Bimetallic Fenton-like Catalysts in the Remediation of Dyes
Abstract
:1. Introduction
1.1. Oxidative Degradation Processes
1.2. Classic Fenton Chemistry
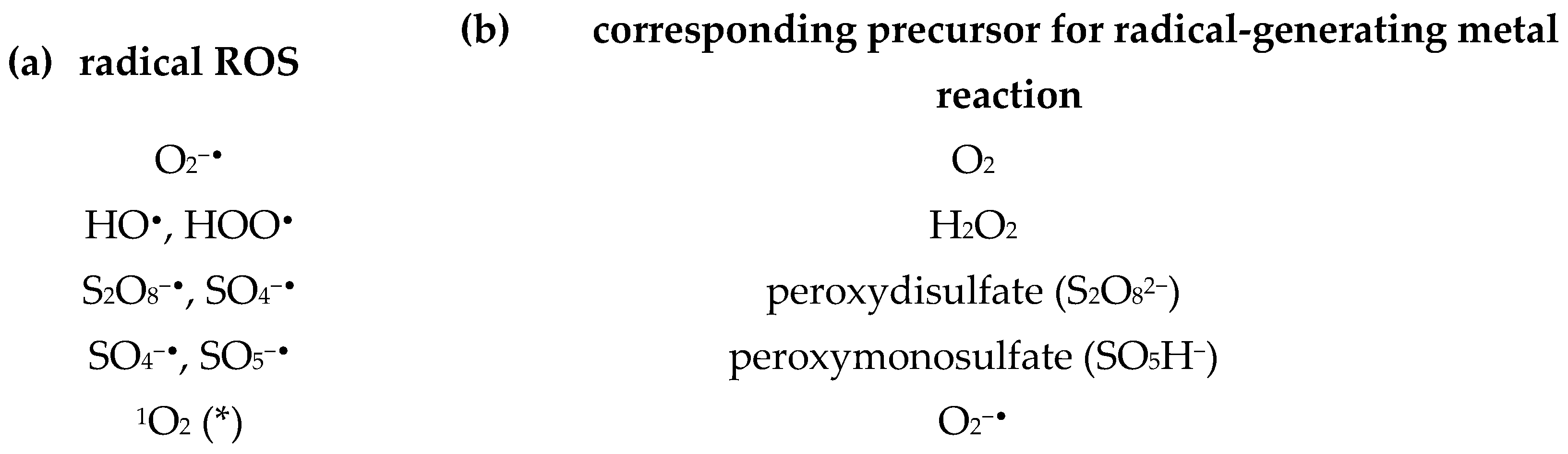
1.3. Radical and Reactive Oxygen Species
1.4. The Challenges of Fenton Cycling with Homogeneous Iron Catalysts
1.5. Bimetallic Catalyst Systems and the Modularity of Fenton-like Reactions
1.5.1. Homogeneous and Heterogeneous Fenton-like Processes
1.5.2. The Radical-Generating Metal Reaction
1.5.3. The Reactive Oxygen Species
1.5.4. The Cycling Reduction Reaction
1.6. The Advantages and Prospects of Bimetallic Fenton-like Catalysts
2. Metal Catalysts for Dye Degradation
2.1. A Co-Catalytic Effect for the Cycling of Iron
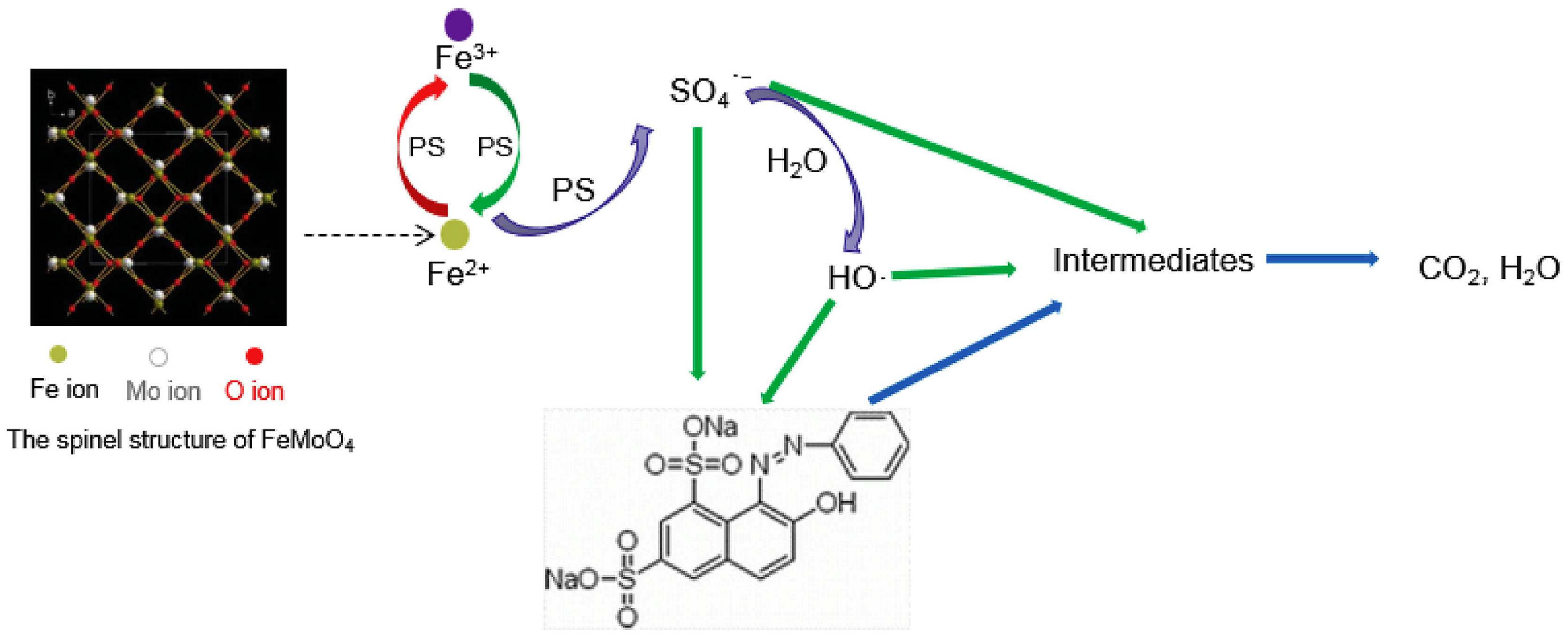
2.1.1. Iron with Molybdenum
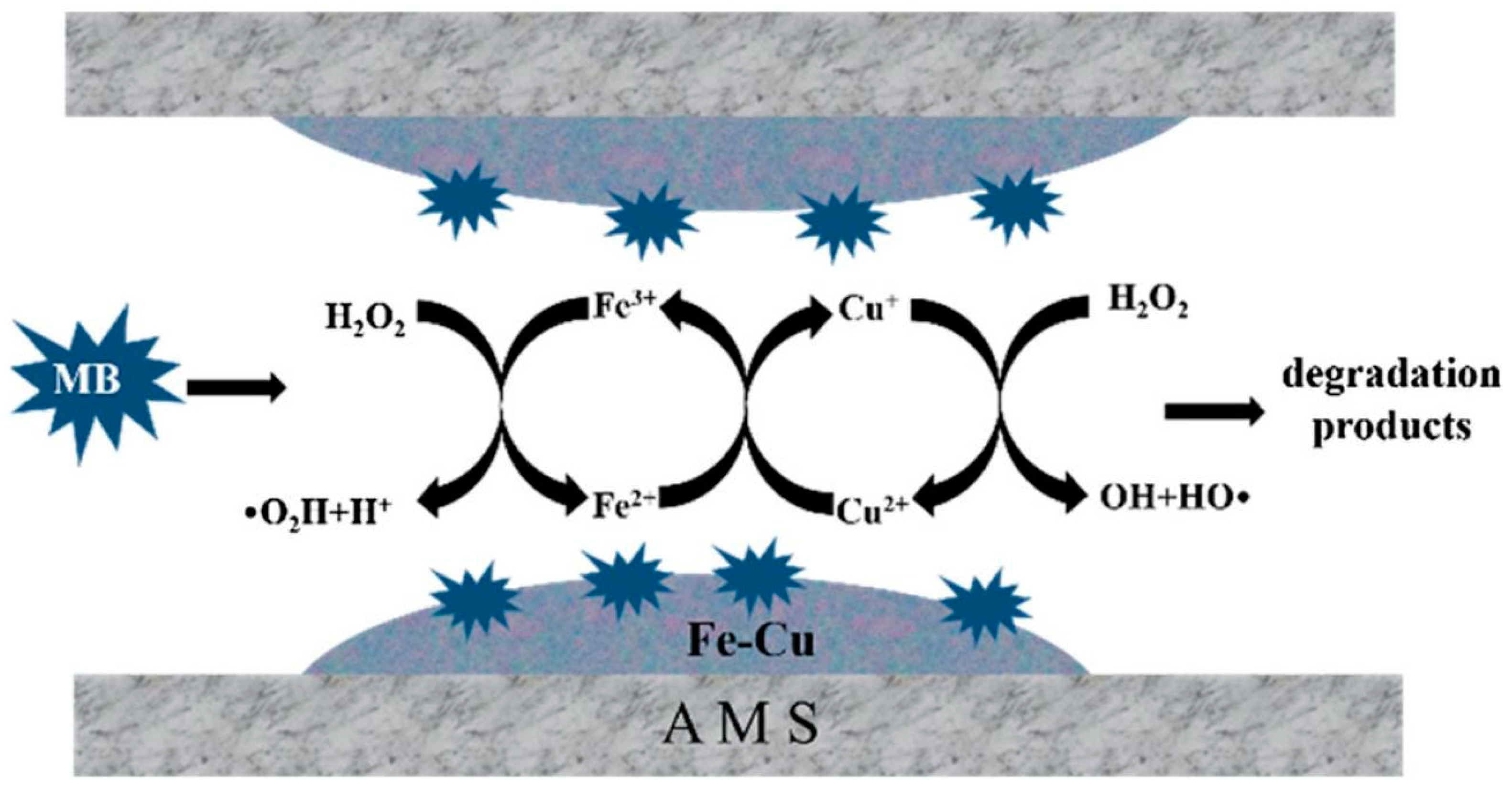
2.1.2. Iron with Copper
2.1.3. Iron with Vanadium
2.1.4. Iron with Magnesium
2.2. The Systems of Two Radical-Generating Metals
2.2.1. Iron with Copper
2.2.2. Iron with Molybdenum
2.3. Iron-Free Bimetallic Catalysts
2.3.1. Copper and Molybdenum
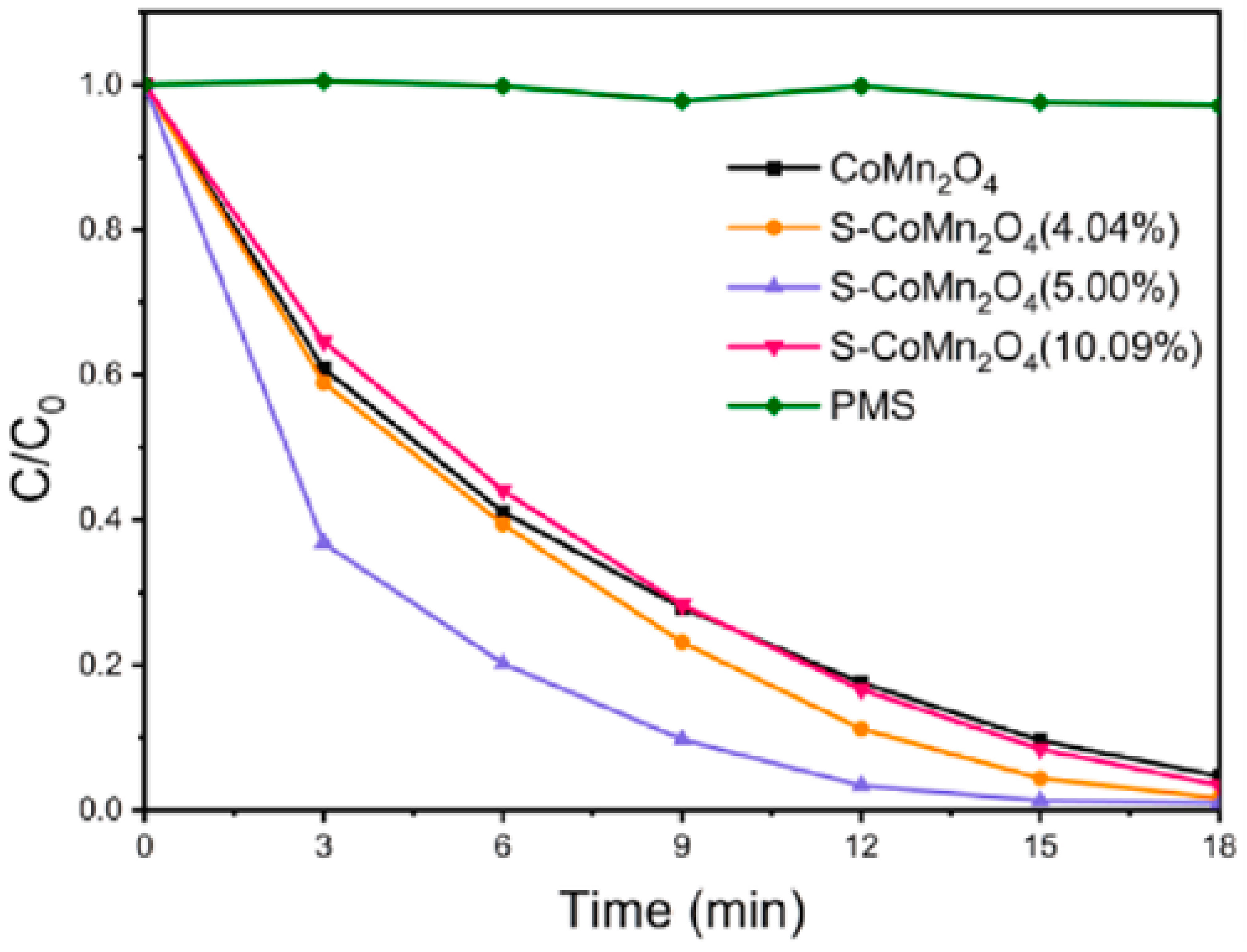
2.3.2. Manganese and Cobalt
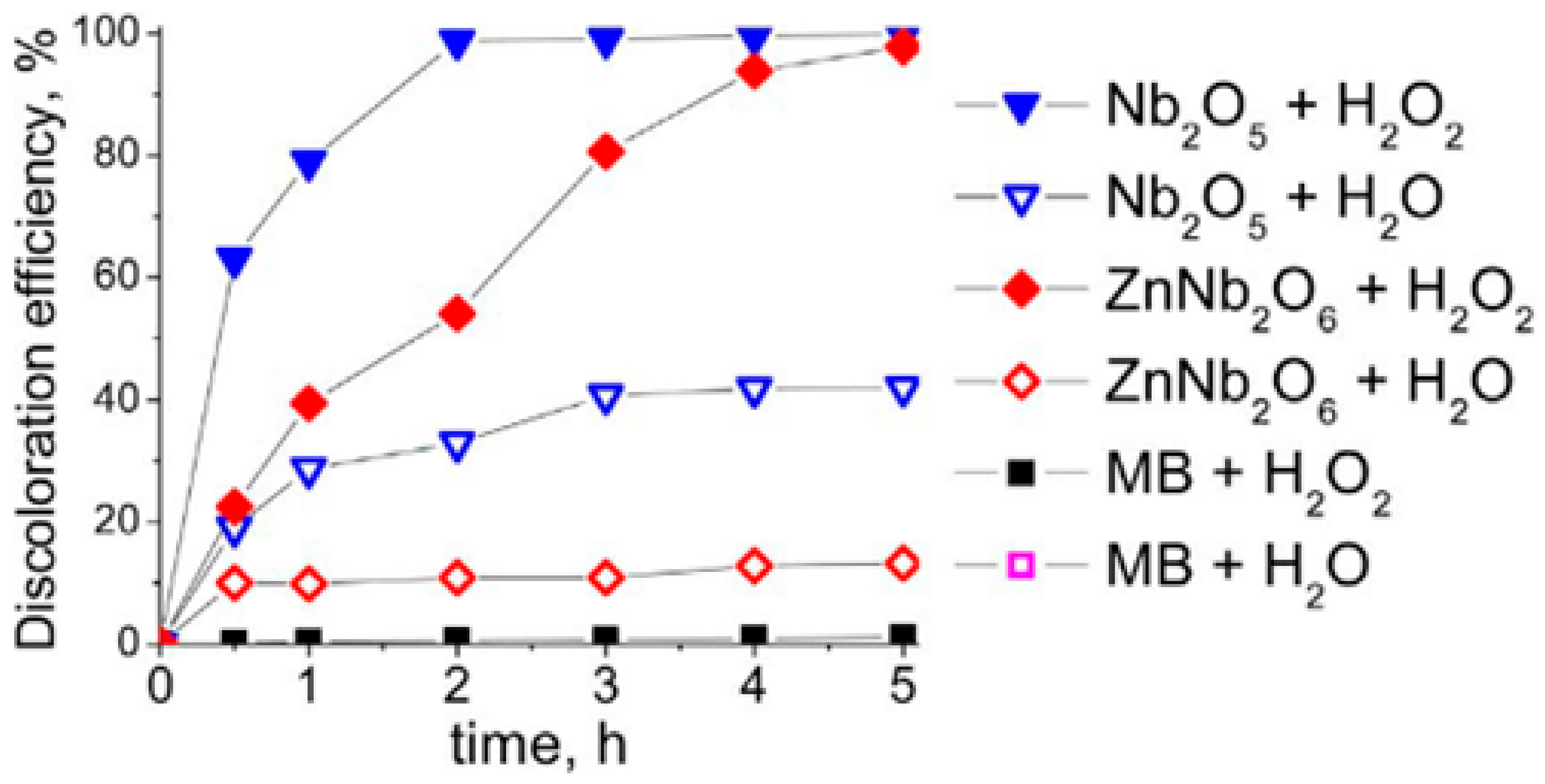
2.3.3. Niobium and Zinc
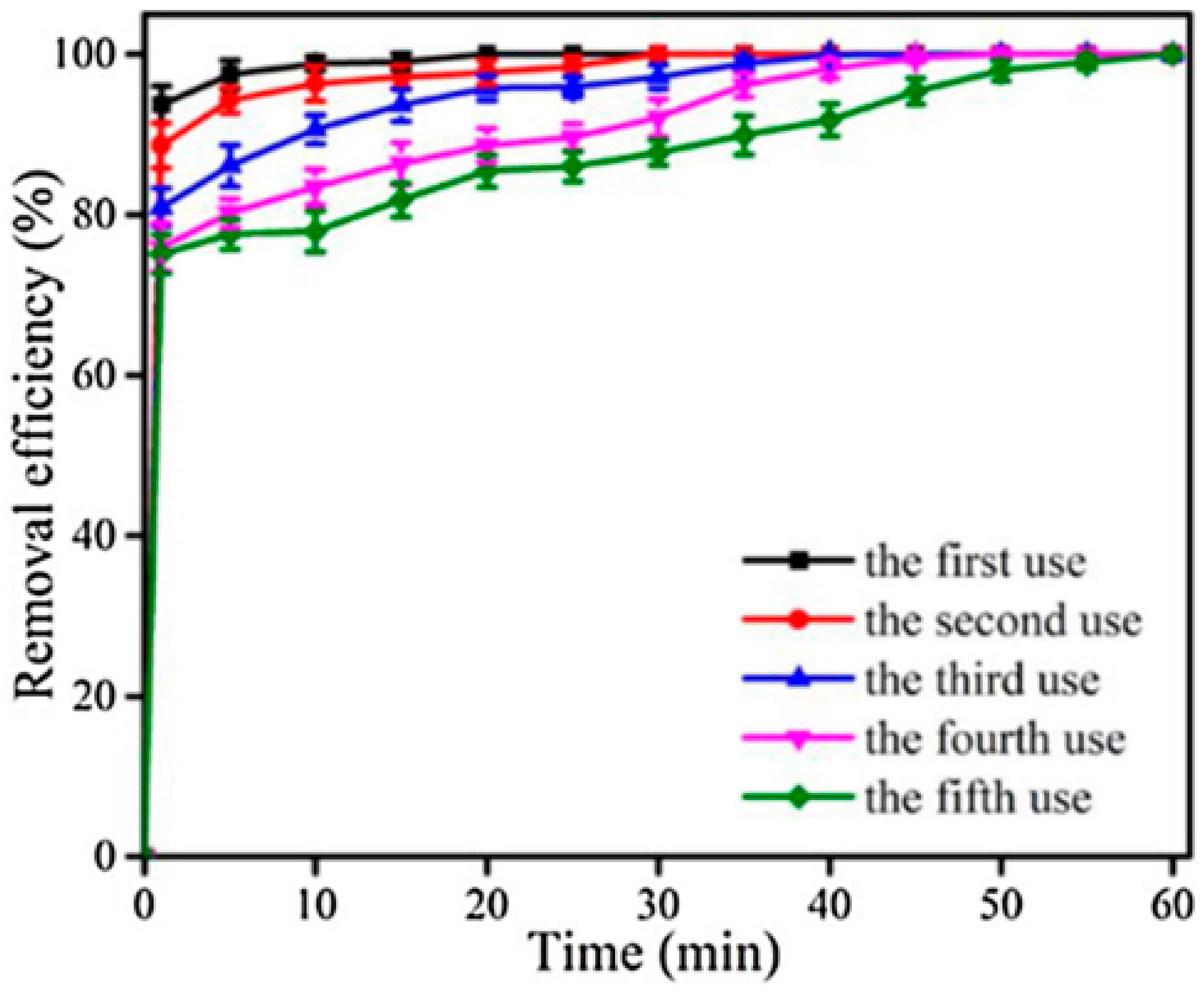
2.4. Case Studies: Iron–Cobalt and Iron–Copper Heterogeneous Systems Exploiting Metal–Metal Synergy and the Facile Reclamation of Catalysts
2.4.1. A Fe3O4−CoS2−(S, O)x/HSO5− Catalyst System with Oxygen or Sulfur Vacancies
2.4.2. A Reduced Iron–Copper Spinel Catalyst Containing Zerovalent Iron and Copper
3. Dye Degradation Mechanisms and Products
3.1. Effects of Reaction Conditions on the Catalysis
3.2. Dye Degradation Reactions and Products
3.3. Matrix Effects
