Synthesis of High Near-Infrared Reflective Black Pigment Based on YMn2O5
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization
3. Results and Discussion
3.1. Y(Mn0.95M0.05)2O5 (M = Al, Fe, Ga, Ti, and Zr)
3.1.1. X-ray Powder Diffraction (XRD)
3.1.2. Ultraviolet–Visible–Near-Infrared (UV–Vis–NIR) Reflectance Spectra
3.1.3. Color Properties
3.2. Y(Mn1−xFex)2O5 (0 ≤ x ≤ 0.15)
3.3. Y(Mn1−xGax)2O5 (0 ≤ x ≤ 0.20)
3.3.1. X-ray Powder Diffraction (XRD)
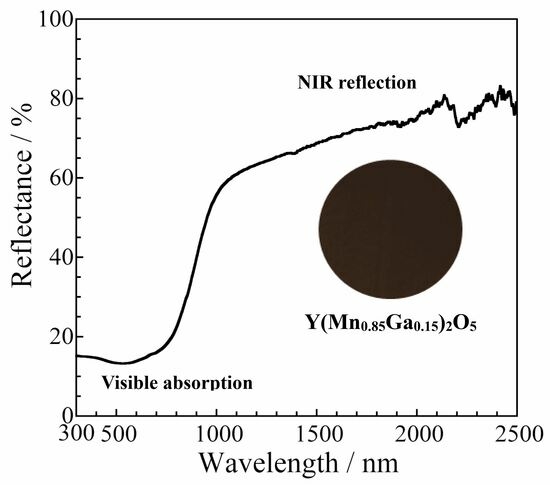
3.3.2. Ultraviolet–Visible–Near-Infrared (UV–Vis–NIR) Reflectance Spectra
3.3.3. Color Properties
3.3.4. Chemical Stability Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faulkner, E.B.; Schwartz, R.J. High Performance Pigments, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Oka, R.; Masui, T. Synthesis and characterization of black pigments based on calcium manganese oxides for high near-infrared (NIR) reflectance. RSC Adv. 2016, 6, 90952–90957. [Google Scholar] [CrossRef]
- Oka, R.; Iwasaki, S.; Masui, T. Improvement of near-infrared (NIR) reflectivity and black color tone by doping Zn2+ into the Ca2Mn0.85Ti0.15O4 structure. RSC Adv. 2019, 9, 38822–38827. [Google Scholar] [CrossRef]
- Oka, R.; Hayakawa, T. Raman spectroscopic investigation and electronic state calculation for Ca2(Mn,Ti)O4 black pigments with high near-Infrared (NIR) reflectivity. Inorg. Chem. 2022, 61, 6500–6507. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Oka, R.; Minagawa, K.; Masui, T. Novel near-infrared reflective black inorganic pigment based on cerium vanadate. RSC Adv. 2022, 12, 16570–16575. [Google Scholar] [CrossRef]
- Alonso, J.A.; Casais, M.T.; Martínez-Lope, M.J.; Martínez, J.L.; Ferandez-Díaz, M.T. A structural study from neutron diffraction data and magnetic properties of RMn2O5 (R = La, rare earth). J. Phys. Condens. Matter 1997, 9, 8515–8526. [Google Scholar] [CrossRef]
- Kagomiya, I.; Kakimoto, K.; Ohsato, H. Precursor phenomenon on ferroelectric transition in multiferroic YMn2O5. J. Eur. Ceram. Soc. 2010, 30, 255–258. [Google Scholar] [CrossRef]
- Yin, C.; Xia, Q.; Zhou, J.; Li, B.; Guo, Y.; Khan, A.; Li, X.; Xu, A. Direct electron transfer process-based peroxymonosulfate activation via surface labile oxygen over mullite oxide YMn2O5 for effective removal of bisphenol A. Sep. Purif. Technol. 2022, 280, 119924. [Google Scholar] [CrossRef]
- Zhang, T.; Li, H.; Ynag, Z.; Cao, F.; Chen, H.; Liu, H.; Xiong, K.; Wu, J.; Hong, Z.; Wnag, W. Electrospun YMn2O5 nanofibers: A highly catalytic activity for NO oxidation. Appl. Catal. B Environ. 2019, 247, 133–141. [Google Scholar] [CrossRef]
