Hot Corrosion Resistance of Laser-Sealed Thermal-Sprayed Cermet Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Powders and Coatings Deposition
2.2. Laser Post-Treatment
2.3. Hot Corrosion Tests
2.4. Coatings Characterization
3. Results and Discussion
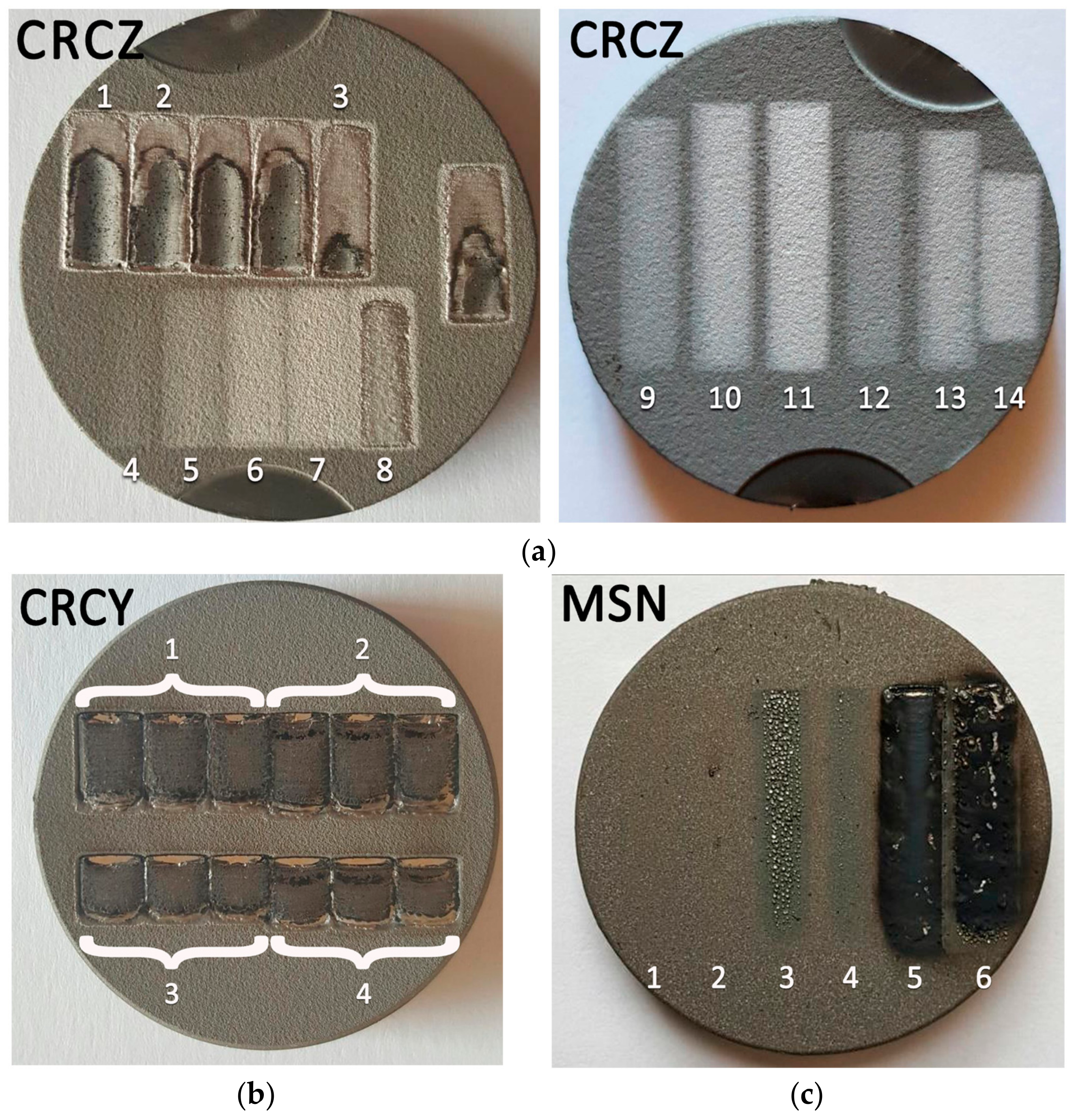
3.1. Optimization of the Post-Deposition Laser Treatment
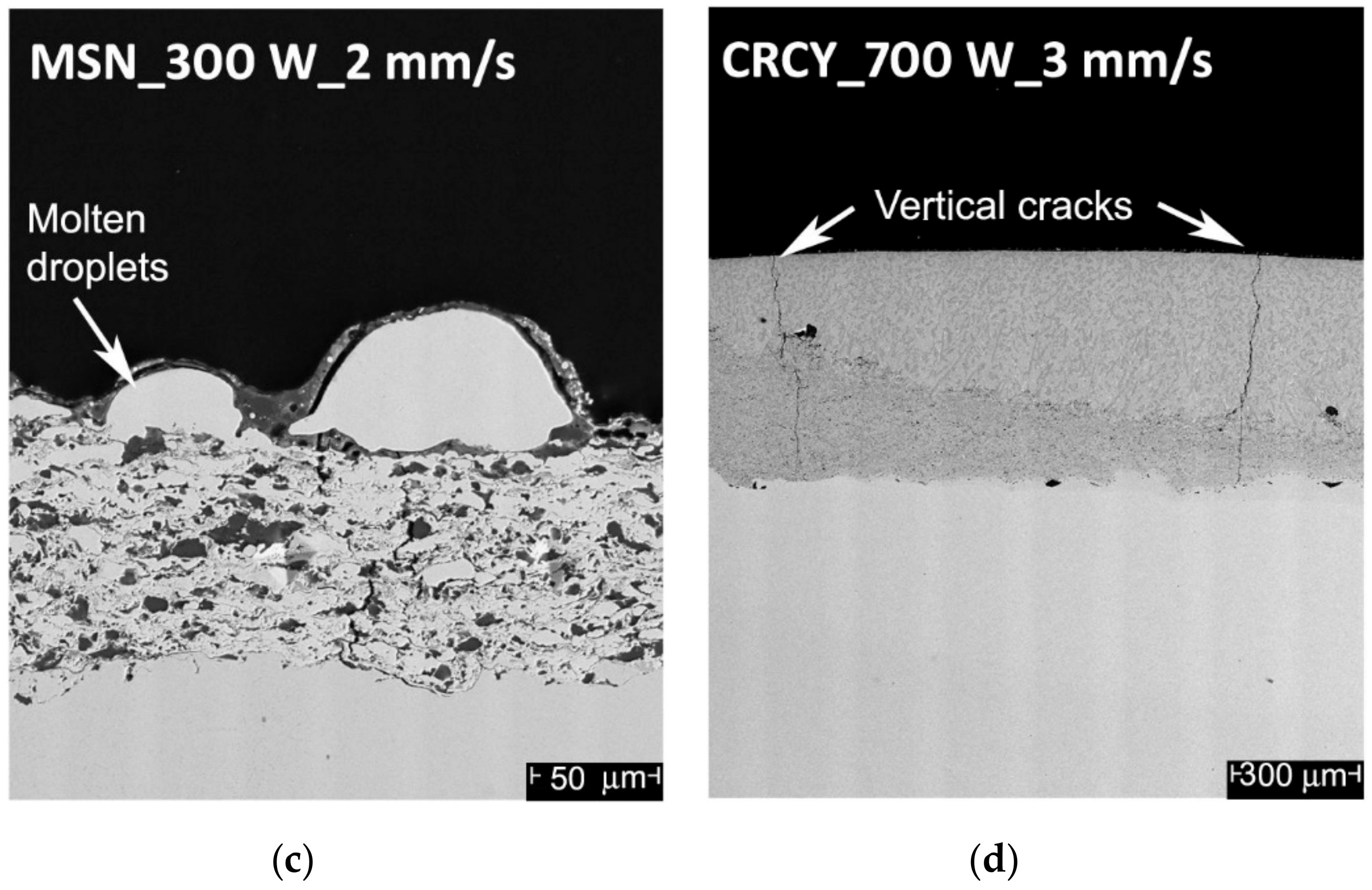
- Iincorporation of large amounts of the shielding gas inside the molten material, with formation of residual macro-porosities