Array-Designed Triboelectric Nanogenerator for Healthcare Diagnostics: Current Progress and Future Perspectives
Abstract
:1. Introduction
2. Array Configuration Amplifies TENG Energy Collection
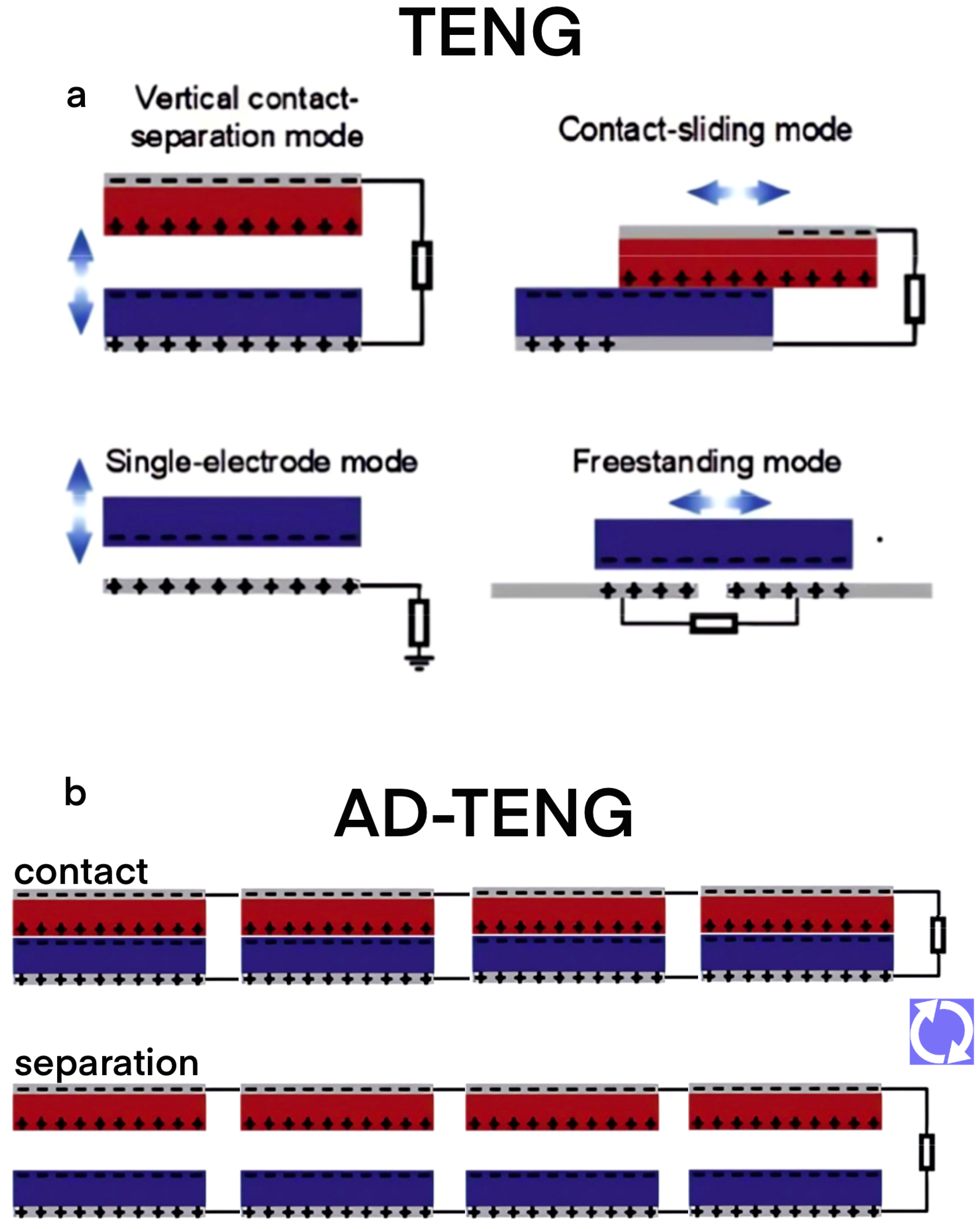
2.1. The Principle TENG
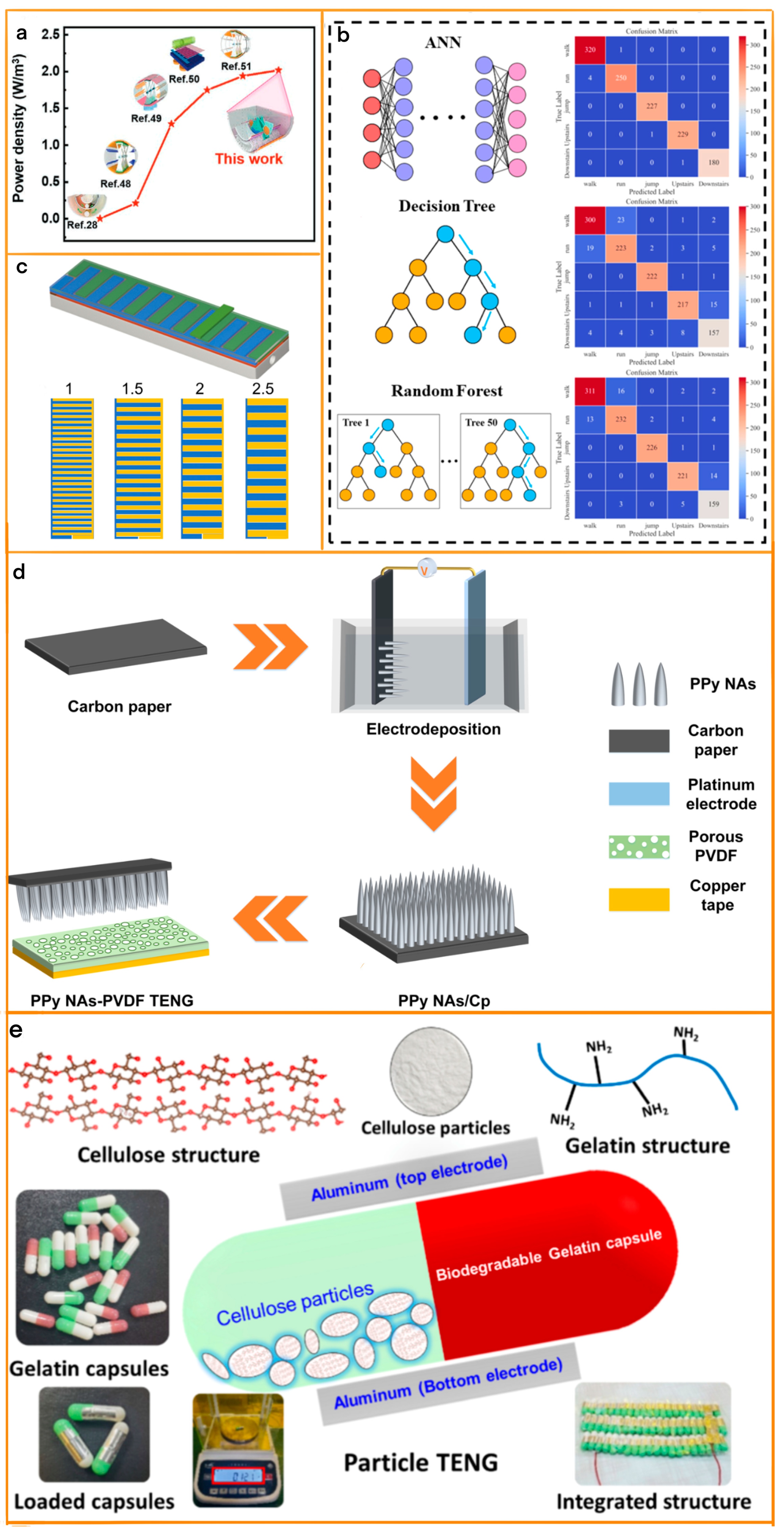
2.2. Design and Optimization of Array-Designed TENG
2.3. Improving Energy Collection Efficiency
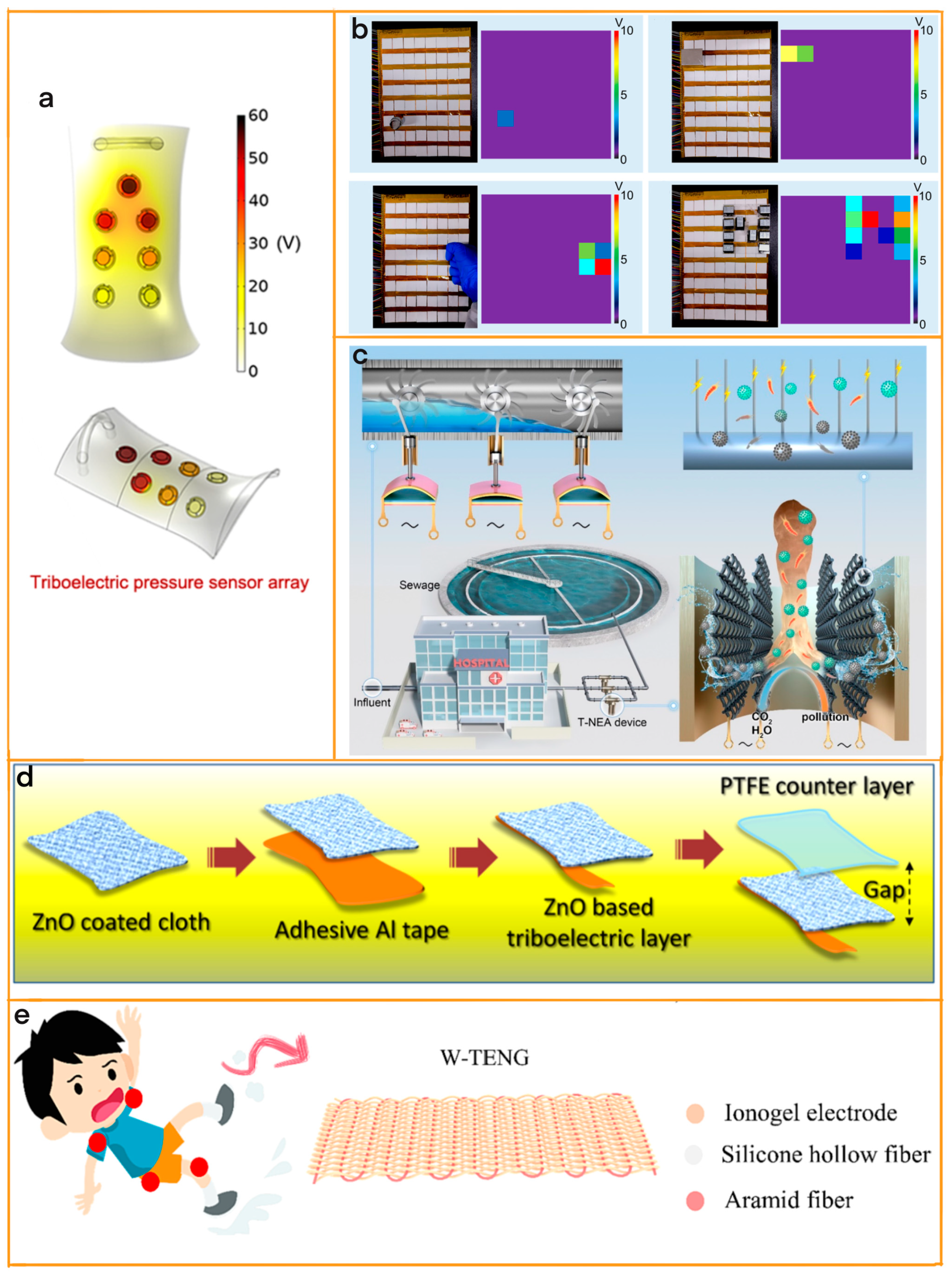
3. Array Configuration Enhances TENG Sensitivity
3.1. Matrix-Designed TENG
3.1.1. Matrix Design Enhances Motion Sensing
3.1.2. Matrix Design Enhances Touch Perception
3.2. Array Textile-Based TENG
3.2.1. Improve Motion Sensing through Array Textile Design
3.2.2. Improving Disease Prevention through Array Textile Design
4. Array Configuration Expands TENG Applications
4.1. Extreme Environmental Applications
4.2. Medical Applications
5. Conclusions and Prospect
5.1. Enhancing AD-TENG’s Power Generation Capacity
