Abstract
In this research work, Co-B/SiC composite coatings were synthesized by electrochemical deposition from colloidal suspensions with different content of SiC. The Co-B/SiC films obtained were heat treatment at 350 °C. The composition, morphology, and structure of the Co-B/SiC composite coatings were analyzed using glow discharge spectrometry (GDS), scanning electron microscopy (SEM) coupled with energy-dispersive spectroscopy (EDS), and X-ray diffraction (XRD). Hardness and tribological properties were also studied. The results showed that an increase in the SiC concentration in the colloidal suspensions resulted in both an increase in the SiC content and a decrease in the B content in the obtained Co-B/SiC coatings. The Co-B/SiC coatings were adherent, glossy, and soft, and exhibited a homogeneous composition in all thicknesses. Besides, an increase in the SiC particle content of the Co-B/SiC composite coating from 0 to 2.56 at.% SiC reduced the hardness of the film from 680 to 360 HV and decreased the wear volume values from 1180 to 23 μm3 N−1 m−1, respectively (that is, the wear resistance increased). Moreover, when the Co-B/SiC coatings with SiC content ranging from 0 to 2.56 at.% SiC were subjected to a heat treatment process, the obtained coating hardness values were in the range of 1200 to 1500 HV, and the wear volume values were in the range of 382 to 19 μm3 N−1 m−1.
1. Introduction
Surface degradation is one of the main damages experienced by metallic parts of mechanical devices which are subjected to extreme working conditions, e.g., high friction, high temperatures, corrosive environments. The deterioration of the surface of these metallic parts causes the continuous decrease in their tribological properties (i.e., friction, wear resistance) and hardness, causing failures in the operation of the machinery. For many decades, hard coatings such as cadmium (Cd), nickel (Ni), or chromium (Cr) have been widely used to protect metal components and tools against wear. Although Cd, Ni, and Cr coatings are the most commonly protective coatings due to their excellent mechanical properties such as wear resistance, friction and hardness, the Environmental Protection Agency (EPA) of the United States, list these elements as priority pollutants, and they are considered to be among the 17 most toxic heavy metals [1]. For these reasons, alternative materials have been studied for many years. In this sense, composite coatings have recently been studied as an alternative to hard Cr coatings. Composite coatings are formed by two phases: The metal matrix as the main phase, and as the second phase, micro- or nanoparticles occluded in the metal matrix [2,3]. The insoluble micro- or nanoparticles occluded in the metal matrix can be nitrides, oxides, and carbides (Si3N4, SiO2, Al2O3, TiO2, SiC, WC, graphite), and their function is to increase the wear resistance and hardness of the coatings [4,5,6,7,8,9]. These phenomena are mainly attributed to the hardening of the metal matrix by finely dispersed ceramic particles. Ogihara et al. [10] have reported that composite films with Ni-B as the matrix material and SiC particles as the second phase exhibit hardness values of 845 HV without heat treatment and 1490 HV with heat treatment. Balaraju and Seshadri have shown that, when the content of Si3N4 particles occluded in a Ni-P metal matrix is increased, the wear resistance of the Ni-P/Si3N4 coating increases substantially [11]. Min-Chieh et al. [9] have reported that the addition of SiC particles to a Ni-P metal matrix reduces the residual stress of the deposits and therefore eliminates surface cracking. In addition, several studies on Ni/SiC composite coatings have reported significant improvement in wear resistance when SiC particles are added to the nickel matrix [12,13]. In another study, the addition of B4C particles to the matrix of the metallic Ni-P(9%) alloy was found to increase the wear resistance of Ni-P(9%)/B4C composites [14].
Recently, nanocrystalline cobalt (Co) coatings and Co alloys have been identified as good candidates for replacing the hard coatings of Cr, Cd, or Ni because of their similar or improved mechanical properties [15,16]. Additionally, the EPA does not classify the Co as dangerous for human health [17]. In a previous work, Martinez-Hernández et al. [18] reported the formation for electrodeposition of Co-B hard coatings with hardness values between 710 and 820 HV (very similar to those of hard Cr), by increasing the content of B in the range of 0 to 3.0 wt.%.
The novelty of the present work consisted of the preparation of Co-B/SiC composite coatings through an electrodeposition method due to the need for coatings with low friction and improved wear resistance for their application in the fabrication of machines, parts, and metal structures exposed to high stress and severe erosion conditions. The aim was to elucidate the effect of incorporated SiC particles in the Co-B metal matrix on wear volume, the friction coefficient, and hardness. The effect of thermally treated Co-B/SiC composite coatings was also studied.
2. Materials and Methods
2.1. Study of the Stability of SiC Particles in Colloidal Suspension
The study of the stability of SiC particles suspended in electrolytic baths containing the dispersant cetyltrimethylammonium bromide (CTAB) was carried out using a colloidal suspension of composition S0 (= 0.14 M CoCl2·6H2O + 2.8 M KCl + 0.32 M H3BO3 + 0.19 M dimethylamine borane (DMAB) (DMAB was used as a boron source) + 27.4 mM CTAB) + x g L−1 SiC (x = 0.0, 0.15, 0.6, 2.5, 5.0, 10.0, 15.0) at pH 5.0. The colloidal suspensions were made using distilled ultrafiltered water (18 MΩ cm resistivity) and analytical-grade reagents (Sigma-Aldrich, St. Louis, MO, USA). The size of the SiC particles used was 100 nm (SkySpring Nanomaterials, Inc., Houston, TX, USA).
