1. Introduction
Magnesium alloys are lightweight alloys, and could be utilized in various fields [
1,
2,
3]. The corrosion resistance of magnesium alloys is poor [
4,
5,
6], which limits further utilization of magnesium alloys.
Coatings are fabricated on the surface to enhance the corrosion resistance of magnesium alloys [
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19]. Among which, the zinc coating is an effective one. It is reported that the weight loss of pure Zn is about half of the weight loss of pure Mg in the same duration after exposed to humid air [
20]. Adding a little magnesium into pure zinc would reduce the weight loss further [
20].
Moreover, zinc-rich coatings are frequently utilized in the electroplating method to fabricate protective coatings on magnesium alloys [
17,
21]. Magnesium alloys are active, and they are prone to react with oxygen and water to form MgO and Mg(OH)
2 on the surface, which would lead to a weak adhesion of as-electrodeposited coatings [
17,
21]. Thus, before electroplating, magnesium alloys should be pretreated. Zinc immersion is one of the general processes to pretreat magnesium alloys before plating.
Before the zinc immersion process, several pretreatment processes, including surface cleaning, alkaline cleaning, acid pickle, and acid activation, are commonly enlisted to remove soil, debris, oil, grease, oxides, etc. [
17]. Then the magnesium alloy is immersed in special solutions for the zinc film to form [
21].
The zinc-rich coating could also be fabricated by a thermal evaporation method [
18]. In this method, a vacuum environment is required, and the temperature for the evaporation of the original Zn powder is 600 °C. Similarly, the magnesium specimen could be obtained by thermal diffusion, which buries the magnesium alloy specimen into the pure Zn powder, and keeps the diffusion system at an elevated temperature for the zinc-rich coating to grow. In this method, the diffusion system could be buried in compact carbon powder to further prevent oxidation [
19].
Thus, it is crucial to clean the surface of a magnesium alloy matrix to prohibit the formation of the magnesium oxide film in the zinc immersion method, and it is also crucial to keep a relatively inert atmosphere, to prohibit the oxidation of pure metals in the thermal evaporation method and the thermal diffusion method. It seems as though oxides are always unfavorable, and should be avoided.
In this research, a method based on the diffusion alloying process, but utilizing zinc oxide as the diffusion source instead of pure Zn powder to fabricate zinc-rich coatings on magnesium alloys, is raised. The pretreatment process of the magnesium alloy matrix is simplified effectively by this method. Meanwhile, the environment requirement, such as a vacuum or inert atmosphere, becomes unnecessary.
2. Experimental Procedures
An as-cast AZ91D magnesium alloy with the composition of 9.1 wt.% Al, 0.52 wt.% Zn, 0.26 wt.% Mn and Mg as balance, was utilized as the matrix. Samples were cut into specimens with a dimension of 15 mm × 10 mm × 3 mm from the AZ91D magnesium alloy sheets with an electrical discharge cutting machine, and then polished with SiC papers to 1000 grit, rinsed with alcohol, and naturally dried up.
The diffusion source is a mixture of 1 g NH
4Cl powder and 10 g ZnO powder. Photographs of the NH
4Cl powder and the ZnO powder are shown in
Figure 1. The magnesium alloy specimens were inserted in the diffusion source. The diffusion source and the magnesium alloy specimens were put into a 30 mL crucible for the following heat treatment. A schemetic diagram of the diffusion system is shown in
Figure 2.
After the furnace temperature reached 430 °C and was kept stable for minutes, the filled crucible was put into the furnace as quickly as possible, and kept at 430 °C for 2 h for the surface alloying process on the magnesium alloy specimen to proceed. The whole surface alloying process was carried out in the air; neither vacuum nor inert gases were utilized. After the surface alloying process, specimens were taken out from the furnace and cooled down naturally in the air.
Then the residual powder on the magnesium alloy specimens was cleaned up, and the as-surface alloyed specimens were washed with running water and alcohol successively, and then naturally dried up. Cross-sections of the as-surface alloyed magnesium alloy specimens were obtained with a saw. Then the cross-sections were polished and etched successively for microstructure observation. The polished cross-sections were etched until the as-surface alloyed coating showed up clearly on the specimen surface, and then washed with alcohol and naturally dried up. Microstructure photographs and composition analysis results of the as-diffusion-alloyed specimens were obtained by a scanning electronic microscope (S-3400N, Hitachi, Tokyo, Japan). Phase identification was carried out utilizing an X-ray diffractometer (Ultima IV, Rigaku, Tokyo, Japan). The Gibbs free energies of relative chemical reactions were calculated by a software (HSC 6.0).