in the coating (mainly caused by too high a laser power, in the presence of high scan rates, where the viscosity of the molten material was drastically reduced and the interaction time was not sufficient to allow for proper degassing); Figure 2b, CRCZ coating, track 1, 800 W, 4 mm/s;
- Selective remelting or evaporation and recondensation in the shape of surface droplets of the lower melting fraction of the material (too long interaction times); Figure 2c, MSN coating, track 3, 300 W, 2 mm/s.
3.2. Hot Corrosion Results
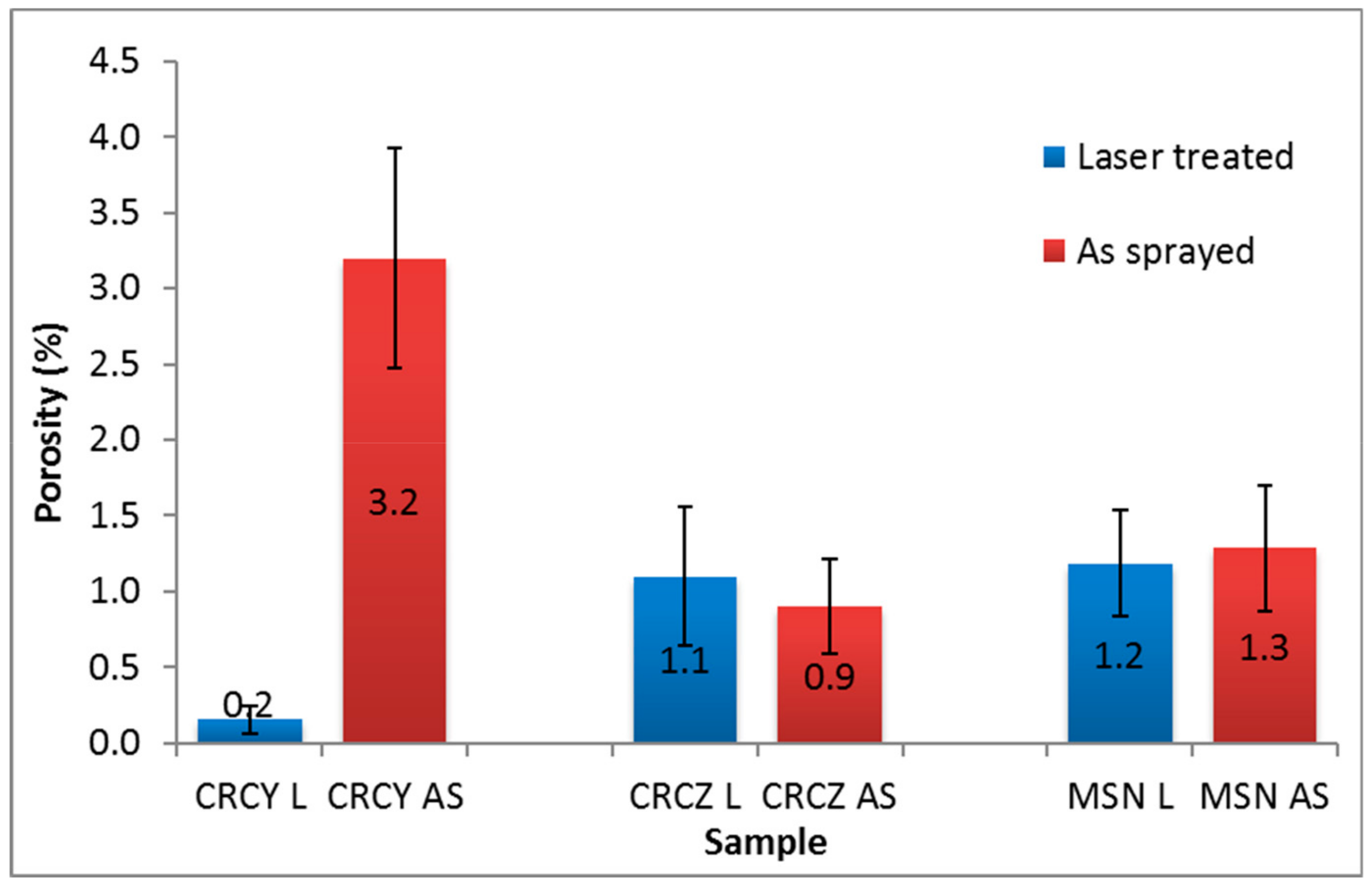
3.2.1. Microstructure
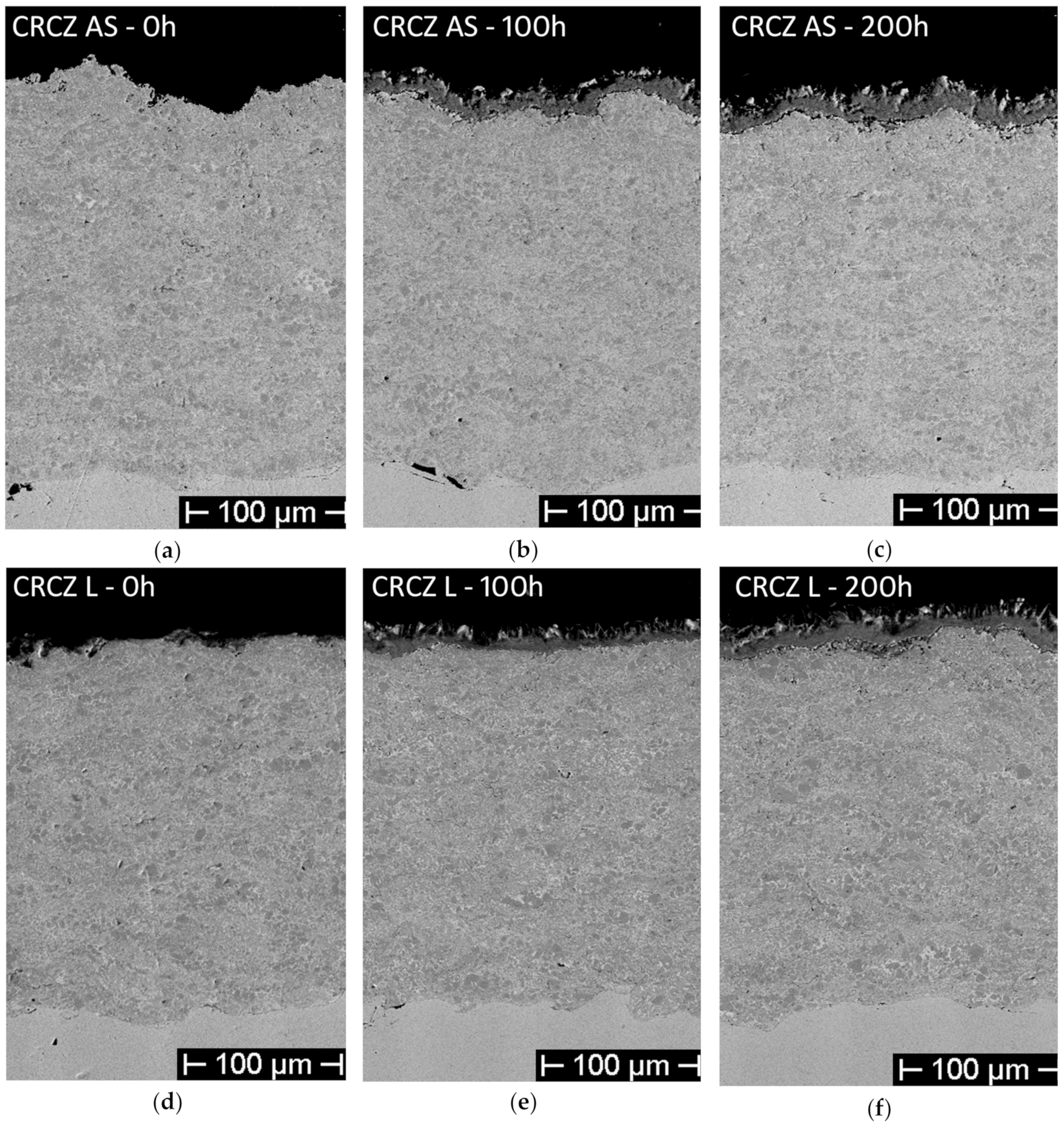
- For the CRCZ as-sprayed coatings (Figure 3a–c), a scale of about 25 µm was formed after 100 h, made of an inner layer of about 10 µm, compact and continuous, and an outer, less dense, coat, characterized by the presence of both acicular and sharp-cornered non-cohesive phases. The same corrosion scale morphology was highlighted in the CRCZ laser-treated samples, after 100 h of exposure test (Figure 11a).