This study was carried out with a Turbiscan analyzer (mod. Lab. Expert, Formulation Co., Toulouse, France) using cylinder-shaped vials. The analysis of the obtained transmittance signals versus vial height was performed as described in Reference [19]. The scanning of the transmittance signal (T) along the height vial were periodically measured at pauses of 5 min for 8 h. Figure 1 shows a typical graph of the transmittance signals (T) vs. vial height. From the graphs T vs. vial height, the thickness of the diluted phase (ΔH (mm) = Hsup − Hinf) was calculated (see Figure 1). The Hinf (mm) value was acquired by measuring in the vial, the point of intersection between the transmittance (T) signal (as a function of vial height) obtained during the scan and the Tf value (Tf = 1% T). Tf is the maximum limit value of transmittance accepted. In this work, Tf was fixed arbitrarily at 1% of the transmittance (Tf = 1% T). Hsup, the height of the sample in the glass vial, was equal to 43 mm. The difference between the two distances measured in the glass vial (ΔH = Hsup − Hinf) was indicative of the precipitation of the SiC particles. High values of the thickness of the diluted phase (ΔH) were indicative of an unstable suspension, whereas small values were indicative of a stable suspension.
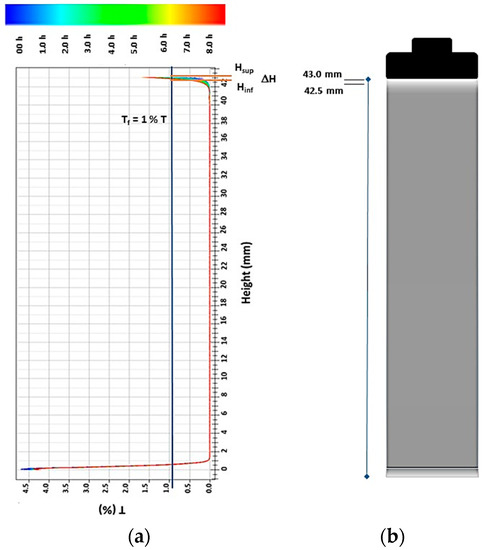
Figure 1.
(a) Transmittance profiles of the colloidal suspension S0 (= 0.14 M CoCl2·6H2O + 2.8 M KCl + 0.32 M H3BO3 + 0.19 M dimethylamine borane (DMAB) + 27.4 mM cetyltrimethylammonium bromide (CTAB)) with 10 g L−1 SiC. Data are reported as a function of time (0 to 8 h) and sample height (0 to 43 mm). (b) Schematic representation of the dispersion of SiC particles in the S0 solution with 10 g L−1 SiC.
2.2. Electrodeposition and Characterization of the Co-B/SiC Composite Coatings
The Co-B/SiC composite coatings were electrodeposited from colloidal suspensions S0 + x g L−1 SiC (x = 0.0, 0.15, 0.6, 2.5, 5.0, 10.0, 15.0) at pH 5.0 using a parallel-plate cell of 50 mL capacity and with an interelectrode distance of 5 cm. AISI 1018 steel plates (2.5 × 5.5 cm2 of the exposed area) were used as the cathodes and graphite plates as the anodes. The Co-B/SiC composite coatings were obtained by galvanostatic electrodeposition by applying 8.6 mA cm−2 for 56 min at 25 °C. Additional tests using the Hull cell technique (not presented here) were performed to select the applied current density.
The deposited phases of the Co-B/SiC composite coatings were analyzed by X-ray diffraction (XRD) using a Bruker diffractometer (model D8 Advance, Coventry, UK) equipped with a Bragg–Brentano arrangement, a Cu Kα radiation source (α = 1.54 Å) and scanning from 30° to 150° of 2θ° with a scan rate of 0.2° s−1. Scanning electron microscopy (SEM) (JEOL JSM-6510 LV, Tokyo, Japan) coupled to an energy-dispersive spectroscopy (EDS) analyzer (Bruker Quantax 200, Osaka, Japan) was used to evaluate the morphology and chemical composition of coatings. The elemental composition profiles of the Co-B/SiC composite coatings as a function of the thickness of the coatings were obtained using a glow discharge spectrometer (GDS) (Horiba, model GD Profiler 2, Montpellier, France).
Hardness tests of the obtained Co-B/SiC composite coatings were carried out using an automatic hardness testing system (Matsuzawa, mod. MXT-ALFA, Tokyo, Japan) under an applied load of 25 g for 15 s. For each coating, measurements were performed at six locations to obtain an average value.
Wear volume tests were made in the air with the ball-on-disk scheme (CSM instruments, Needham, MA, USA) at 25 °C and 39% of relative humidity without lubrication. As the counter body, WC Balls (Anton-Paar, Graz, Austria), 6 mm in diameter and with a hardness of 3500 HV, were used. The normal load was 2 N and sliding speed was of 4.2 cm s−1. The wear volume tests were performed in triplicate and in accordance with standard ASTM G99 [20]. During the tests, the sliding time and friction coefficient were automatically recorded.