3. Results
After being diffusion alloyed at 430 °C for 2 h with 10 g ZnO and 1 g NH
4Cl as the diffusion source, a coating was fabricated on the AZ91D magnesium alloy specimen as shown in
Figure 3a. It could be figured out that the thickness of the as-diffusion alloyed coating can be hundreds of micrometers. From a magnified photograph of the coating shown in
Figure 3b, it could be observed that the coating contains multiple phases. Some phases have a network shape morphology, while other phases are embedded in the network. Dendrites could be observed clearly. The size of the dendrites embedded in the networks is estimated to be tens of micrometers to two hundred micrometers.
From the microstructure observation of the coating, it could be inferred that the formation of the coating is not a simple solid diffusion process, liquid phase should also be involved in the surface alloying process, since a pure solid diffusion process is driven by the concentration gradient which commonly results in a layer structure, while solidification of a molten phase is prone to result in a network shape eutectic multiple-phase-microstructure [
10]. The existence of a liquid phase in the diffusion alloying process is reasonable [
22], because the eutectic temperature in the Mg–Zn binary system could even be 341 °C, according to the Mg–Zn binary phase diagram [
20].
From the EDS results shown in
Figure 4, it could be figured out that there are mainly Mg, Zn, Al, and O elements in the coating. Mg is the dominant element in the AZ91D magnesium alloy matrix. Compared to the original content which was 0.52 wt.% in the AZ91D magnesium matrix, the relative content of Zn increased significantly in the magnesium alloy surface, which could be attributed to the continuous input of Zn atoms from the diffusion source. There was 9.1 wt.% Al in the original AZ91D magnesium specimen, hence Al could also be found in the coating. The existence of the O element is inferred to be related to the oxidation of active metallic atoms on the cross-section of the AZ91D magnesium alloy specimen.
Major elements in the surface-alloyed coating on the AZ91D magnesium alloy specimen could be figured out from the element maps shown in
Figure 5. Comparing with which in the matrix, the relative content of Mg is obviously less in the coating. Whereas, Zn is richer in the coating compared with which in the matrix, since the main source of Zn is the diffusion source, which was composed of the ZnO powder and the NH
4Cl powder outside the specimen. Besides, the Al element and the O element could also be observed in the coating.
From the XRD pattern shown in
Figure 6, it could be figured out that there are Mg
0.97Zn
0.03, MgO, Mg
32(Al, Zn)
49, Mg
7Zn
3, AlMg
4Zn
11, Al
2Mg phases in the as-diffusion-alloyed coating. MgO is inferred to be formed in the duration when the cross-section of the coating was exposed in the air, and other phases including Mg
0.97Zn
0.03, Mg
32(Al, Zn)
49, Mg
7Zn
3, AlMg
4Zn
11, Al
2Mg are inferred to be in the original coating. Comparing with a binary alloy system, the constituent of a ternary alloy system is much more complex. According to the melting points, these phases would solidify one by one from the melt in the cooling process [
16].
4. Discussion
Since the Zn element in the coating is significantly increased compared with which in the AZ91D magnesium matrix, there should be a Zn element input continuously from the diffusion source. As there was only ZnO, which contains Zn in the diffusion source, ZnO is inferred to be reduced into Zn atoms, and Zn atoms were transferred and dissolved into the surface of the AZ91D magnesium alloy specimen. Relative chemical reactions are listed as follows [
23].
NH
4Cl is unstable at the diffusion alloying temperature of 430 °C, and it would decompose into NH
3 gas and HCl gas [
23,
24] as shown in Equation (1). HCl gas could promote the oxidation of Mg atoms at the AZ91D magnesium alloy surface as shown in Equations (2) and (3). The oxide film on the AZ91D magnesium alloy specimen surface could be compact or porous. Whereas even a compact oxide film formed initially, this compact film could be converted into a porous one with the help of gaseous HCl [
24] as shown in Equation (3). A porous oxide film on the AZ91D magnesium alloy specimen is much more helpful for the mass transport between the AZ91D magnesium alloy matrix and the diffusion source.
It is reported that in Al-rich magnesium alloys, the oxidation of Mg is more preferred than Al [
4], and the content of Al could increase at some positions on the magnesium alloy surface, thus as a result, local melts could emerge. The existence of liquid Mg has the possibility of releasing gaseous Mg further [
4], as shown in Equation (4), and the sublimation of solid Mg may also happen [
25] as shown in Equation (5). Liquid Mg, gaseous Mg and Mg atoms could react with ZnO to produce Zn atoms as shown in Equation (6).
