Protective Performance of Zn-Al-Mg-TiO2 Coating Prepared by Cold Spraying on Marine Steel Equipment
Abstract
:1. Introduction
2. Experimental Methods
2.1. Preparation of Coatings
2.2. Testing and Analysis of Coatings
3. Results and Discussion
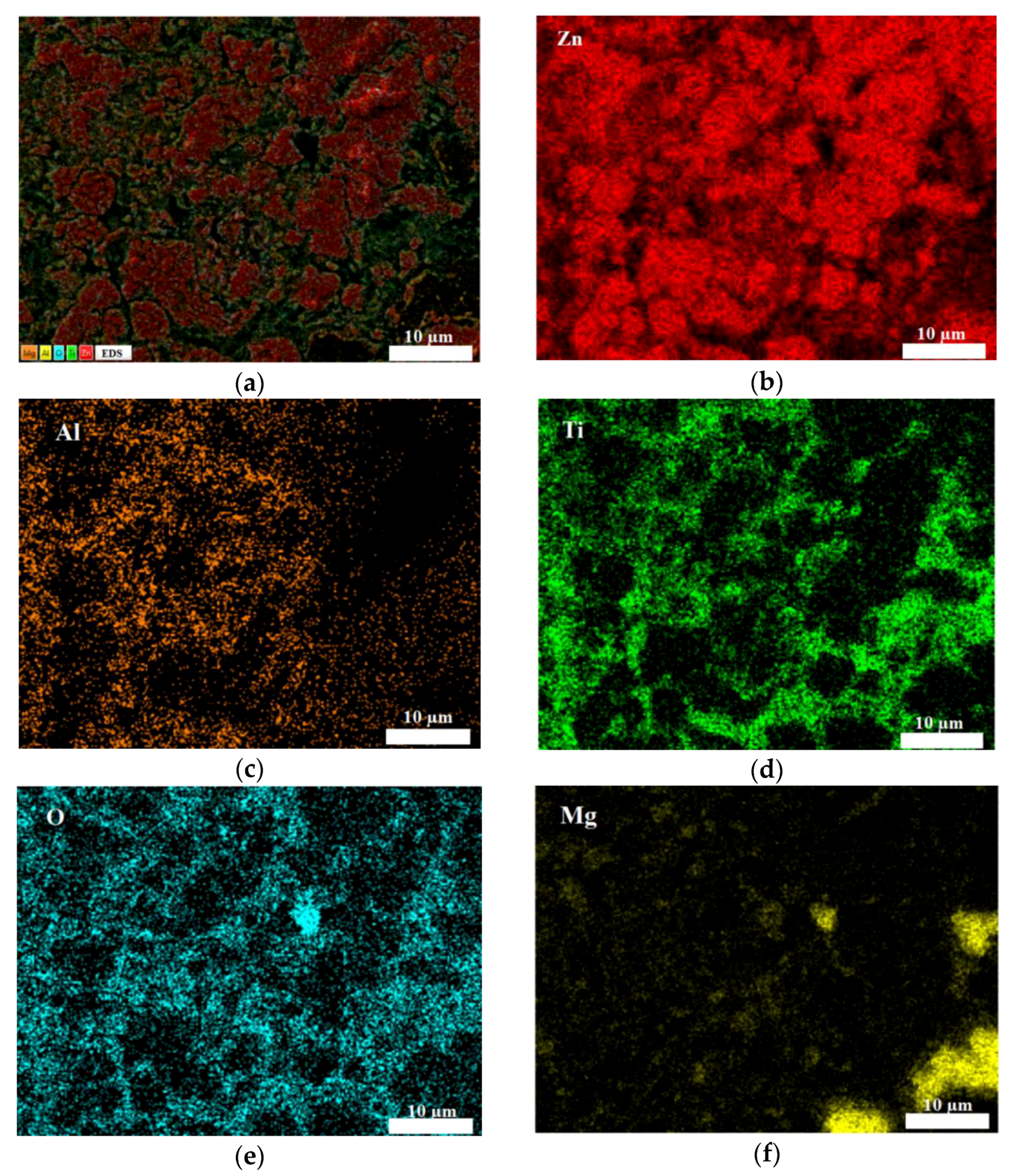
3.1. Microstructure of the Coating
3.2. Analysis of the Results of Dynamic Salt Water Corrosion Testing
3.3. Analysis of the Results of Electrochemical Testing
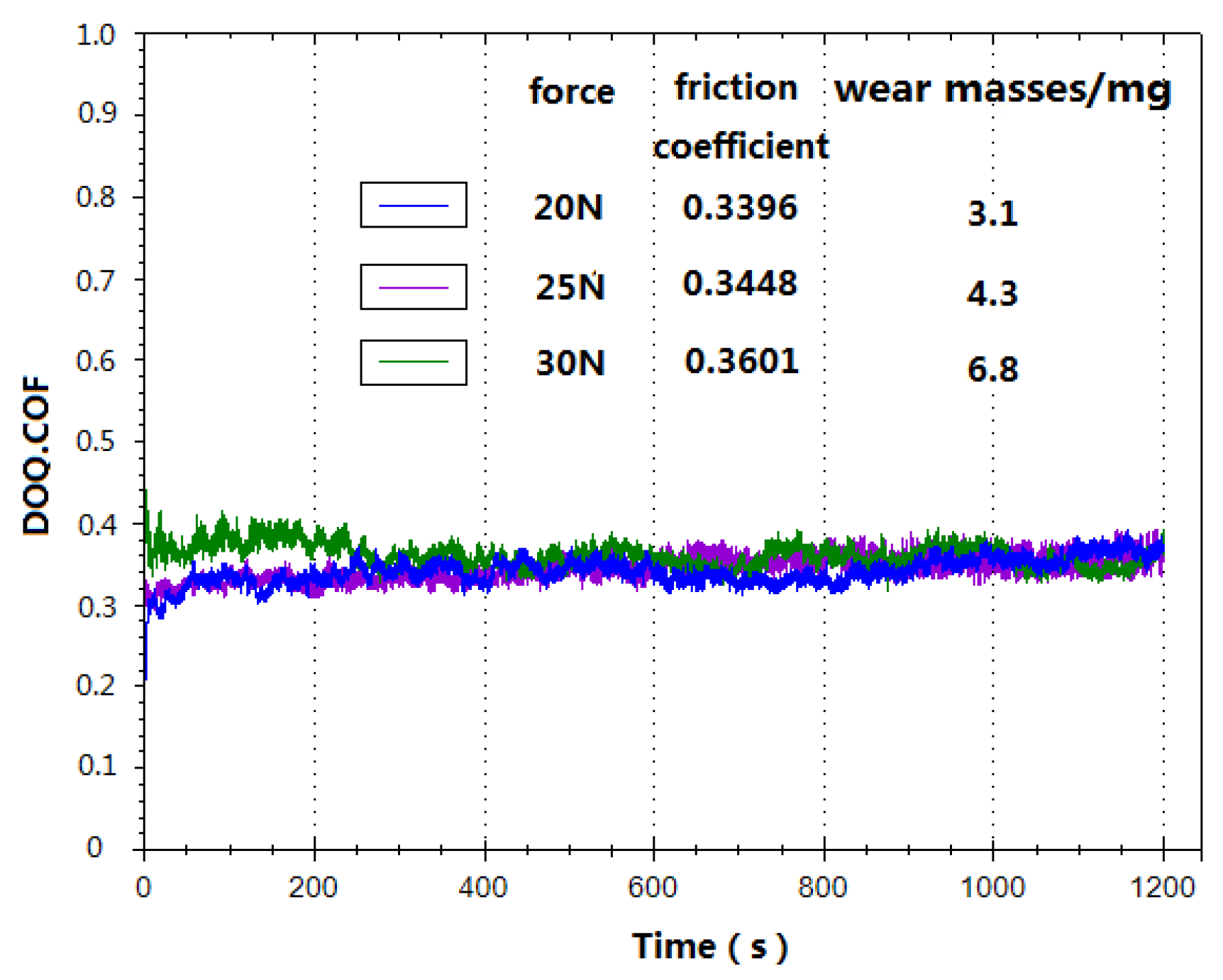
3.4. Analysis of the Wear Resistance of the Coating
4. Conclusions
- In a corrosive medium, the corrosion rate of Zn-Al-Mg-TiO2 first increases and then decreases, with the Zn-rich phase of the coating consumed first and with a high corrosion rate. After this, the passivation film formed by the corrosion product of Al and Mg covers the coating surface, which can reduce the corrosion rate of the coating.
- TiO2 has excellent photocatalytic self-cleaning performance, the corrosion current density for an ultraviolet radiation experiment is always lower than a no ultraviolet radiation experiment, and the water film attached to the surface of the coating can provide physical shielding for the coating.