- Han, T.-C.; Lin, J.G. Enhancement of magnetic moment in Ca-doped helimagnetic YMn2O5. J. Magn. Magn. Mater. 2006, 304, e424–e426. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, S.-H.; Park, S.I.; Kenzelmann, M.; Harris, A.B.; Schefer, J.; Chung, J.-H.; Majkrzak, C.F.; Takeda, M.; Wakimoto, S.; et al. Spiral spin structures and origin of the magnetoelectric coupling in YMn2O5. Phys. Rev. B 2008, 78, 245115. [Google Scholar] [CrossRef]
- Han, A.; Zhao, M.; Ye, M.; Liao, J.; Zhang, Z.; Li, N. Crystal structure and optical properties of YMnO3 compound with high near-infrared reflectance. Sol. Energy 2013, 91, 32–36. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Y.; Wang, Z.; Wang, X.; Liu, H.; Cheng, G.J. Molten salt synthesis of YMnO3 powder with high near-infrared reflectivity. Mater. Lett. 2018, 229, 171–173. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, R.-J.; Ma, C.; Qin, Y.-B.; Shi, H.-L.; Wang, Z.-W.; Song, Y.-J.; Wang, Z.; Tian, H.-F.; Yang, H.-X.; et al. Electronic structure of YMn2O5 studied by EELS and first-principles calculations. Front. Phys. 2012, 7, 429–434. [Google Scholar] [CrossRef]
- Raj, A.K.V.; Rao, P.P.; Sameera, S.; Divya, S. Pigments based on terbium-doped yttrium cerate with high NIR reflectance for cool roof and surface coating applications. Dyes Pigm. 2015, 122, 116–125. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Tamilarasan, S.; Laha, S.; Natarajan, S.; Gopalakrishnan, J. Li2MnO3: A Rare Red-Coloured Manganese (IV) Oxide Exhibiting Tunable Red−Yellow−Green Emission. J. Mater. Chem. C 2015, 3, 4794–4800. [Google Scholar] [CrossRef]
- Kovaleva, N.N.; Gavartin, J.L.; Shluger, A.L.; Boris, A.V.; Stoneham, A.M. Lattice relaxation and charge-transfer optical transitions due to self-trapped holes in nonstoichiometric LaMnO3 crystal. J. Exp. Theor. Phys. 2002, 94, 178–190. [Google Scholar] [CrossRef]
- Kim, T.G.; Kim, S.J.; Lin, C.C.; Liu, R.S.; Chan, T.S.; Im, S.J. Melilite-type blue chromophores based on Mn3+ in a trigonal-bipyramidal coordination induced by interstitial oxygen. J. Mater. Chem. C 2013, 1, 5843–5848. [Google Scholar] [CrossRef]
- Wang, D.; Su, D.; Zhong, M. Chromatic and near-infrared reflective properties of Fe3+ doped KZnPO4. Sol Energy 2014, 110, 1–6. [Google Scholar] [CrossRef]











| Sample | Y(NO3)3·6H2O | (CH3COO)2Mn·4H2O | Reagent of Dopant | CA |
|---|---|---|---|---|
| YMn2O5 | 1.3739 g | 1.7582 g | — | 4.1350 g |
| Y(Mn0.95Al0.05)2O5 | 1.3879 g | 1.6872 g | Al(NO3)3·9H2O 0.1360 g | 4.1766 g |
| Y(Mn0.95Fe0.05)2O5 | 1.3735 g | 1.6699 g | Fe(NO3)3·9H2O 0.1448 g | 4.1335 g |
| Y(Mn0.95Ga0.05)2O5 | 1.3666 g | 1.6617 g | Ga(NO3)·8H2O 0.1426 g | 4.1129 g |
| Y(Mn0.95Ti0.05)2O5 | 1.3775 g | 1.6746 g | [(CH3)2CHO]4Ti 0.1022 g | 4.1455 g |
| Y(Mn0.95Zr0.05)2O5 | 1.3564 g | 1.6489 g | ZrO(NO3)2·2H2O 0.0946 g | 4.0819 g |
| Sample | Y(NO3)3·6H2O | (CH3COO)2Mn·4H2O | Fe(NO3)3·9H2O | CA |
|---|---|---|---|---|