4. Implementation and Environmental Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ammar, M.; Yousef, E.; Mahmoud, M.A.; Ashraf, S.; Baltrusaitis, J. A Comprehensive Review of the Developments in Electrocoagulation for the Removal of Contaminants from Wastewater. Separations 2023, 10, 337. [Google Scholar] [CrossRef]
- United Nations Department of Economic and Social Affairs. Sustainable Development. Available online: https://sdgs.un.org/goals (accessed on 9 December 2023).
- Steffen, W.; Richardson, K.; Rockstrom, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Rabbi, M.R.; Sufian, M.A.; Mahjebin, S. Effects of textile dyeing effluent on the environment and its treatment: A review. Eng. Appl. Sci. Lett. 2022, 5, 1–17. [Google Scholar] [CrossRef]
- Lin, X.; Ma, Y.; Wan, J.; Wang, Y.; Li, Y. Efficient degradation of Orange G with persulfate activated by recyclable FeMoO4. Chemosphere 2019, 214, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Piaskowski, K.; Świderska-Dąbrowska, R.; Zarzycki, P.K. Dye Removal from Water and Wastewater Using Various Physical, Chemical, and Biological Processes. J. AOAC Int. 2019, 101, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Javaid, R.; Qazi, U.Y. Catalytic Oxidation Process for the Degradation of Synthetic Dyes: An Overview. Int. J. Environ. Res. Public Health 2019, 16, 2066. [Google Scholar] [CrossRef] [PubMed]
- Omiri, J.; Snoussi, Y.; Bhakta, A.K.; Truong, S.; Ammar, S.; Khalil, A.M.; Jouini, M.; Chehimi, M.M. Citric-Acid-Assisted Preparation of Biochar Loaded with Copper/Nickel Bimetallic Nanoparticles for Dye Degradation. Colloids Interfaces 2022, 6, 18. [Google Scholar] [CrossRef]