- The friction coefficient of the Zn-Al-Mg-TiO2 coating is about 0.35 with a Q235 friction pair. After friction and wear testing, wear marks are relatively smooth, and no bulk material falls off. These results indicate that the Zn-Al-Mg-TiO2 coating has a dense structure, high bonding strength, and great wear resistance.
Author Contributions
Funding
Conflicts of Interest
References
- Traverso, P.; Canepa, E. A review of studies on corrosion of metals and alloys in deep-sea environment. Ocean Eng. 2014, 87, 10–15. [Google Scholar] [CrossRef]
- Liang, Y.L.; Wang, Z.B.; Zhang, J.B.; Lu, K. Formation of interfacial compounds and the effects on stripping behaviors of a cold-sprayed Zn–Al coating on interstitial-free steel. Appl. Surf. Sci. 2015, 340, 89–95. [Google Scholar] [CrossRef]
- Chen, T.C.; Chou, C.C.; Yung, T.Y.; Tsai, K.C.; Huang, J.Y. Wear behavior of thermally sprayed Zn/15Al, Al and Inconel 625 coatings on carbon steel. Surf. Coat. Technol. 2016, 303, 78–85. [Google Scholar] [CrossRef]
- Gancarz, T.; Mech, K.; Guśpiel, J.; Berent, K. Corrosion studies of Li, Na and Si doped Zn-Al alloy immersed in NaCl solutions. J. Alloy. Compd. 2018, 767, 1225–1237. [Google Scholar] [CrossRef]
- Katayama, H.; Kuroda, S. Long-term atmospheric corrosion properties of thermally sprayed Zn, Al and Zn-Al coatings exposed in a coastal area. Corros. Sci. 2013, 76, 35–41. [Google Scholar] [CrossRef]
- Gou, J.; Wang, G.; Ning, Y.; Guan, L.; Zhang, Y.; Liao, J.; Wang, Y. Preparation and corrosion resistance of chromium-free Zn-Al coatings with two different silane coupling agents. Surf. Coat. Technol. 2019, 366, 1–6. [Google Scholar] [CrossRef]
- Perez, A.; Billard, A.; Rébéré, C.; Berziou, C.; Touzain, S.; Creus, J. Influence of metallurgical states on the corrosion behaviour of Al–Zn PVD coatings in saline solution. Corros. Sci. 2013, 74, 240–249. [Google Scholar] [CrossRef]
- Li, W.; Liu, A.; Tian, H.; Wang, D. Controlled release of nitrate and molybdate intercalated in Zn-Al-layered double hydroxide nanocontainers towards marine anticorrosion applications. Colloid Interface Sci. Commun. 2018, 24, 18–23. [Google Scholar] [CrossRef]
- Liu, W.; Li, Q.; Li, M.C. Corrosion behaviour of hot-dip Al–Zn–Si and Al–Zn–Si–3Mg coatings in NaCl solution. Corros. Sci. 2017, 121, 72–83. [Google Scholar] [CrossRef]
- Tokuda, S.; Muto, I.; Sugawara, Y.; Takahashi, M.; Matsumoto, M.; Hara, N. Micro-electrochemical investigation on the role of Mg in sacrificial corrosion protection of 55mass% Al-Zn-Mg coated steel. Corros. Sci. 2017, 129, 126–135. [Google Scholar] [CrossRef]
- Dutta, M.; Halder, A.K.; Singh, S.B. Morphology and properties of hot dip Zn–Mg and Zn–Mg–Al alloy coatings on steel sheet. Surf. Coat. Technol. 2010, 205, 2578–2584. [Google Scholar] [CrossRef]
- Yao, C.; Lv, H.; Zhu, T.; Zheng, W.; Yuan, X.; Gao, W. Effect of Mg content on microstructure and corrosion behavior of hot dipped Zn–Al–Mg coatings. J. Alloy. Compd. 2016, 670, 239–248. [Google Scholar] [CrossRef]
- La, J.; Lee, S.; Hong, S. Synthesis of Zn–Mg coatings using unbalanced magnetron sputtering and theirs corrosion resistance. Surf. Coat. Technol. 2014, 259, 56–61. [Google Scholar] [CrossRef]
- Baghery, P.; Farzam, M.; Mousavi, A.B.; Hosseini, M. Ni-TiO2 nanocomposite coating with high resistance to corrosion and wear. Surf. Coat. Technol. 2010, 204, 3804–3810. [Google Scholar] [CrossRef]