- For CRCY, the thickness of the scale increased with the number of hot corrosion cycles for, both, the as-sprayed (Figure 4a–c) and laser-treated coatings (Figure 4d–f), but in this case, a more evident beneficial effect of the sealing treatment could be observed—after 200 h the as-sprayed coatings exhibited a spalled, detached, and thick (≈50 μm) corrosion scale, whereas, the laser-treated samples showed thinner and more compact surface layers formed by hot corrosion products.
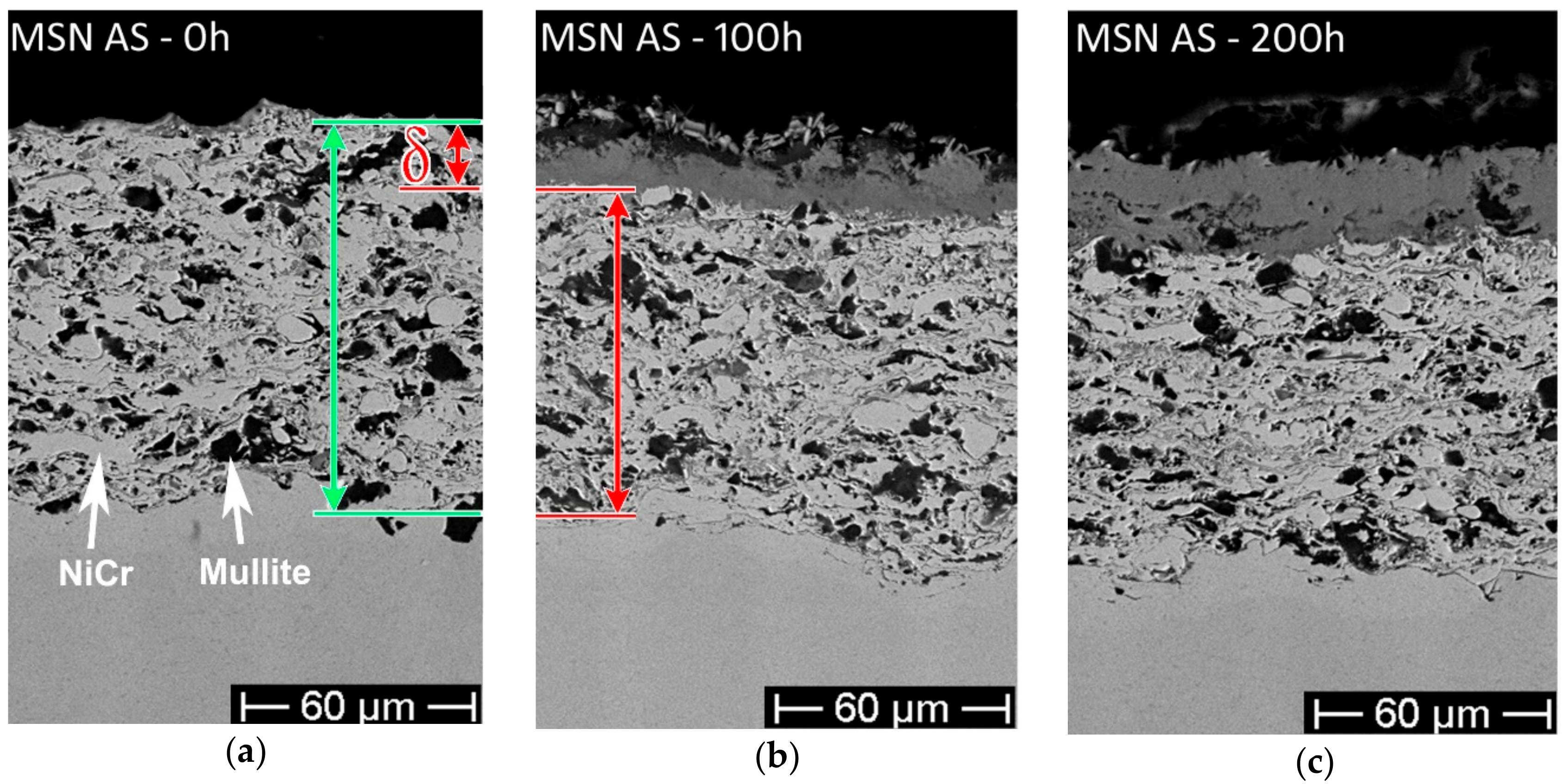
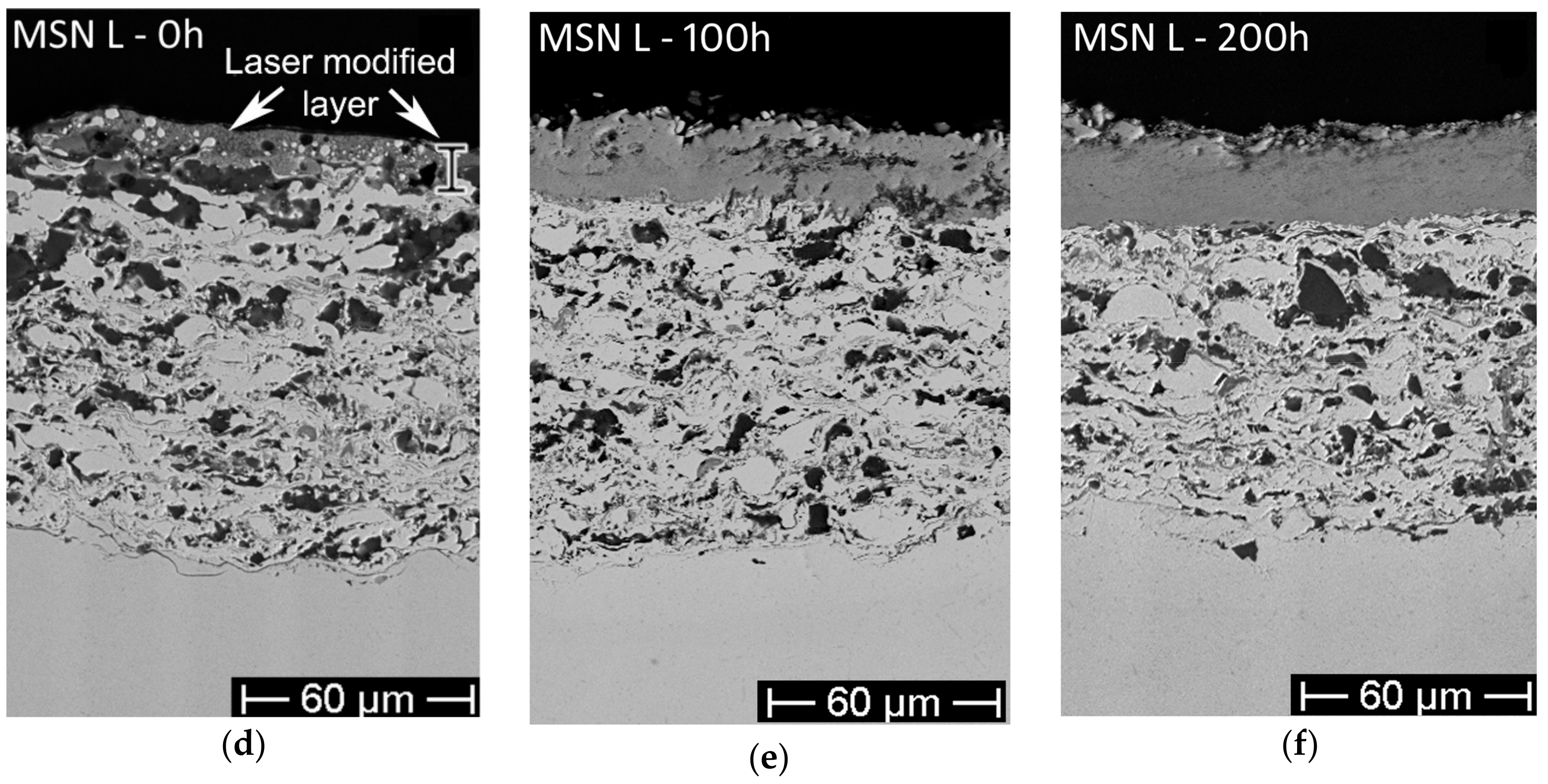
- The as-sprayed (Figure 5a,c) and laser-treated (Figure 5d–f) MSN samples did not show significant differences in terms of hot corrosion behavior—the scale thickness was comparable for each exposure time, while the corrosion layers of the laser-treated samples seemed to be slightly more compact and cohesive than the untreated.
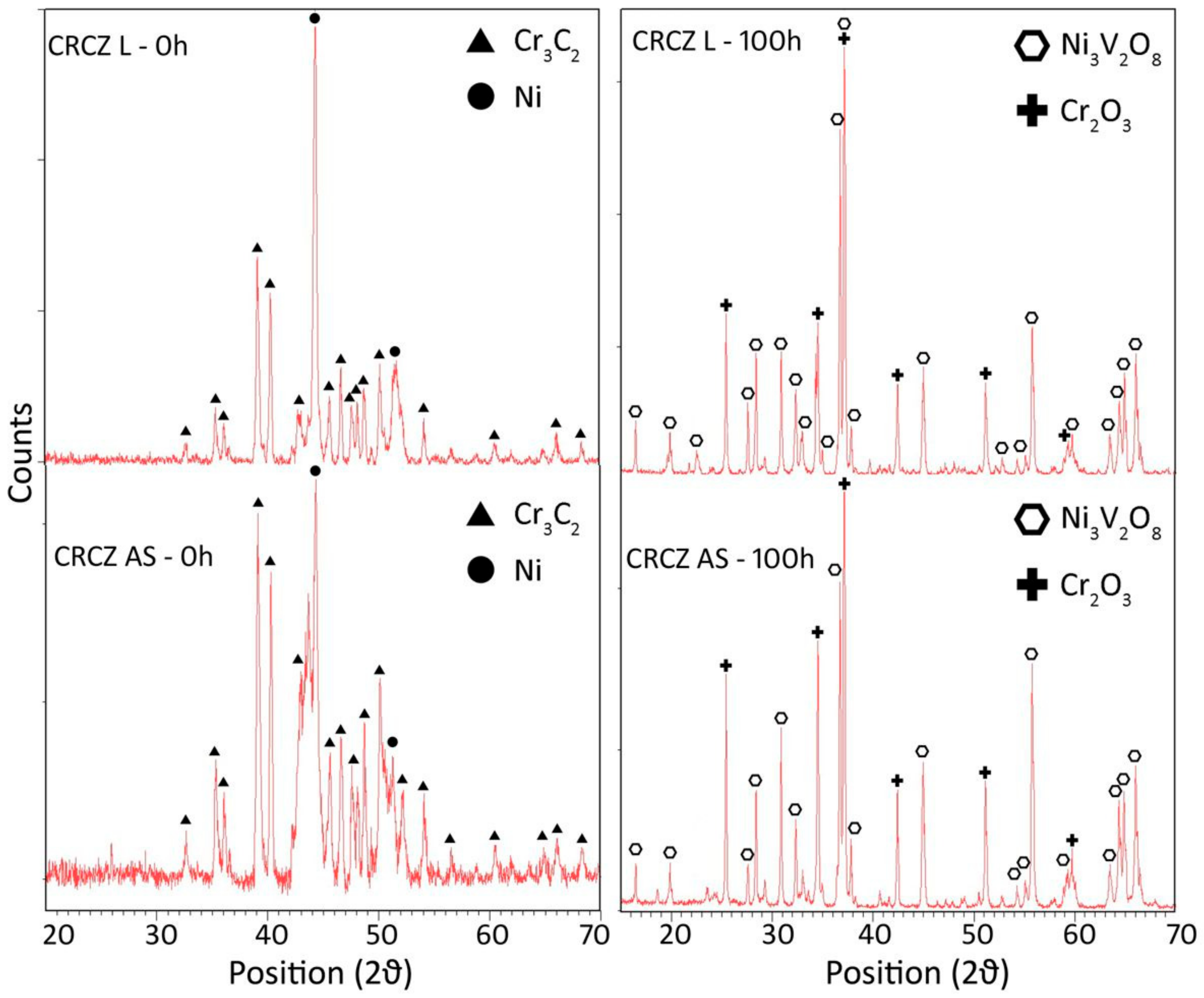
3.2.2. Composition of the Scale
3.2.3. Recession
4. Conclusions
- The mechanism of the degradation occurring in typical type II hot corrosion conditions was not substantially altered by the formation of a surface-laser-densified layer, as confirmed by the analysis of the corrosion products of the treated and as-sprayed samples exhibiting an analogous microstructure and composition;
- The formation of surface compact layers (with overall thickness and microstructure very much dependent on coating composition) was responsible for a considerable increase of surface hardness of all coatings, and for a consistent improvement in the hot corrosion resistance, promoting the formation of thinner and more compact corrosion scales, and considerably reducing the surface recession rate (up to 60%, after 200 h exposure for Cr3C2–25% CoNiCrAlY coatings).
Author Contributions
Funding
Conflicts of Interest
References
- Eliaz, N.; Shemesh, G.; Latanision, R.M. Hot corrosion in gas turbine components. Eng. Fail. Anal. 2002, 9, 31–43. [Google Scholar] [CrossRef]
- Lai, G.Y. High-Temperature Corrosion of Engineering Alloys. ASM International: Metals Park, OH, USA, 1990. [Google Scholar]
- Pettit, F. Hot corrosion of metals and alloys. Oxid. Met. 2011, 76, 1–21. [Google Scholar] [CrossRef]
- Bornstein, N.S. Reviewing sulfidation corrosion—Yesterday and today. J. Met. 1996, 11, 37–39. [Google Scholar]