- Santos, B.L.C.; Parpot, P.; Soares, O.S.G.P.; Pereira, M.F.R.; Rombi, E.; Fonseca, A.M.; Neves, I.C. Fenton-type bimetallic catalysts for degradation of dyes in aqueous solutions. Catalysts 2021, 11, 32. [Google Scholar] [CrossRef]
- Mathur, N.; Bhatnagar, P.; Sharma, P. Review of the mutagenicity of textile dye products. Univers. J. Environ. Res. Technol. 2012, 2, 1–18. [Google Scholar]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Anisuzzaman, S.M.; Joseph, C.G.; Pang, C.K.; Affandi, N.A.; Maruja, S.N.; Vijayan, V. Current Trends in the Utilization of Photolysis and Photocatalysis Treatment Processes for the Remediation of Dye Wastewater: A Short Review. ChemEngineering 2022, 6, 58. [Google Scholar] [CrossRef]
- Dong, C.; Fang, W.; Yi, Q.; Zhang, J. A comprehensive review on reactive oxygen species (ROS) in advanced oxidation processes (AOPs). Chemosphere 2022, 308 Pt 1, 136205. [Google Scholar] [CrossRef] [PubMed]
- Fenton, H.J.H. LXXIII.—Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Wolski, L.; Ziolek, M. Insight into pathways of Methylene Blue degradation with H2O2 over mono and bimetallic Nb, Zn oxides. Appl. Catal. B 2018, 224, 634–647. [Google Scholar] [CrossRef]
- Su, S.S.; Liu, Y.Y.; Liu, X.M.; Jin, W.; Zhao, Y.P. Transformation pathway and degradation mechanism of methylene blue through beta-FeOOH@GO catalyzed photo-Fenton-like system. Chemosphere 2019, 218, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Feng, S.; Zhou, J.; Chen, R.; Wei, Y.; Liu, H.; Wang, S. Hydrogen peroxide decomposition by co-catalytic effect of iron phosphide on Fenton reaction for degradation of methylene blue. Appl. Catal. B 2019, 259, 118015. [Google Scholar] [CrossRef]