3. Results and Discussion
3.1. Stability Analyses of the System SiC Particles/CTAB in Colloidal Suspensions
To uniformly occlude the SiC particles in the metallic matrix during the electrodeposition process of Co-B/SiC composite coatings, it is necessary to form a stable colloidal suspension between the SiC particles and the electrolytic bath, for this, we use a cationic type surfactant (i.e., CTAB). Therefore, before the electrodeposition of the Co-B/SiC composite coatings, the stability of the SiC particles in an electrolytic bath with CTAB as a dispersant was studied.
The stability of the SiC particles suspended in colloidal suspensions S0 + x g L−1 SiC (x = 0.0, 0.15, 0.6, 2.5, 5.0, 10.0, 15.0) at pH 5.0 was analyzed at 25 °C using the Turbiscan analyzer, an instrument that allows analyzing the stability of colloidal suspensions for long periods of time. The stability analysis was based on the graphics of the transmittance (T) profiles versus the vial height, obtained at 5 min intervals for 8 h. Typical transmittance profiles (obtained at different times) versus the vial height of the SiC particles in a colloidal suspension are shown in Figure 1. In the lower part of the vial, in the range from 0 to 1.0 mm, an increase of ~5% T was observed, indicative of the formation of a diluted phase in this region. Subsequently, in the range from 1 to 42 mm, transmittance values close to zero (~0% T) were observed during the 8 h of the experiment, indicating that the solution was opaque in this range because the SiC particles formed a stable suspension. Finally, a small clarifying zone (diluted phase) (ΔH) was formed in the range from 42.5 to 43 mm (top of the vial). Therefore, when CTAB was used as a dispersant, stable colloidal suspensions of SiC particles were obtained during a period of 8 h.
In Figure 2 it is possible to observe the thickness variation of the diluted phase (ΔH) as a function of time, in colloidal suspensions with different concentrations of SiC particles. The results indicate that an increase in the concentration of SiC particles in the colloidal suspensions cause a significant decrease in the values of the thickness variation of the clarifying layer (ΔH) as a function of time. Lower values of ΔH were obtained for the highest concentrations of SiC particles (15 and 10 g L−1). After 8 h of experimentation, the ΔH value of the colloidal suspensions with both 15 and 10 g L−1 SiC was only 0.5 mm, whereas, for the suspension with 0.15 g L−1 SiC, ΔH was 37 mm in the same period.
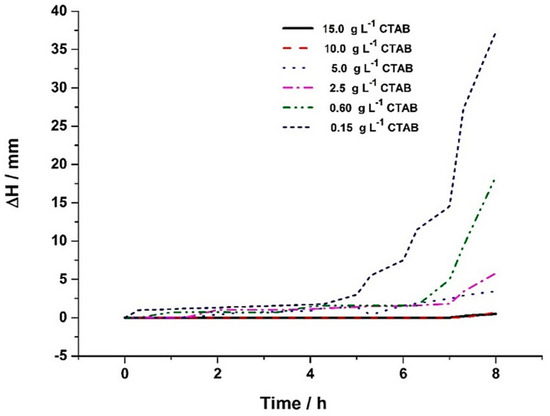
Figure 2.
Effect of SiC concentration in the colloidal suspensions on the diluted phase thickness (ΔH) as a function of time (0 to 8 h).
The greater stability of the SiC particles in the colloidal suspensions was associated with the adsorption of CTAB molecules on their surface. Due to its hydrophilic functional groups and its spatial structure, the adsorbed CTAB increased the steric hindrance and electrostatic repulsion among the SiC particles [21].
3.2. Co-B/SiC Composite Coating Composition
The Co-B/SiC composite coatings were obtained by applying a current density of 8.6 mA cm−2 for 56 min from colloidal suspensions S0 + x g L−1 SiCP (x = 0.0, 0.15, 0.60, 2.5, 5.0, 10.0, 15.0) at pH = 5.0. The thickness of the coatings was 7.3 ± 1.3 μm, which was measured by X-ray fluorescence.
GDS was used to analyze the influence of the SiC particle concentration in the colloidal suspensions, on the distribution and relative amount (atomic percentage (at.%)) of Co, B, SiC in the obtained Co-B/SiC composite coatings. For this analysis, we assumed that the detected Si signal corresponded to SiC. Moreover, when the substrate signal, Fe, was close to 100 at.%, the analysis was finished. Figure 3 shows GDS profiles of the elemental composition (at.%) variation of Co, B, Si, O, and C when varying the depth of a Co-B/SiC composite coating obtained from a colloidal suspension S0 + 2.5 g L−1 SiC. In Figure 3, to better observe the behavior of the elements, the signal corresponding to B was five times amplified, and the signal corresponding to Si was ten times amplified. On the surface of the coating, the formation of a surface oxide film of approximately 0.45 μm in thickness was observed. After the surface oxide layer was removed, the signals of C (~5.0 at.%), O (~7.0 at.%), Si (~1.7 at.%), Co (~75.0 at.%), and B (~7.0 at.%) were detected. These signals were practically constant throughout the thickness of the Co-B/SiC coating (approximately 7 μm). Subsequently, they decreased rapidly, while the substrate signal (Fe) increased, forming an interface zone between the substrate (Fe) and the Co-B/SiC coating. At depths greater than 8 μm, the substrate signal (Fe) remained constant and close to 100 at.%. In addition, the behaviors of the signals corresponding to Si, C, and B were similar to those of the Co signal. This behavior demonstrated the simultaneous co-position of Co, B, and Si. Moreover, Figure 3 clearly shows the homogeneous distribution of all the elements inside the coating. It is important to mention that for all samples a similar behavior was observed.