- Zhang, W.-J.; Sharghi-Moshtaghin, R. Revisit the type II corrosion mechanism. Metall. Mater. Trans. A 2018, 49, 4362–4372. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High Temperature Oxidation of Metals, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Young, D.J. High Temperature Oxidation and Corrosion of Metals, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Rajendran, R. Gas turbine coatings–An overview. Eng. Fail. Anal. 2008, 26, 355–369. [Google Scholar] [CrossRef]
- Pawlowski, L. The Science and Engineering of Thermal Spray Coatings, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Saini, H.; Kumar, D.; Shukla, V.N. Hot corrosion behaviour of nanostructured cermet based coatings deposited by different thermal spray techniques: A review. Mater. Today Proc. 2017, 4, 541–545. [Google Scholar] [CrossRef]
- Valente, T.; Bartuli, C.; Tului, M. Thermal Sprayed Hard Cr3C2-Ni Cr Coatings for Wear Protection. In Thermal Spray 2001: New Surfaces for a New Millennium; ASM International: Materials Park, OH, USA, 2001; pp. 1075–1084. [Google Scholar]
- Kamal, S.; Jayaganthan, R.; Prakash, S. Evaluation of cyclic hot corrosion behaviour of detonation gun sprayed Cr3C2-25%NiCr coatings on nickel- and iron-based superalloys. Surf. Coat. Technol. 2009, 203, 1004–1013. [Google Scholar]
- Bluni, S.T.; Marder, A.R. Effects of thermal spray coating composition and microstructure on coating response and substrate protection at high temperatures. Corrosion 1996, 52, 213–218. [Google Scholar] [CrossRef]
- Di Girolamo, G.; Pilloni, L.; Pulci, G.; Marra, F. Tribological characterization of WC-Co plasma sprayed coatings. J. Am. Ceram. Soc. 2009, 92, 1118–1124. [Google Scholar] [CrossRef]
- Bartuli, C.; Valente, T.; Cipri, F.; Bemporad, E.; Tului, M. Parametric study of an HVOF process for the deposition of nanostructured WC-Co coatings. J. Therm. Spray Technol. 2005, 14, 187–195. [Google Scholar] [CrossRef]
- Bartuli, C.; Cipri, F.; Valente, T.; Verdone, N. CFD Simulation of an HVOF Process for the Optimization of WC-Co Protective Coatings. WIT Trans. Eng. Sci. 2003, 39, 71–83. [Google Scholar]
- Bartuli, C.; Valente, T.; Cipri, F.; Bemporad, E. Nanostructured Wear Resistant Coatings Deposited by HVOF. In Surface Engineering: Coatings and Heat Treatments; Popoola, O., Dahotre, N.B., Midea, S., Kopech, H., Eds.; ASM International: Materials Park, OH, USA, 2003; pp. 480–488. [Google Scholar]
- Rickerby, D.S.; Eckold, G.; Scott, K.T.; Buckley-Golder, I.M. The interrelationship between internal stress, processing parameters and microstructure of physically vapour deposited and thermally sprayed coatings. Thin Solid Films 1987, 154, 125–141. [Google Scholar] [CrossRef]