- Cheng, F.; Tong, Y.; Liu, Y.; Yuan, Y.; Chen, Z.; Liang, J.; Zhang, Y.; Zhou, P.; Duan, X.; Lai, B. Vanadium as co-catalyst for exceptionally boosted Fenton and Fenton-like oxidation: Vanadium species mediated direct and indirect routes. J. Hazard. Mater. 2023, 446, 130719. [Google Scholar] [CrossRef]
- Haber, F.; Weiss, J.; Pope, W.J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc. R. Soc. London. Ser. A Math. Phys. Sci. 1934, 147, 332–351. [Google Scholar] [CrossRef]
- Weinstein, J.; Bielski, B.H.J. Kinetics of the interaction of perhydroxyl and superoxide radicals with hydrogen peroxide. The Haber-Weiss reaction. J. Am. Chem. Soc. 1979, 101, 58–62. [Google Scholar] [CrossRef]
- Barb, W.G.; Baxendale, J.H.; George, P.; Hargrave, K.R. Reactions of Ferrous and Ferric Ions with Hydrogen Peroxide. Nature 1949, 163, 692–694. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Jiang, F.; Liu, Q.; Lang, D. Co-Catalytic Effect of Molybdenum Disulfide on Fenton Reaction for the Degradation of Acid Orange 7 Dye: Reaction Mechanism, Performance Optimization, and Toxicity Evaluation. Environ. Eng. Sci. 2021, 38, 1149–1157. [Google Scholar] [CrossRef]
- Li, X.; Kong, Y.; Zhou, S.; Wang, B. In situ incorporation of well-dispersed Cu-Fe oxides in the mesochannels of AMS and their utilization as catalysts towards the Fenton-like degradation of methylene blue. J. Mater. Sci. 2017, 52, 1432–1445. [Google Scholar] [CrossRef]
- Cai, F.; Sun, C.; Sun, Z.; Lai, Y.; Ding, H. Sulfur-functionalized CoMn2O4 as a Fenton-like catalyst for the efficient rhodamine B degradation. Appl. Surf. Sci. 2023, 623, 157044. [Google Scholar] [CrossRef]