Figure 3.
Glow discharge spectrometry (GDS) elemental composition profiles of a Co-B/SiC composite coating obtained for electrodeposition from colloidal suspension S0 (= 0.14 M CoCl2·6H2O + 2.8 M KCl + 0.32 M H3BO3 + 0.19 M DMAB + 27.4 mM CTAB) with 10 g L−1 SiC.
From the GDS analyses of the obtained coatings, Figure 4 indicates the variation of the atomic percentage (at.%) of the elements B and Si in the Co-B/SiC composite coatings by increasing the concentration of the SiC particles in the colloidal suspensions. This figure also indicates that the Si content in the coating increased and reached a saturation point of 2.5 ± 0.06 at.% Si for [SiC] ≥ 5 g L−1 in the colloidal suspensions. In contrast, the content of B in the coating decreased as the concentration of SiC increased. At concentrations greater than or equal to 10 g L−1, the B content remained constant at 1.5 ± 0.08 at.%. An equivalent behavior for the variation in P during the electrodeposition of the Ni-P-SiC composite has been observed by other authors [9,22,23].

Figure 4.
Changes in the B (at.%) and Si (at.%) contents in the Co-B/SiC composite coatings according to the concentration of SiC in the colloidal suspensions.
3.3. Co-B/SiC Composite Coating Morphology
Figure 5 shows SEM micrographs of the Co-B/SiC composite coatings formed from colloidal suspensions with variation in SiC content (i.e., Co-B/SiC coatings with different at.% SiC). The results indicate that from electrolytic solutions without SiC (Figure 5a) (0 at.% SiC in the coating), a compact, smooth, adherent, and shiny Co-B coating was formed. Additionally, microcracks were observed on the surface of the coating, indicative of high internal stress in the coating possibly due to the evolution of hydrogen during electrodeposition. The inset of Figure 5a shows the amorphous structure of the Co-B coating. In contrast, occlusion of SiC particles in the metal matrix caused a transition in morphology toward a more crystalline structure of the Co-B/SiC composite coatings obtained (see Figure 5b (1.76 at.% SiC), Figure 5c (2.56 at.% SiC)). Semispherical structures of nanometric size were formed on top of the clusters. In addition, as a result of increasing the content of SiC in the coatings, the size and amount of the surface microcracks decreased, which was indicative of a lower level of stress inside the coatings. This behavior was associated with an inhibition of the hydrogen evolution reaction, due to the adsorption of the CTAB dispersant on the surface of the substrate.
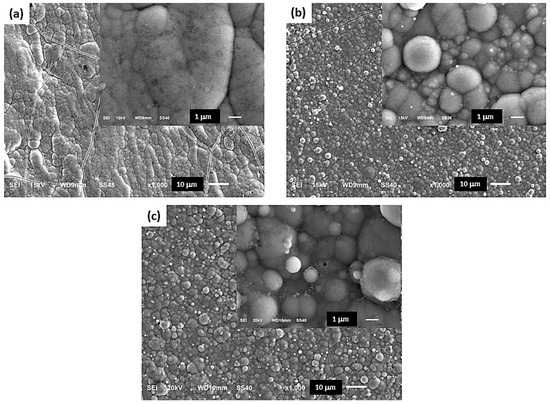
Figure 5.
(a) SEM images of the Co-B coatings obtained from colloidal suspension S0 (= 0.14 M CoCl2·6H2O + 2.8 M KCl + 0.32 M H3BO3 + 0.19 M DMAB + 27.4 mM CTAB); (b) SEM images of the Co-B/SiC composite coatings obtained from the colloidal suspension S0 + 2.5 g L−1 SiC; and (c) SEM images of the Co-B/SiC composite coatings obtained from the colloidal suspension S0 + 15 g L−1 SiC.
The elementary mapping analysis obtained from the surface of the Co-B/SiC (2.56 at.% SiC) coating (Figure 6) evidenced the homogeneous distribution of the SiC particles on the surface of the coating. These results, together with the GDS results shown in the previous section, confirmed the homogeneous incorporation of the SiC particles in the Co-B metal matrix: For all Co-B/SiC composite coatings with different SiC content, the same behavior was observed. This behavior was the result of improving the migration of the SiC particles toward the negatively charged electrode surface (cathode) due to the increase in the positive electric charge of the SiC particles that occurred because of the adsorption of the cationic dispersant CTAB on the surface of the SiC particles [24].