- Lih, W.-C.; Yang, S.H.; Su, C.Y.; Huang, S.C.; Hsu, I.C.; Leu, M.S. Effects of process parameters on molten particle speed and surface temperature and the properties of HVOF CrC/NiCr coatings. Surf. Coat. Technol. 2000, 133, 54–60. [Google Scholar] [CrossRef]
- Sidhu, T.S.; Prakash, S.; Agrawal, R.D. State of the art of HVOF coating investigations-A review. Mar. Technol. Soc. J. 2005, 39, 53–64. [Google Scholar] [CrossRef]
- Sreenivasulu, V.; Manikandan, M. Hot corrosion studies of HVOF sprayed carbide and metallic powder coatings on alloy 80A at 900 °C. Mater. Res. Express 2019, 6, 036519. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, K.; Deng, C.; Zeng, K.; Li, Y. Hot corrosion behaviour of HVOF-sprayed Cr3C2-NiCrMoNbAl coating. Surf. Coat. Technol. 2017, 309, 849–859. [Google Scholar] [CrossRef]
- Sidhu, T.S.; Prakash, S.; Agrawal, R.D. Hot corrosion studies of HVOF sprayed Cr3C2-NiCr and Ni-20Cr coatings on nickel—based superalloy at 900 °C. Surf. Coat. Technol. 2006, 201, 792–800. [Google Scholar] [CrossRef]
- Chatha, S.S.; Sidhu, H.S.; Sidhu, B.S. The effects of post-treatment on the hot corrosion behavior of the HVOF-sprayed Cr3C2-NiCr coating. Surf. Coat. Technol. 2012, 206, 4212–4224. [Google Scholar] [CrossRef]
- Houdková, S.; Smazalová, E.; Vostřák, M.; Schubert, J. Properties of NiCrBSi coating, as sprayed and remelted by different technologies. Surf. Coat. Technol. 2014, 253, 14–26. [Google Scholar] [CrossRef]
- Smurov, I.; Uglov, A.; Krivongov, Y.; Sturlese, S.; Bartuli, C. Pulsed laser treatment of plasma sprayed thermal barrier coatings: Effect of pulse duration and energy input. J. Mater. Sci. 1992, 27, 4523–4530. [Google Scholar]
- Garcia-Alonso, D.; Serres, N.; Demian, C.; Costil, S.; Langlade, C.; Coddet, C. Pre-/during-/post-laser processes to enhance the adhesion and mechanical properties of thermal-sprayed coatings with a reduced environmental impact. J. Therm. Spray Technol. 2011, 20, 719–735. [Google Scholar] [CrossRef]
- Koivuluoto, H.; Milanti, A.; Bolelli, G.; Latokartano, J.; Marra, F.; Pulci, G.; Vihinen, J.; Lusvarghi, L.; Vuoristo, P. Structures and properties of laser-assisted cold-sprayed aluminum coatings. Mater. Sci. Forum 2017, 879, 984–989. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.; Viejo, F.; Aburas, Z.; Rakhes, M. Laser-induced microstructural modification for corrosion protection. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2010, 224, 1073–1085. [Google Scholar] [CrossRef]