- Merkofer, M.; Kissner, R.; Hider, R.C.; Brunk, U.T.; Koppenol, W.H. Fenton Chemistry and Iron Chelation under Physiologically Relevant Conditions: Electrochemistry and Kinetics. Chem. Res. Toxicol. 2006, 19, 1263–1269. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Ji, Y.; Shi, Y.; Wang, L.; Lu, J. Denitration and renitration processes in sulfate radical-mediated degradation of nitrobenzene. Chem. Eng. J. 2017, 315, 591–597. [Google Scholar] [CrossRef]
- Xia, X.; Zhu, F.; Li, J.; Yang, H.; Wei, L.; Li, Q.; Jiang, J.; Zhang, G.; Zhao, Q. A Review Study on Sulfate-Radical-Based Advanced Oxidation Processes for Domestic/Industrial Wastewater Treatment: Degradation, Efficiency, and Mechanism. Front. Chem. 2020, 8, 592056. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Cui, Y.; Zhang, D.; Liu, M.; Huang, H.; Sha, X.; Deng, F.; Zhou, N.; Zhang, X.; Wei, Y. Biomimetic preparation of MoS2-Fe3O4 MNPs as heterogeneous catalysts for the degradation of methylene blue. J. Environ. Chem. Eng. 2020, 8, 104125. [Google Scholar] [CrossRef]
- Zheng, N.; Tang, X.; Lian, Y.; Ou, Z.; Zhou, Q.; Wang, R.; Hu, Z. Low-valent copper on molybdenum triggers molecular oxygen activation to selectively generate singlet oxygen for advanced oxidation processes. J. Hazard. Mater. 2023, 452, 131210. [Google Scholar] [CrossRef]
- Yi, Q.; Ji, J.; Shen, B.; Dong, C.; Liu, J.; Zhang, J.; Xing, M. Singlet Oxygen Triggered by Superoxide Radicals in a Molybdenum Cocatalytic Fenton Reaction with Enhanced REDOX Activity in the Environment. Environ. Sci. Technol. 2019, 53, 9725–9733. [Google Scholar] [CrossRef]