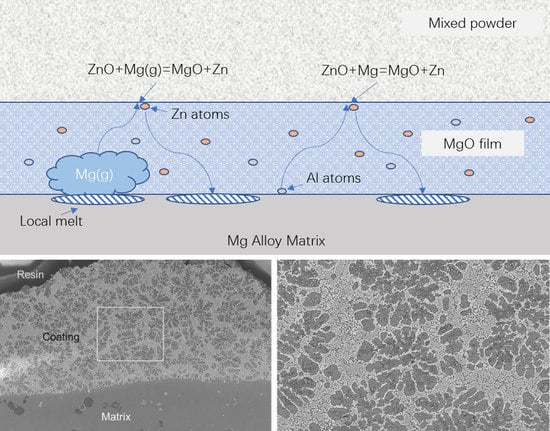
Meanwhile, oxidation of Mg is reported to occur at the interface between the MgO film and the air in a regular oxidation process of magnesium alloys [
4,
5]. In the diffusion alloying process here, the MgO film contacted with the mixed powder which contains ZnO directly, thus it is possible for the active Mg atoms to pass through the MgO film and react with ZnO to produce Zn atoms as shown in
Figure 7.
Zn atoms are transported into the magnesium alloy surface continuously through vacancies in the MgO film, and a Zn-rich layer would form on this magnesium alloy. Since the eutectic temperature of the Mg–Zn binary system could be 341 °C which is below the diffusion alloying temperature, the Zn-rich layer would be molten in the diffusion alloying process. In the cooling process, the melt solidified gradually, and the coating which composed of various phases formed.
The Gibbs free energies of relative chemical equations are shown in
Figure 8. It could be found that most of the chemical equations are preferred, except Equations (4) and (5). Although pure liquid Mg and pure solid Mg both have difficulty becoming pure gaseous Mg in the thermal calculations which describe the preference of reactions under ideal conditions as shown in
Figure 8, the Gibbs energies of Equations (4) and (5) may change in a real sophisticated diffusion alloying system. In fact, these reactions are reported in some literatures [
25,
26]. The existence of Al and Zn both in the intermetallic melt and in the magnesium alloy matrix could affect the evaporation of liquid Mg and the sublimation of solid Mg. It could be easier for an Mg atom in the liquid or solid phase in the magnesium alloy surface to become an Mg atom in the gaseous phase, considering that the melting point of Mg could be reduced by adding Al and Zn into the Mg solid solution.
5. Conclusions
To conclude, it is possible to fabricate a Zn-rich coating on magnesium alloys by the powder thermal diffusion alloying method with ZnO powder as the only Zn donor. Some ZnO powder could be reduced to produce Zn atoms by taking advantage of the intrinsic activity of Mg atoms in the magnesium alloy matrix. As the MgO film is full of vacancies, the as-reduced Zn atoms would diffuse into the magnesium alloy matrix across the porous MgO film.
This method is an economic method to fabricate Zn-rich coatings on magnesium alloys. Since stable ZnO is utilized as the raw material, transport and storage of the diffusion source would be much easier.
Author Contributions
Conceptualization, D.L.; Methodology, D.L.; Software, D.L.; Validation, D.L.; Formal Analysis, D.L.; Investigation, D.L.; Resources, D.L.; Data Curation, D.L.; Writing–Original Draft Preparation, D.L.; Writing–Review & Editing, D.L.; Visualization, D.L.; Supervision, Y.H., J.D. and B.H.; Project Administration, D.L.; Funding Acquisition, D.L.
Funding
This research was funded by the Key Research & Development Program of Shandong Province, China [No: 2018GSF117039] and the Applied Research Program of Qingdao, China [No: 17-1-1-22-jch].
Acknowledgments
The authors would like to thank all the anonymous reviewers for their kind help and useful advice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Szklarz, Z.; Bisztyga, M.; Krawiec, H.; Lityńska-Dobrzyńska, L.; Rogal, Ł. Global and local investigations of the electrochemical behavior the T6 heat treated Mg–Zn–RE magnesium alloy thixo-cast. Appl. Surf. Sci. 2017, 405, 529–539. [Google Scholar] [CrossRef]
- Wan, Y.; Gao, Y.; Jiang, S.; Liu, C.; Chen, Z. Manufacturing high-performance Mg alloy through hot extrusion. Mater. Manuf. Process. 2018, 33, 863–866. [Google Scholar] [CrossRef]
- Lu, D.; Huang, Y.; Jiang, Q.; Zheng, M.; Duan, J.; Hou, B. An approach to fabricating protective coatings on a magnesium alloy utilising alumina. Surf. Coat. Technol. 2019, 367, 336–340. [Google Scholar] [CrossRef]
- Tan, Q.; Atrens, A.; Mo, N.; Zhang, M.X. Oxidation of magnesium alloys at elevated temperatures in air: A review. Corro. Sci. 2016, 112, 734–759. [Google Scholar] [CrossRef]