- Gisario, A.; Barletta, M.; Veniali, F. Laser surface modification (LSM) of thermally-sprayed Diamalloy 2002 coating. Opt. Laser Technol. 2012, 44, 1942–1958. [Google Scholar] [CrossRef]
- Gisario, A.; Puopolo, M.; Venettacci, S.; Veniali, F. Improvement of thermally sprayed WC-Co/NiCr coatings by surface laser processing. Int. J. Refract. Met. Hard Mater. 2015, 52, 123–130. [Google Scholar] [CrossRef]
- Morimoto, J.; Sasaki, Y.; Fukuhara, S.; Abe, N.; Tukamoto, M. Surface modification of Cr3C2-NiCr cermet coatings by direct diode laser. Vacuum 2006, 80, 1400–1405. [Google Scholar] [CrossRef]
- Ghasemi, R.; Shoja-Razavi, R.; Mozafarinia, R.; Jamali, H.; Hajizadeh-Oghaz, M.; Ahmadi-Pidani, R. The influence of laser treatment on hot corrosion behavior of plasma-sprayed nanostructured yttria stabilized zirconia thermal barrier coatings. J. Eur. Ceram. Soc. 2014, 34, 2013–2021. [Google Scholar] [CrossRef]
- Ahmadi, M.S.; Shoja-Razavi, R.; Valefi, Z.; Jamali, H. Evaluation of hot corrosion behavior of plasma sprayed and laser glazed YSZ–Al2O3 thermal barrier composite. Opt. Laser Technol. 2019, 111, 687–695. [Google Scholar] [CrossRef]
- Yi, P.; Mostaghimi, J.; Pershin, L.; Xu, P.; Zhan, X.; Jia, D.; Yi, H.; Liu, Y. Effects of laser surface remelting on the molten salt corrosion resistance of yttria-stabilized zirconia coatings. Ceram. Int. 2018, 44, 22645–22655. [Google Scholar] [CrossRef]
- Cai, J.; Gao, C.; Lv, P.; Zhang, C.; Guan, Q.; Lu, J.; Xu, X. Hot corrosion behaviour of thermally sprayed CoCrAlY coating irradiated by high-current pulsed electron beam. J. Alloy. Compd. 2019, 784, 1221–1233. [Google Scholar] [CrossRef]
- Dharamendara, M.; Jegadeeswaran, N. Development of laser heat treatment HVOF coating to combat oxidation on gas turbines. Mater. Today Proc. 2018, 5, 24937–24943. [Google Scholar] [CrossRef]
- Baiamonte, L.; Marra, F.; Gazzola, S.; Giovanetto, P.; Bartuli, C.; Valente, T.; Pulci, G. Thermal sprayed coatings for hot corrosion protection of exhaust valves in naval diesel engines. Surf. Coat. Technol. 2016, 295, 78–87. [Google Scholar] [CrossRef]
- Verlotski, V.; Stanglmaier, R.H.; Moormann, G.; Geraets, R. Mineral-metal, multiphase coatings to protect combustion chamber components against hot-gas corrosion and thermal loading. J. Eng. Gas Turbines Power 2011, 133, 1–5. [Google Scholar] [CrossRef]