- Likodimos, V. Photonic crystal-assisted visible light activated TiO2 photocatalysis. Appl. Catal. B 2018, 230, 269–303. [Google Scholar] [CrossRef]
- Xu, F.; Wang, T.; Chen, H.; Bohling, J.; Maurice, A.M.; Wu, L.; Zhou, S. Preparation of photocatalytic TiO2-based self-cleaning coatings for painted surface without interlayer. Prog. Org. Coat. 2017, 113, 15–24. [Google Scholar] [CrossRef]
- Min, K.S.; Manivannan, R.; Son, Y.A. Porphyrin Dye/TiO2 imbedded PET to improve visible-light photocatalytic activity and organosilicon attachment to enrich hydrophobicity to attain an efficient self-cleaning material. Dyes Pigment. 2019, 162, 8–17. [Google Scholar] [CrossRef]
- Natarajan, S.; Lakshmi, D.S.; Thiagarajan, V.; Mrudula, P.; Chandrasekaran, N.; Mukherjee, A. Antifouling and anti-algal effects of chitosan nanocomposite (TiO2/Ag) and pristine (TiO2 and Ag) films on marine microalgae Dunaliella salina. J. Environ. Chem. Eng. 2018, 6, 6870–6880. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, M.; Luo, C.; Chen, X.; Kong, J.; Zhou, T. A novel composite paint (TiO2/fluorinated acrylic nanocomposite) for antifouling application in marine environments. J. Environ. Chem. Eng. 2016, 4, 2545–2555. [Google Scholar] [CrossRef]
- Raoelison, R.N.; Xie, Y.; Sapanathan, T.; Planche, M.P.; Kromer, R.; Costil, S.; Langlade, C. Cold gas dynamic spray technology: A comprehensive review of processing conditions for various technological developments till to date. Addit. Manuf. 2018, 19, 134–159. [Google Scholar] [CrossRef]
- Yin, S.; Cavaliere, P.; Aldwell, B.; Jenkins, R.; Liao, H.; Li, W.; Lupoi, R. Cold spray additive manufacturing and repair: Fundamentals and applications. Addit. Manuf. 2018, 21, 628–650. [Google Scholar] [CrossRef]
- Xie, X.; Chen, C.; Xie, Y.; Aubry, E.; Ren, Z.; Ji, G.; Liao, H. Comparative investigation of microstructure and properties of Ni-coated FeSiAl soft magnetic composite coatings produced by cold spraying and HVOF. Surf. Coat. Technol. 2018, in press. [Google Scholar] [CrossRef]
- Tanaka, H.; Nagano, S.; Ishikawa, T.; Nakayama, T. Simulating study of atmospheric corrosion of Zn-Al alloy coating in industrial zone: Structure and properties of zinc hydroxysulfate rust particles prepared in the presence of Al(III). Adv. Powder Technol. 2019, 30, 807–814. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Wang, S.; Xiong, T.; Wen, D.; Wang, G.; Liu, W.; Du, H. Protective Performance of Zn-Al-Mg-TiO2 Coating Prepared by Cold Spraying on Marine Steel Equipment. Coatings 2019, 9, 339. https://doi.org/10.3390/coatings9050339
Wang K, Wang S, Xiong T, Wen D, Wang G, Liu W, Du H. Protective Performance of Zn-Al-Mg-TiO2 Coating Prepared by Cold Spraying on Marine Steel Equipment. Coatings. 2019; 9(5):339. https://doi.org/10.3390/coatings9050339
Chicago/Turabian StyleWang, Kunkun, Shouren Wang, Tianying Xiong, Daosheng Wen, Gaoqi Wang, Wentao Liu, and Hao Du. 2019. "Protective Performance of Zn-Al-Mg-TiO2 Coating Prepared by Cold Spraying on Marine Steel Equipment" Coatings 9, no. 5: 339. https://doi.org/10.3390/coatings9050339
APA StyleWang, K., Wang, S., Xiong, T., Wen, D., Wang, G., Liu, W., & Du, H. (2019). Protective Performance of Zn-Al-Mg-TiO2 Coating Prepared by Cold Spraying on Marine Steel Equipment. Coatings, 9(5), 339. https://doi.org/10.3390/coatings9050339