- Shen, B.; Dong, C.; Ji, J.; Xing, M.; Zhang, J. Efficient Fe(III)/Fe(II) cycling triggered by MoO2 in Fenton reaction for the degradation of dye molecules and the reduction of Cr(VI). Chin. Chem. Lett. 2019, 30, 2205–2210. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Cong, S.; Xia, S.; Zou, D. Review of Mo-based materials in heterogeneous catalytic oxidation for wastewater purification. Sep. Purif. Technol. 2023, 312, 123345. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, K.; Zhai, Z.; Zhao, T.; Yuan, D.; Jiao, T.; Zhang, Q.; Tang, S. Molybdenum co-catalytic promotion for Fe3+/peroxydisulfate process: Performance, mechanism, and immobilization. Chem. Eng. J. 2022, 438, 135656. [Google Scholar] [CrossRef]
- Ji, J.; Aleisa, R.M.; Duan, H.; Zhang, J.; Yin, Y.; Xing, M. Metallic Active Sites on MoO2(110) Surface to Catalyze Advanced Oxidation Processes for Efficient Pollutant Removal. iScience 2020, 23, 100861. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Xu, W.; Dong, C.; Bai, Y.; Zeng, J.; Zhou, Y.; Zhang, J.; Yin, Y. Metal Sulfides as Excellent Co-catalysts for H2O2 Decomposition in Advanced Oxidation Processes. Chem 2018, 4, 1359–1372. [Google Scholar] [CrossRef]
- Dong, C.; Ji, J.; Shen, B.; Xing, M.; Zhang, J. Enhancement of H2O2 Decomposition by the Co-catalytic Effect of WS2 on the Fenton Reaction for the Synchronous Reduction of Cr(VI) and Remediation of Phenol. Environ. Sci. Technol. 2018, 52, 11297–11308. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Fu, L.; Peng, X.; Lei, M.; Wang, C.; Jiang, J. Copper catalysts for radical and nonradical persulfate based advanced oxidation processes: Certainties and uncertainties. Chem. Eng. J. 2022, 427, 131776. [Google Scholar] [CrossRef]
- Liang, L.; Duan, Y.; Xiong, Y.; Zuo, W.; Ye, F.; Zhao, S. Synergistic cocatalytic effect of MoO3 and creatinine on Cu–Fenton reactions for efficient decomposition of H2O2. Mater. Today Chem. 2022, 24, 100805. [Google Scholar] [CrossRef]