- Verlotski, V. Thermally sprayed gastight protective layer for metal substrates. US patent No. US8784979B2, 30 May 2018. [Google Scholar]
- ASTM E384-89 Standard Test Method for Microhardness of Materials; ASTM International: West Conshohocken, PA, USA, 1989.







| Name | Composition | Particle Size | Trade Name |
|---|---|---|---|
| CRCZ | 75 wt % Cr3C2 | −45 + 15 μm | Sulzer-WOKA 7302 |
| 25wt % Ni20Cr | |||
| CRCY | 75 wt % Cr3C2 | −45 + 15 μm | FST-K880.23 |
| 25wt % CoNiCrAlY (Co 9.50 Ni 7.50 Al 1.75 Y 0.20 C10 Cr bal.) | |||
| MSN | 10wt % nano-SiO2 | −53 + 20 μm (sieved) | Purposely produced |
| 30wt % mullite (3Al2O3-2SIO2) | |||
| 60wt % Ni20Cr |
| Composition | CRCZ | CRCY | MSN |
|---|---|---|---|
| Kerosene feed rate (gph) | 6.5 | 6.5 | 6 |
| O2 feed rate (scfh) | 2000 | 2000 | 1850 |
| Spray distance (mm) | 350 | 370 | 350 |
| Test No. | Power P (W) | Scan Rate V (mm/s) | Fluence H (J/mm2) |
|---|---|---|---|
| 1 | 800 | 4 | 53.08 |
| 2 | 800 | 3 | 70.77 |
| 3 | 700 | 3 | 61.92 |
| 4 | 200 | 2 | 26.54 |
| 5 | 300 | 2 | 39.81 |
| 6 | 400 | 2 | 53.08 |
| 7 | 500 | 2.5 | 52.08 |
| 8 | 600 | 2.5 | 63.69 |
| 9 | 300 | 3 | 26.54 |
| 10 | 400 | 4 | 26.54 |
| 11 | 500 | 5 | 26.54 |
| 12 | 300 | 4 | 20.51 |
| 13 | 400 | 5 | 20.51 |
| 14 | 500 | 6.5 | 20.51 |
| Test No. | Power P (W) | Scan Rate V (mm/s) | Fluence H (J/mm2) |
|---|---|---|---|
| 1 | 800 | 4 | 53.08 |
| 2 | 800 | 3 | 70.77 |
| 3 | 700 | 4 | 46.44 |
| 4 | 700 | 3 | 61.92 |
| Test No. | Power P (W) | Scan Rate V (mm/s) | Fluence H (J/mm2) |
|---|---|---|---|
| 1 | 200 | 2 | 26.54 |
| 2 | 200 | 4 | 13.27 |
| 3 | 300 | 2 | 39.81 |
| 4 | 300 | 4 | 20.51 |
| 5 | 400 | 2 | 53.08 |
| 6 | 400 | 4 | 26.54 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baiamonte, L.; Bartuli, C.; Marra, F.; Gisario, A.; Pulci, G. Hot Corrosion Resistance of Laser-Sealed Thermal-Sprayed Cermet Coatings. Coatings 2019, 9, 347. https://doi.org/10.3390/coatings9060347
Baiamonte L, Bartuli C, Marra F, Gisario A, Pulci G. Hot Corrosion Resistance of Laser-Sealed Thermal-Sprayed Cermet Coatings. Coatings. 2019; 9(6):347. https://doi.org/10.3390/coatings9060347
Chicago/Turabian StyleBaiamonte, Lidia, Cecilia Bartuli, Francesco Marra, Annamaria Gisario, and Giovanni Pulci. 2019. "Hot Corrosion Resistance of Laser-Sealed Thermal-Sprayed Cermet Coatings" Coatings 9, no. 6: 347. https://doi.org/10.3390/coatings9060347






