-Dip Zn–Al–Mg Alloy Coated Steel Sheet. In Proceedings of the Galvatech’01, Brussels, Belgium, 26 June 2001; Volume 1, pp. 145–152. [Google Scholar]
- Tanaka, S.; Honda, K.; Takahashi, A.; Morimoto, Y.; Kurosaki, M.; Shindo, H.; Sugiyama, M. The Performance of Zn–Al–Mg–Si Hot-Dip Galvanized Steel Sheet. In Proceedings of the Galvatech’01, Brussels, Belgium, 28 June 2001; Volume 1, pp. 153–160. [Google Scholar]
- Hosking, N.C.; Ström, M.A.; Shipway, P.H.; Rudd, C.D. Corrosion resistance of zinc-magnesium coated steel. Corros. Sci. 2007, 49, 3669–3695. [Google Scholar] [CrossRef]
- Kawafuku, J.; Katoh, J.; Toyama, M.; Nishimoto, H.; Ikeda, K.; Satoh, H. Structure and corrosion resistance of zinc alloy coated steel sheets obtained by continuous vapor deposition apparatus. Tetsu-to-Hagane J. Iron Steel Inst. Jpn. 1991, 77, 995–1002. [Google Scholar] [CrossRef]
- Shindo, H.; Nishimura, K.; Kato, K. Anti-Corrosion in Atmospheric Exposure of Zn–Mg–Al Hot-Dip Galvanized Steel Sheet. In Proceedings of the Galvatech’98, Chiba, Japan, 23 September 1998; pp. 433–436. [Google Scholar]
- Tanaka, J.; Ono, K.; Hayashi, S.; Ohsasa, K.; Narita, T. Effect of Mg and Si on the microstructure and corrosion behavior of Zn–Al hot dip coatings on low carbon steel. ISIJ Int. 2002, 42, 80–85. [Google Scholar] [CrossRef]
- Zhu, G.L. Study on Mg and RE in the Modification of Zn–23Al–0.3Si Coating. Master’s Thesis, Dissertation, Northeastern University, Shengyang, China, 16 June 2014. (In Chinese). [Google Scholar]
- GB/T 19746-2005. Corrosion of Metals and Alloys-Alternate Immersion Test in Salt Solution. Available online: http://www.gb688.cn/bzgk/gb/newGbInfo?hcno=A1AB7892E1199C9D7F91DB6D23B83D2F (accessed on 13 May 2015). (In Chinese).
- GB/T 16545-1996. Corrosion of Metals and Alloys-Removal of Corrosion Products from Corrosion Test Specimens. Available online: http://www.csres.com/detail/56299.html (accessed on 27 September 1996). (In Chinese).
- Chen, R.Y.; Willis, D. The behavior of silicon in the solidification of Zn–55Al–1.6Si coating on steel. Metall. Mater. Trans. A 2005, 36, 117–128. [Google Scholar] [CrossRef]
- Peng, W.J.; Du, Q.; Wu, G.X.; Dan, W.D.; Hu, W.D.; Zhang, J.Y. The role of Ti and Si in the nucleation of α-Al during hot dip coating of steel with Al–43.4 wt % Zn–1.6 wt % Si alloy. Surf. Coat. Technol. 2016, 299, 56–64. [Google Scholar] [CrossRef]
- Peng, W.J.; Wu, G.X.; Dai, X.; Zhang, J.Y.; Hu, W.D. Grain refinement behavior of Al–Zn–Si alloy by inoculation in hot-dip coating. Light Met. 2015, 2015, 301–306. [Google Scholar]
- Cao, W.S.; Chen, S.L.; Zhang, F.; Wu, K.; Yang, Y.; Chang, Y.A.; Schmin-Fetzer, R.; Oates, W.A. PANDAT software with PanEngine, PanOptimizer and PanPrecipitation for multi-component phase diagram calculation and materials property simulation. Calphad 2009, 33, 328–342. [Google Scholar] [CrossRef]
- PanAl-Aluminium Alloy Thermodynamic DaTablease; CompuTherm: Madison, WI, USA, 2015.