- Liu, G.; Guan, W.; Chen, D.; Liu, W.; Mi, H.; Liu, Y.; Xiong, J. Efficient activation of peroxymonosulfate via Cu2+/Cu+ cycle enhanced by hydroxylamine for the degradation of Rhodamine B. Environ. Sci. Pollut. Res. 2023, 30, 33133–33141. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Dong, C.-D.; Huang, C.P.; Chen, C.-W.; Hsieh, S.-L.; Hsieh, S. Fe-Cu bimetallic catalyst for the degradation of hazardous organic chemicals exemplified by methylene blue in Fenton-like reaction. J. Environ. Chem. Eng. 2020, 8, 104139. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Y.; Xing, S. Comparative study of Cu-based bimetallic oxides for Fenton-like degradation of organic pollutants. Chemosphere 2018, 203, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, X.; Pu, S.; Ni, R.; Lin, Y.; Liu, Y. Novel Fenton-like system (Mg/FeO2) for degradation of 4-chlorophenol. Environ. Pollut. 2019, 250, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhang, D.; Yao, Z.; Jiang, Z. Revealing the enhancing mechanisms of Fe-Cu bimetallic catalysts for the Fenton-like degradation of phenol. Chemosphere 2022, 289, 133195. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Liu, Y.; Li, X.; Sun, T.; Xu, Y. Enhanced heterogeneous Fenton-like degradation of methylene blue by reduced CuFe2O4. RSC Adv. 2018, 8, 1071–1077. [Google Scholar] [CrossRef]
- Hazarika, K.K.; Talukdar, H.; Sudarsanam, P.; Bhargava, S.K.; Bharali, P. Highly dispersed Mn2O3-Co3O4 nanostructures on carbon matrix as heterogeneous Fenton-like catalyst. Appl. Organomet. Chem. 2020, 34, e5512. [Google Scholar] [CrossRef]