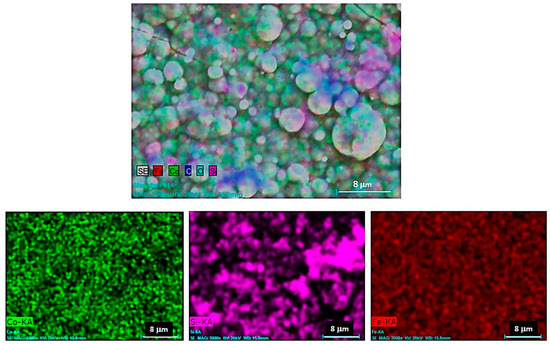
Figure 6.
Elemental mapping profile of a Co-B/SiC composite coating obtained from a colloidal suspension S0 with 10 g L−1 SiC.
3.4. XRD Analysis
The structural changes of the Co-B/SiC composite coatings were characterized by XRD. Figure 7 present the results of the XRD analyses obtained from Co-B/SiC coatings with different atomic percentages of SiC (at.% SiC). For the Co-B (0 at.% SiC) coating, the XRD diffractogram shows a broad peak at 2θ = 44.44°, which is typical of an amorphous phase. This behavior was modified when the SiC particles were incorporated into the Co-B metal matrix to form the Co-B/SiC composite coating. When the amount of SiC particles in the Co-B matrix was increased, the crystallinity of the Co-B/SiC composite increased. These results corroborated the behavior observed in the SEM micrographs in Figure 5a–c. Bozzini et al. observed a similar phenomenon during the occlusion of B4C particles in the matrix of a Ni-P alloy: This behavior was tentatively explained by the particles promoting crystallization at low temperatures by providing nucleation sites for the precipitation of nuclei in the amorphous matrix [14,25,26].

Figure 7.
Normalized XRD diffractograms obtained from Co-B/SiC composite coatings with different SiC atomic percentage (at.% SiC) formed under galvanostatic conditions (8.6 mA cm−2 for 56 min): βCo (JCPDS 01-089-4308), CoB (JCPDS 01-089-4308), SiC (JCPDS 00-042-1360).
3.5. Microhardness of the Co-B/SiC Composite Coatings
Figure 8 shows the behavior of the microhardness of the Co-B/SiC composite coatings produced as a function of the content of SiC particles in the coating. The microhardness of the Co-B/SiC coatings decreased with increasing SiC content in the Co-B/SiC composite. This behavior could be associated with a decrease in the content of B by increasing the content of SiC particles in the coating (see Figure 4). In reported works, it has been shown that an increase in the B content of the Co-B coatings, provokes an increase in their hardness. This behavior is associated with the formation of the hard intermetallic compound Co-B [18,27]. In the present study, the results in Figure 4 show that increasing the content of SiC particles in the colloidal suspensions increased the content of SiC particles and reduced the B content in the coating. Thus, the observed decrease in microhardness could be caused by the decrease in the B content in the coating.

Figure 8.
Variation in Co-B/SiC composite coating hardness as a function of the SiC content in the coatings.
3.6. Tribological Behavior
Table 1 shows the average values of the wear volume measured at the end of the test (300 m sliding distance) as a function of the amount of SiC in the obtained Co-B/SiC coatings. It is observed that by increasing the amount of SiC in the Co-B/SiC coatings, the volume of wear decreases. That is, the wear resistance is increased by the SiC occlusion in the Co-B phase. The lowest wear volume value (23.0 μm3/Nm) was obtained for the Co-B/SiC (2.5 at.% SiC) coating: This value was lower than what has been reported for different systems, such as an Ni-P-FGD composite [28], Ni-B [29], NiP/SiC [30], Ni-P(9%)/B4C [14], Co-P [15], or Co-B [18], and was similar to that of a hard Cr coating [28].

Table 1.
The relationship between the SiC content in the Co-B/SiC composite coatings and the wear volume and friction coefficients of the Co-B/SiC coatings deposited under galvanostatic conditions (8.6 mA cm−2 for 56 min).
To identify the wear mechanism of the coatings, we analyzed the worn surface of the Co-B/SiC composite coatings with different SiC contents by SEM. In the Co-B coatings (Figure 9a), the presence of torn patches and some detachment within the worn tracks was typical evidence of a plastic deformation being carried out in this process, which indicated that the principal mechanism was adhesive wear. Moreover, by increasing the SiC particle content up to 2.5 at.% SiC (Figure 9b), it was possible to observe the formation of the thinner grooves on the worn surface. This behavior was evidence of the beneficial effect of the incorporation of SiC, which improved wear resistance and low friction. Similar behavior was observed in the wear tests of all the Co-B / SiC composite coatings obtained. Additionally, as shown in Figure 9, the Co-B/SiC coating with 2.5 at.% SiC (Figure 9b) exhibited the shallowest ploughed lines and narrowest width. These results indicated that this coating exhibited the best behavior for wear resistance.
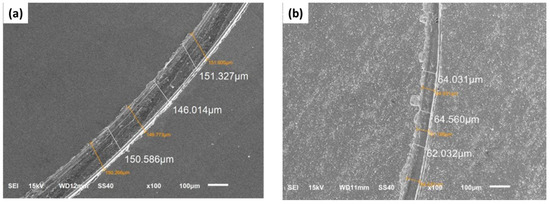
Figure 9.