- Li, Q.; Zhao, Y.Z.; Luo, Q.; Chen, S.L.; Zhang, J.Y.; Chou, K.C. Experimental study and phase diagram calculation in Al-Zn-Mg-Si quaternary system. J. Alloys Compd. 2010, 501, 282–290. [Google Scholar] [CrossRef]
- Mao, F.; Chen, F.; Yan, G.Y.; Wang, T.M.; Cao, Z.Q. Effect of strontium addition on silicon phase and mechanical properties of Zn–27Al–3Si alloy. J. Alloys Compd. 2015, 622, 871–879. [Google Scholar] [CrossRef]
- Peng, W.J.; Wu, G.X.; Peng, H.; Ding, D.J.; Yu, Y.W.; Zhang, J.Y. The effect of Al–5 wt % Ti–0.2 wt % B on the solidification characteristics of 55 wt % Al–Zn–1.6 wt % Si alloy in hot-dip coating. Surf. Coat. Technol. 2016, 360, 379–389. [Google Scholar]
- Kruehong, C.; El-Mahdy, G.A.; Nishikata, A.; Tsuru, T. Influence of second phases on the electrochemical behavior of hot dipped Al–Mg–Si coated steel. Corros. Sci. 2010, 52, 2379–2386. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.C.; Luo Fan, H.Q.; Zhang, J.Y.; Lu, H.S.; Chou, K.C.; Wang, X.L.; Li, Q. Influence of alloyed magnesium on the microstructure and long-term corrosion behavior of hot-dip Al–Zn–Si coating in NaCl solution. Corros. Sci. 2016, 104, 217–226. [Google Scholar] [CrossRef]
- Honda, K.; Sugiyama, M.; Ikematsu, Y.; Ushioda, K. Role of TiAl3 fine precipitate in nucleation of the primary Al dendrite phase during solidification in hot-dip Zn–11% Al–3% Mg–0.2% Si coated steel sheet. Mater. Trans. 2011, 52, 90–95. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, C.T.; Liu, Q.; Smith, R.; Nolan, D. A new quaternary phase observed in a laser treated Zn–Al–Mg–Si coating. J. Alloys Compd. 2014, 589, 226–229. [Google Scholar] [CrossRef]
- Pan, X.H.; Tu, H.; Su, X.P.; Peng, H.P.; Wu, C.J.; Wang, J.H. Effects of temperature and aluminium content on formation of zinc dross in zinc-aluminium bath. Mater. Sci. Eng. Powder Metall. 2015, 20, 258–265. [Google Scholar]
- Nicard, C.; Allély, C.; Volovitch, P. Effect of Zn and Mg alloying on microstructure and anticorrosion mechanisms of Al-Si based coatings for high strength steel. Corros. Sci. 2019, 146, 192–201. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R.G. Electrochemical characteristics of intermetallic phases in aluminum alloys an experimental survey and discussion. J. Electrochem. Soc. 2005, 152, B140–B151. [Google Scholar] [CrossRef]
- Chang, J.K.; Lin, C.S.; Wang, W.R.; Cheng, W.J. Microstructural evaluation and property change of 5 Wt Pct Al–Zn coating on press hardening steel during austenitization. Metall. Mater. Trans. A 2018, 49, 3715–3728. [Google Scholar] [CrossRef]












| Alloy | Al | Zn | Si | Mg | La | Fe |
|---|---|---|---|---|---|---|
| 23AZS | 22.80 | bal | 0.38 | – | – | – |
| 23AZS–1.5Mg | 23.20 | bal | 0.34 | 1.58 | – | – |
| 23AZS–3Mg | 23.10 | bal | 0.35 | 2.87 | – | – |
| 55AZS | 52.697 | 45.7 | 1.31 | – | 0.2 | 0.093 |
| Immersion Days | Detected Phases |
|---|---|
| 4 | Zn5(OH)8Cl2·H2O |
| 8 | Zn5(OH)8Cl2·H2O |
| 13 | Zn5(OH)8Cl2·H2O |
| 19 | Zn5(OH)8Cl2·H2O |
| 30 | Zn5(OH)8Cl2·H2O |
| 38 | Zn0.67Al0.33(OH)2(CO3)0.165·xH2O |
| 47 | Zn0.67Al0.33(OH)2(CO3)0.165·xH2O |
| 59 | Zn0.67Al0.33(OH)2(CO3)0.185·xH2O |
| Alloy | Upper | Middle | Bottom |
|---|---|---|---|
| 55AZS | τ6 | τ6 | τ6, Fe4Al13, τ5 |
| 23AZS | τ5, Fe4Al13 | τ5, τ6, Fe4Al13 | τ5, Fe4Al13 |
| 23AZS–1.5Mg | Mg2Si, τ6, τ5, x | Mg2Si, τ6, τ5, x | Mg2Si, τ6, τ5, x |
| 23AZS–3Mg | τ5, Mg2Si | Mg2Si, τ5, Fe4Al13 | Mg2Si, τ5 |
| Alloy name | Solubility/wt % | Alloy | Solubility/wt % |
|---|---|---|---|
| 23AZS | 0.002 | 23ASZ–3Mg | 0.004 |
| 23ASZ–1.5Mg | 0.003 | 55AZS | 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, W.; Wu, G.; Lu, R.; Lian, Q.; Zhang, J. The Evaluation on Corrosion Resistance and Dross Formation of Zn–23 wt % Al–0.3 wt % Si–x wt % Mg Alloy. Coatings 2019, 9, 199. https://doi.org/10.3390/coatings9030199
Peng W, Wu G, Lu R, Lian Q, Zhang J. The Evaluation on Corrosion Resistance and Dross Formation of Zn–23 wt % Al–0.3 wt % Si–x wt % Mg Alloy. Coatings. 2019; 9(3):199. https://doi.org/10.3390/coatings9030199
Chicago/Turabian StylePeng, Wangjun, Guangxin Wu, Rui Lu, Quanyong Lian, and Jieyu Zhang. 2019. "The Evaluation on Corrosion Resistance and Dross Formation of Zn–23 wt % Al–0.3 wt % Si–x wt % Mg Alloy" Coatings 9, no. 3: 199. https://doi.org/10.3390/coatings9030199