- Czerwinski, F. The reactive element effect on high-temperature oxidation of magnesium. Inter. Mater. Rev. 2015, 60, 264–296. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.; Fajardo, S.; Birbilis, N.; Frankel, G.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, X.; Liu, B.; Liu, J.; Han, X.; Deng, Y.; Hu, W.; Zhong, C. One-step fabrication and localized electrochemical characterization of continuous Al-alloyed intermetallic surface layer on magnesium alloy. Coatings 2018, 8, 148. [Google Scholar] [CrossRef]
- Han, J.; Blawert, C.; Tang, S.; Yang, J.; Hu, J.; Zheludkevich, M.L. Effect of surface pre-treatments on the formation and degradation behaviour of a calcium phosphate coating on pure magnesium. Coatings 2019, 9, 259. [Google Scholar] [CrossRef]
- Song, J.; Cui, X.; Jin, G.; Cai, Z.; Liu, E.; Li, X.; Chen, Y.; Lu, B. Self-healing conversion coating with gelatin–chitosan microcapsules containing inhibitor on AZ91D alloy. Surf. Eng. 2018, 34, 79–84. [Google Scholar] [CrossRef]
- Gu, C.; Wang, L.; Hu, X.; Dong, W.; DaCosta, H. Borate’s effects on coatings by PEO on AZ91D alloy. Surf. Eng. 2017, 33, 773–778. [Google Scholar] [CrossRef]
- Shigematsu, I.; Nakamura, M.; Saitou, N.; Shimojima, K. Surface treatment of AZ91D magnesium alloy by aluminum diffusion coating. J. Mater. Sci. Lett. 2000, 19, 473–475. [Google Scholar] [CrossRef]
- Zhang, M.; Kelly, P. Surface alloying of AZ91D alloy by diffusion coating. J. Mater. Res. 2002, 17, 2477–2479. [Google Scholar] [CrossRef]
- Youping, M.; Xu, K.; Wen, W.; He, X.; Liu, P. The effect of solid diffusion surface alloying on properties of ZM5 magnesium alloy. Surf. Coat. Technol. 2005, 190, 165–170. [Google Scholar] [CrossRef]
- Zhu, L.; Song, G. Improved corrosion resistance of AZ91D magnesium alloy by an aluminium-alloyed coating. Surf. Coat. Technol. 2006, 200, 2834–2840. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, Q.; Wang, X.; Yang, L.; Ma, X.; Wang, W.; Huang, Y. Intermetallic layer obtained by the compact powder diffusion alloying method on AZ91D magnesium alloy in air. Surf. Coat. Tech. 2017, 309, 986–993. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, Q.; Wang, W.; Guan, F.; Ma, X.; Yang, L.; Wang, X.; Huang, Y.; Hou, B. Effect of cooling rate and the original matrix on the thermal diffusion alloyed intermetallic layer on magnesium alloys. Mater. Design 2017, 120, 75–82. [Google Scholar] [CrossRef]
- Gray, J.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloy. Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Wang, D.; Zhao, M.; Han, F. A novel approach to fabricate Zn coating on Mg foam through a modified thermal evaporation technique. J. Mater. Sci. Tech. 2018, 34, 1558–1563. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, T.; Lu, L.; Wang, Z. Thermally diffused antimony and zinc coatings on magnesium alloys AZ31. Surf. Eng. 2012, 28, 382–386. [Google Scholar] [CrossRef]
- Prosek, T.; Nazarov, A.; Bexell, U.; Thierry, D.; Serak, J. Corrosion mechanism of model zinc–magnesium alloys in atmospheric conditions. Corro. Sci. 2008, 50, 2216–2231. [Google Scholar] [CrossRef]
- Zhao, M.; Cai, C.; Wang, L.; Zhang, Z.; Zhang, J. Effect of zinc immersion pretreatment on the electro-deposition of Ni onto AZ91Dmagnesium alloy. Surf. Coat. Technol. 2010, 205, 2160–2166. [Google Scholar] [CrossRef]
- Hirmke, J.; Zhang, M.X.; St John, D.H. Surface alloying of AZ91E alloy by Al–Zn packed powder diffusion coating. Surf. Coat. Technol. 2011, 206, 425–433. [Google Scholar] [CrossRef]
- Luo, B.; Zhu, Q.; Cheng, Y.; Wang, L.; Li, X. The integrated cleaning process of soda and vinyl chloride on the chemical looping of NH4Cl decomposition. Chem. React. Eng. Technol. 2015, 31, 449–458. (In Chinese) [Google Scholar]
- Lu, D.; Jiang, Q.; Zheng, M.; Zhang, J.; Huang, Y.; Hou, B. The role of ammonium chloride in the powder thermal diffusion alloying process on a magnesium alloy. Coatings 2019, 9, 252. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, X.; Mao, M.; Yu, Q.; Mao, S.; Li, J.; Zhang, Z. In situ observation of sublimation-enhanced magnesium oxidation at elevated temperature. Nano Res. 2016, 9, 2796–2802. [Google Scholar] [CrossRef]
- Czerwinski, F. Oxidation characteristics of magnesium alloys. JOM 2012, 64, 1477–1483. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).