Typical SEM micrographs of the wear track pattern of coatings in their as-deposited condition: (a) Co-B coating and (b) Co-B/SiC (2.5 at.% SiC) composite coating.
Likewise, the friction coefficients average values (μ) of the Co-B/SiC coatings, which were obtained simultaneously during the wear test, decreased as the atomic percentage (at.%) of SiC in the coating increased and reached a constant value of ≈0.31 at the highest concentrations of SiC. The obtained friction coefficients values were lower than other reported for systems such as Ni-P/SiC [12,23,30], Ni-B [31], Ni-B-diamond [32], Ni-P/B4C [14], and Ni-SiC [33], but were higher than those reported for Ni-P-GDF and hard Cr coatings [19]. The above results imply that the SiC particles occluded in the coating matrix acted as a lubricant since when the content of SiC particles in the Co-B/SiC coatings increased, the lubricating properties of the coatings increased.
3.7. Study of Heat Treatment (HT) onto Hardness, Wear Volume, and Friction Coefficients (μ)
The CoB/SiC coatings with different atomic percentage (at.%) of SiC, were thermally treated for 1 h in air atmosphere, and subsequently, their hardness, wear volume and friction coefficient were evaluated.
The microhardness results are shown in Figure 10. As expected, the hardness of the coatings increased considerably after they were thermally treated. Hardness values from 1200 to 1500 HV were obtained over the entire range of SiC concentrations (0 to 2.56 at.% SiC). Thus, the presence of SiC particles in the coatings had little influence on their hardness.

Figure 10.
Variation in the Co-B/SiC composite coating hardness as a function of the SiC atomic percentage (at.%) in the coatings after heat treatment at 350 °C for 1 h.
Therefore, the increase in hardness was mainly associated with the crystallization of the hard intermetallic species Co3B, which occurred between 200 and 400 °C and was further confirmed by XRD patterns (Figure 11). In Figure 11, the XRD patterns show that when the Co-B/SiC (2.56 at.% SiC) coating was thermally treated at 350 °C, a material more crystalline is obtained and peaks corresponding to crystalline Co3B and Co3O4 appeared. Similar behavior was obtained from all Co-B/SiC coatings analyzed.
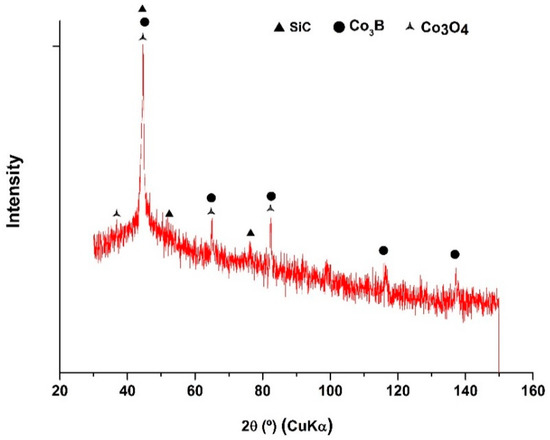
Figure 11.
Normalized XRD pattern of the Co-B/SiC (2.56 at.% SiC) composite coating after heat treatment at 350 °C: SiC (JCPDS 00-042-1360), Co3B (JCPDS 03-065-2414), Co3O4 (JCPDS 00-043-1003).
The GDS analysis performed on the Co-B/SiC (2.56 at.% SiC) composite coating after being heat-treated at 350 °C presented the following composition: 2.13 ± 0.3 at.% SiC, 1.66 ± 0.13 at.% B, 0.7 ± 0.2 at.% O.
The values of the wear volume and friction coefficients, obtained from Co-B/SiC coatings thermally treated at 350 °C for 1 h, are shown in Table 2. Both values decreased with increasing SiC content in the coating. Additionally, the values obtained were similar to those obtained for Co-B/SiC coatings without heat treatment (see Table 1). Therefore, heat treatment strongly influenced the hardness of the coatings due to the formation of the Co3B intermetallic species but had little influence on the wear volume and the friction coefficient, which were mainly decreased by the occlusion of SiC particles into Co-B metal phase.

Table 2.
The relationship between SiC content in the Co-B/SiC composite coatings and the wear volume and friction coefficients of the Co-B/SiC coatings deposited under galvanostatic conditions (8.6 mA cm−2 for 56 min). Values were obtained after thermal treatment of the coatings at 350 °C for 1 h.
4. Conclusions
The objective of this work was to obtain Co-B/SiC composite coatings with different contents of occluded SiC particles via electrodeposition. The composition, hardness, wear volume (wear resistance), and friction coefficient of the coatings before and after heat treatment at 350 °C for 1 h were analyzed. The results showed that using CTAB as a dispersant was possible to obtain Co-B/SiC composite coatings of homogeneous composition. When studying the influence of the concentration of SiC particles in the colloidal suspensions on the composition of the obtained Co-B/SiC coatings, we found that the amount of occluded SiC particles in the coatings increased and the content of B decreased with increasing concentrations of SiC particles in the colloidal suspensions. The variation in the composition of the coatings substantially affected their physical characteristics. Co-B/SiC coatings with a higher B content and a lower SiC particle content presented a higher hardness and lower wear resistance, whereas coatings with a lower B content and a higher SiC particle content exhibited lower hardness and greater wear resistance. Therefore, the occlusion of SiC particles in the metal matrix increased the wear resistance of the coatings.