- Qiu, F.; Pan, Y.; Wang, L.; Song, H.; Liu, X.; Fan, Y.; Zhang, S. Ultrafast degradation of organic pollutants in an Fe3O4-CoS2–x activated persulfate catalytic system with an Fe-Co catalytic cycle involving oxygen and sulfur vacancies. Sep. Purif. Technol. 2024, 330, 125139. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.F.; Zhang, L.; Xu, H.Y. Enhancement strategies for efficient activation of persulfate by heterogeneous cobalt-containing catalysts: A review. Chemosphere 2022, 291, 132954. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Huang, Y.; Hua, J.; Qu, W.; Xia, D.; He, C.; Sharma, V.K.; Shu, D. Overlooked self-catalytic mechanism in phenolic moiety-mediated Fenton-like system: Formation of Fe(III) hydroperoxide complex and co-treatment of refractory pollutants. Appl. Catal. B Environ. 2023, 321, 122062. [Google Scholar] [CrossRef]
- Liang, J.; Huang, W.; Wei, S.; Tian, C.; Zhang, X.; Nong, G.; Wang, S.; Song, H. Photodegradation performance and mechanism of sulfadiazine in Fe(III)-EDDS-activated persulfate system. Environ. Technol. 2022, 44, 3518–3531. [Google Scholar] [CrossRef]

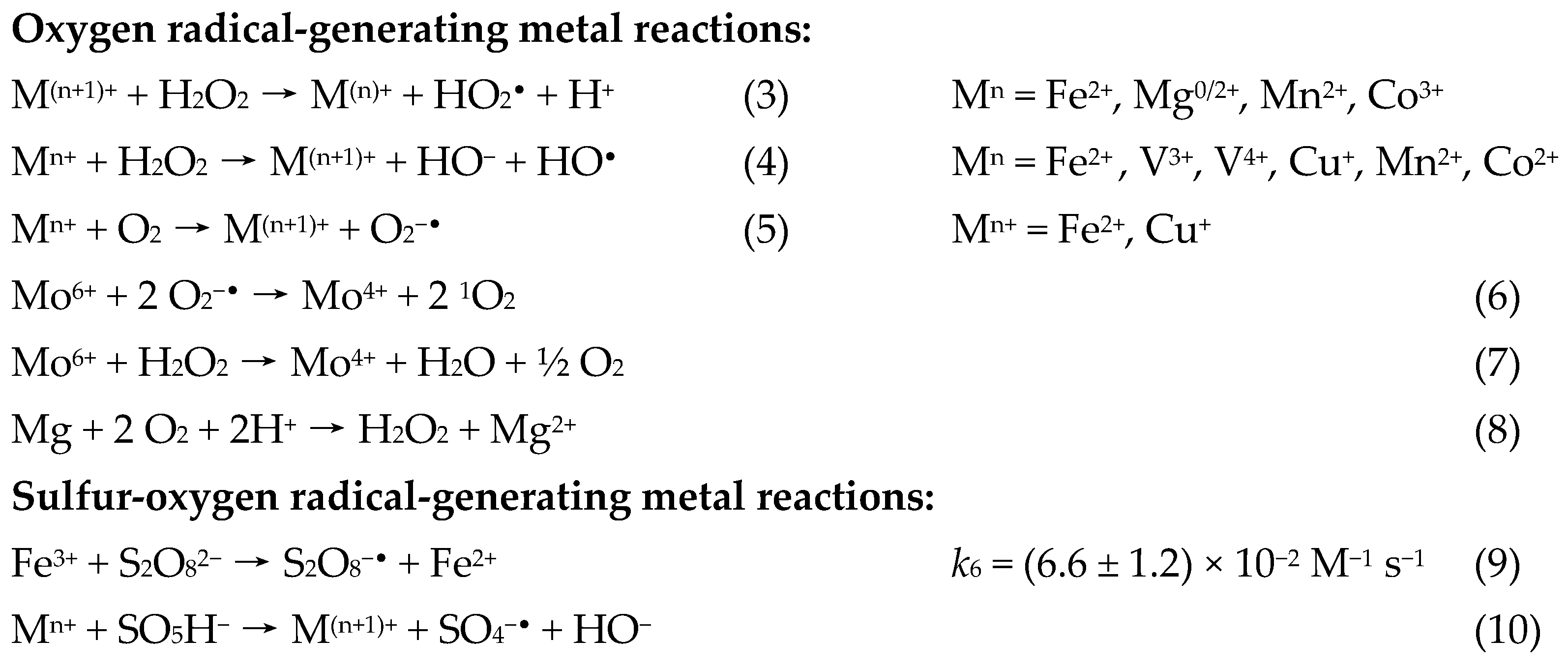


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milam, L.R.; Planalp, R.P. Bimetallic Fenton-like Catalysts in the Remediation of Dyes. Colorants 2024, 3, 1-16. https://doi.org/10.3390/colorants3010001
Milam LR, Planalp RP. Bimetallic Fenton-like Catalysts in the Remediation of Dyes. Colorants. 2024; 3(1):1-16. https://doi.org/10.3390/colorants3010001
Chicago/Turabian StyleMilam, Lydia R., and Roy P. Planalp. 2024. "Bimetallic Fenton-like Catalysts in the Remediation of Dyes" Colorants 3, no. 1: 1-16. https://doi.org/10.3390/colorants3010001