| Y(Mn0.95Fe0.05)2O5 | 1.3735 g | 1.6699 g | 0.1448 g | 4.0973 g |
| Y(Mn0.90Fe0.10)2O5 | 1.3729 g | 1.5813 g | 0.2895 g | 4.1322 g |
| Y(Mn0.85Fe0.15)2O5 | 1.3725 g | 1.4930 g | 0.4343 g | 4.1308 g |
| Sample | Y(NO3)3·6H2O | (CH3COO)2Mn·4H2O | Ga(NO3)·8H2O | CA |
|---|---|---|---|---|
| Y(Mn0.95Ga0.05)2O5 | 1.3666 g | 1.6617 g | 0.1426 g | 4.1129 g |
| Y(Mn0.90Ga0.10)2O5 | 1.3595 g | 1.5658 g | 0.2839 g | 4.0914 g |
| Y(Mn0.85Ga0.15)2O5 | 1.3523 g | 1.4710 g | 0.4236 g | 4.0702 g |
| Y(Mn0.83Ga0.17)2O5 | 1.3494 g | 1.4335 g | 0.4790 g | 4.0616 g |
| Y(Mn0.80Ga0.20)2O5 | 1.3452 g | 1.3773 g | 0.5617 g | 4.0489 g |
| Sample | L* | a* | b* | C | R |
|---|---|---|---|---|---|
| YMn2O5 | 28.2 | +2.83 | −0.44 | 2.86 | 42.9 |
| Y(Mn0.95Al0.05)2O5 | 27.5 | +4.95 | +1.73 | 5.24 | 39.5 |
| Y(Mn0.95Fe0.05)2O5 | 25.5 | +3.24 | +0.10 | 3.24 | 43.5 |
| Y(Mn0.95Ga0.05)2O5 | 25.8 | +3.93 | +0.53 | 3.97 | 43.7 |
| Y(Mn0.95Ti0.05)2O5 | 25.2 | +2.58 | +0.20 | 2.59 | 31.2 |
| Sample | L* | a* | b* | C | R |
|---|---|---|---|---|---|
| YMn2O5 | 28.2 | +2.83 | −0.44 | 2.86 | 42.9 |
| Y(Mn0.95Fe0.05)2O5 | 25.3 | +3.24 | +0.10 | 3.24 | 43.5 |
| Y(Mn0.90Fe0.10)2O5 | 26.2 | +2.07 | −0.98 | 2.50 | 37.7 |
| Y(Mn0.85Fe0.15)2O5 | 24.7 | +1.58 | +1.02 | 1.88 | 28.1 |
| Sample | L* | a* | b* | C | R |
|---|---|---|---|---|---|
| YMn2O5 | 28.2 | +2.83 | −0.44 | 2.86 | 42.9 |
| Y(Mn0.95Ga0.05)2O5 | 25.8 | +3.93 | +0.53 | 3.97 | 43.7 |
| Y(Mn0.90Ga0.10)2O5 | 27.0 | +4.30 | +0.74 | 4.36 | 45.4 |
| Y(Mn0.85Ga0.15)2O5 | 25.8 | +4.82 | +1.25 | 4.98 | 47.6 |
| Sample | L* | a* | b* | C | R |
|---|---|---|---|---|---|
| Y(Mn0.85Ga0.15)2O5 | 25.8 | +4.82 | +1.25 | 4.98 | 47.6 |
| YMnO3 | 22.6 | −2.17 | −9.04 | 9.30 | 43.9 |
| Black 6350 | 25.3 | +1.20 | +4.06 | 4.23 | 41.3 |
| Black 6301 | 23.7 | +0.43 | +1.05 | 1.13 | 38.7 |
| MPT-370 | 23.0 | +0.67 | −0.27 | 0.72 | 44.0 |
| Treatment | L* | a* | b* | C | R |
|---|---|---|---|---|---|
| As synthesized | 25.8 | +4.82 | +1.25 | 4.98 | 47.6 |
| 4% CH3COOH | 25.2 | +4.39 | +1.07 | 4.52 | 42.8 |
| 4% NH4HCO3 | 24.3 | +4.49 | −1.20 | 4.65 | 42.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, K.; Mochizuki, S.; Nagato, Y.; Morimoto, T.; Masui, T. Synthesis of High Near-Infrared Reflective Black Pigment Based on YMn2O5. Colorants 2023, 2, 705-715. https://doi.org/10.3390/colorants2040036
Yamaguchi K, Mochizuki S, Nagato Y, Morimoto T, Masui T. Synthesis of High Near-Infrared Reflective Black Pigment Based on YMn2O5. Colorants. 2023; 2(4):705-715. https://doi.org/10.3390/colorants2040036
Chicago/Turabian StyleYamaguchi, Kazuki, Satoru Mochizuki, Yudai Nagato, Takuro Morimoto, and Toshiyuki Masui. 2023. "Synthesis of High Near-Infrared Reflective Black Pigment Based on YMn2O5" Colorants 2, no. 4: 705-715. https://doi.org/10.3390/colorants2040036