Heat treatment at 350 °C for 1 h had little influence on the wear volume or coefficient of friction of the Co-B/SiC coatings because the values obtained after the heat treatment were similar to those obtained without the heat treatment. However, the hardness of the coatings increased considerably from 1400 to 1500 HV for Co-B/SiC coatings with a SiC particle content in the range of 0 to 2.56 at.%. This behavior was attributed to the formation of the hard intermetallic compound Co3B.
From the obtained results, it is possible to propose that Co-B/SiC composite coatings can be applied to the protection of steel parts against wear. In addition, it is possible to propose that the coatings obtained in this work are an excellent alternative to replace the coatings of Cr.
Author Contributions
Formal Analysis, F.M. and J.J.P.-B.; Investigation, F.J.R.-V. and J.M.-H.; Methodology, A.V.-M. and A.M.-H.; Validation, Y.M. and J.C.B.; Writing—Review and Editing, A.M.-A. and G.T.
Funding
This research was funded by CONACyT, projects: PN/2015-01-248, Sectorial Fund CONACyT-SENER (project 259334) Energy Sustainability, and CONACyT-SENER (project 248090, cluster de bioturbosine), Sustentabilidad Energética 2104-05. A. Villa-Mondragón is grateful to CONACyT for scholarship support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Eskin, S.; Berkh, O.; Rogalsky, G.; Zahavi, J. Co-W alloys for replacement of conventional hard chromium. Plat. Surf. Finish. 1998, 85, 79–83. [Google Scholar]
- Hovestad, A.; Janssen, L.J.J. Electrochemical codeposition of inert particles in a metallic matrix. J. Appl. Electrochem. 1995, 25, 519–527. [Google Scholar] [CrossRef]
- Wang, W.; Hou, F.-Y.; Wang, H.; Guo, H.-T. Fabrication and characterization of Ni-ZrO2 composite nano-coatings by pulse electrodeposition. Scr. Mater. 2005, 53, 613–618. [Google Scholar] [CrossRef]
- Shahri, Z.; Allahkaram, S.R. Effect of plating parameters on microstructure and tribological properties of Co-B(hexagonal) nano composite coatings. Trans. Nonferrous Met. Soc. China 2013, 23, 2929–2938. [Google Scholar] [CrossRef]
- Venkataraman, B.; Sundararajan, G. The sliding wear behavior of Al-SiC particulate composites-I. Macrobehavior. Acta Mater. 1996, 44, 451–460. [Google Scholar] [CrossRef]
- Ortiz-Merino, J.L.; Todd, R.I. Relationship between wear rate, surface pullout and microstructure during abrasive wear of alumina and alumina/SiC nanocomposites. Acta Mater. 2005, 53, 3345–3357. [Google Scholar] [CrossRef]
- Sanchez Egea, A.J.; Martynenko, V.; Abate, G.; Deferrari, N.; Martínez Krahmer, D.; López de Lacalle, L.N. Friction capabilities of graphite-based lubricants at room and over 1400 K temperatures. Int. J. Adv. Manuf. Technol. 2019, 1–11. [Google Scholar] [CrossRef]
- Lee, H.-K.; Lee, H.-Y.; Jeon, J.-M. Electrolytic deposition behaviors of Ni-SiC composite coatings containing submicron-sized SiC particles. Met. Mater. Int. 2008, 14, 599–605. [Google Scholar] [CrossRef]
- Chou, M.-C.; Ger, M.-D.; Ke, S.-T.; Huang, Y.-R.; Wu, S.-T. The Ni–P–SiC composite produced by electro-codeposition. Mater. Chem. Phys. 2005, 92, 146–151. [Google Scholar] [CrossRef]
- Ogihara, H.; Wang, H.; Saji, T. Electrodeposition of Ni-B/SiC composite films with high hardness and wear resistance. Appl. Surf. Sci. 2014, 296, 108–113. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Seshadri, S.K. Synthesis and corrosion behavior of electroless Ni-P-Si3N4 composite coatings. J. Materials. Sci. Lett. 1998, 17, 1297–1299. [Google Scholar] [CrossRef]
- Aslayan, I.R.; Bonino, J.-P.; Celis, J.-P. Effect of submicron SiC particles on the wear of electrolytic NiP coatings Part 1. Uni-directional sliding. Surf. Coat. Technol. 2006, 200, 2909–2916. [Google Scholar] [CrossRef]
- Garcia, I.; Fransaer, J.; Celis, J.-P. Electrodeposition and sliding wear resistance of nickel composite coatings containing micron and submicron SiC particles. Surf. Coat. Technol. 2001, 148, 171–178. [Google Scholar] [CrossRef]
- Bozzini, B.; Martini, C.; Cavalloti, P.L.; Lanzoni, E. Relationships among crystallographic structure, mechanical properties and tribological behavior of electroless Ni-P(9%)/B4C. Wear 1999, 225, 806–813. [Google Scholar] [CrossRef]
- Prado, R.A.; Facchini, D.; Mahalanobis, N.; Gonzalez, F.; Palumbo, G. Electrodeposition of nanocrystalline cobalt alloy coatings as a hard chrome alternative. In Proceedings of the DoD Corrosion Conference, Gaylord National, Washington, DC, USA, 10–14 August 2009. [Google Scholar]
- Friedman, H.; Eidelman, O.; Feldman, Y.; Moshkovich, A.; Perfiliev, V.; Rapoport, I.; Cohen, H.; Yoffe, A.; Tenne, R. Fabrication of self-lubricating cobalt coatings on metal surface. Nanotechnology 2007, 18, 115703–115710. [Google Scholar] [CrossRef]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Martínez-Hernández, A.; Meas, Y.; Pérez-Bueno, J.; Ortíz-Frade, L.; Flores-Segura, J.; Méndez-Albores, A.; Trejo, G. Electrodeposition of Co-B hard coatings: Characterization and tribological properties. Int. J. Electrochem. Sci. 2017, 12, 1863–1873. [Google Scholar] [CrossRef]
- Méndez-Albores, A.; González-Arellano, S.G.; Reyes-Vidal, Y.; Torres, J.; Talu, S.; Cercado, B.; Trejo, G. Electrodeposited chrome/silver nanoparticle (Cr/AgNPs) composite coatings: Characterization and antibacterial activity. J. Alloy. Compd. 2017, 710, 302–311. [Google Scholar] [CrossRef]
- ASTM G99-05 Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus; ASTM International: West Conshohocken, PA, USA, 2005.
- Cerruti, B.; de Souza, C.; Castellan, A.; Ruggiero, R.; Frollini, E. Carboxymethyl lignin as stabilizing agent in aqueous ceramic suspensions. Ind. Crops. Prod. 2012, 36, 108–115. [Google Scholar] [CrossRef]
- Malfati, C.F.; Ferreira, J.Z.; Oliveira, C.T.; Rieder, E.S.; Bonino, J.P. Electrochemical behavior of Ni-P/SiC composite coatings: Effect of heat treatment and SiC particle incorporation. Mater. Corros. 2012, 63, 36–43. [Google Scholar] [CrossRef]
- Hou, K.-H.; Hwu, W.-H.; Ke, S.-T.; Ger, M.-D. Ni-P/SiC composite produced by pulse and direct current plating. Mater. Chem. Phys. 2006, 100, 54–59. [Google Scholar] [CrossRef]
- González-Arellano, S.G.; Avilés-Arellano, L.M.R.; Manríquez, J.; Torres, J.; Ortega, R.; Meas, Y.; Trejo, G.; Méndez-Albores, A. Study of the stability of silver particles suspended using Cetylpyridinium Bromide (CPB) as surfactant. Chem. Sci. Rev. Lett. 2015, 4, 809–816. [Google Scholar]
- Bozzini, B.; Cavallotti, P.L.; Parisi, G. Corrosion and erosion of electrodeposited Ni-P/B4C composites. Brit. Corros. J. 2001, 36, 49–55. [Google Scholar] [CrossRef]
- Bozzini, B.; Lecis, N.; Cavallotti, P.L. Structure and Magnetic properties of nanocrystalline Ni-P prepared by autocatalytic chemical deposition. J. Phys. IV France 1998, 8, 371–374. [Google Scholar] [CrossRef]
- Lim, T.; Kim, J.J. Effect of B and W contents on hardness of electroless Co alloys thin films. Korean Chem. Eng. Res. 2018, 56, 895–900. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Xu, T.; Xue, Q. Corrosion resistance and lubricated sliding wear behavior of novel Ni-P graded alloy as alternative to hard Cr deposits. Appl. Surf. Sci. 2006, 252, 7361–7372. [Google Scholar] [CrossRef]
- Lee, K.H.; Chang, D.S.; Know, C. Properties of electrodeposited nanocrystalline Ni-B alloys films. Electrochim. Acta 2005, 50, 4538–4543. [Google Scholar] [CrossRef]
- Aslanyan, I.R.; Bonino, J.-P.; Celis, J.-P. Effect of reinforcing submicron SiC particles on the wear of electrolytic NiP coatings, Part 2: Bi-directional sliding. Surf. Coat. Technol. 2006, 201, 581–589. [Google Scholar] [CrossRef]
- Krishnaveni, K.; Sankara Narayanan, T.S.N.; Seshadri, S.K. Electrodeposited Ni-B coatings: formation and evaluation of hardness and wear resistance. Mater. Chem. Phys. 2006, 99, 300–308. [Google Scholar] [CrossRef]
- Monteiro, O.R.; Murugesan, S.; Khabashesku, V. Electrodeposited Ni-B and Ni-B metal matrix diamond nanocomposite coatings. Surf. Coat. Technol. 2015, 272, 291–297. [Google Scholar] [CrossRef]
- Benea, L.; Bonora, P.L.; Berello, A.; Martelli, S. Wear corrosion properties of nanostructured SiC nickel composite coatings obtained by electroplating. Wear 2002, 249, 995–1003. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).