5.1.1. Material Innovations
5.1.2. Structural Optimization
5.1.3. Hybrid Energy Harvesting Systems
5.2. Enhancing the AD-TENG Detection Capability
5.2.1. Sensitivity Augmentation
5.2.2. Ensuring Bio-Safety
5.2.3. Reducing Interference and Improving Accuracy
5.3. AD-TENG in Healthcare Detection
5.3.1. Customization for Specific Medical Conditions
5.3.2. Improving Wireless Connectivity and Remote Monitoring
5.3.3. Developing Eco-Friendly and Sustainable Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bai, Q.; Liao, X.-W.; Chen, Z.-W.; Gan, C.-Z.; Zou, H.-X.; Wei, K.-X.; Gu, Z.; Zheng, X.-J. Snap-through Triboelectric Nanogenerator with Magnetic Coupling Buckled Bistable Mechanism for Harvesting Rotational Energy. Nano Energy 2022, 96, 107118. [Google Scholar] [CrossRef]
- Cheedarala, R.K.; Song, J.I. Integrated Electronic Skin (e-Skin) for Harvesting of TENG Energy through Push–Pull Ionic Electrets and Ion-Ion Hopping Mechanism. Sci. Rep. 2022, 12, 3879. [Google Scholar] [CrossRef]
- Chen, B.; Wang, Z.L. Toward a New Era of Sustainable Energy: Advanced Triboelectric Nanogenerator for Harvesting High Entropy Energy. Small 2022, 18, 2107034. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Ma, C.; Chen, W.; Xu, X.; Tang, Q. Flexible and Transparent Triboelectric Nanogenerators Based on Polyoxometalate-Modified Polydimethylsiloxane Composite Films for Harvesting Biomechanical Energy. ACS Appl. Nano Mater. 2022, 5, 15369–15377. [Google Scholar] [CrossRef]
- Feng, H.; Li, H.; Xu, J.; Yin, Y.; Cao, J.; Yu, R.; Wang, B.; Li, R.; Zhu, G. Triboelectric Nanogenerator Based on Direct Image Lithography and Surface Fluorination for Biomechanical Energy Harvesting and Self-Powered Sterilization. Nano Energy 2022, 98, 107279. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Xu, B.; Li, M.; Jiang, C.; Guan, X.; Yang, Y. Scalable Core–Spun Coating Yarn-Based Triboelectric Nanogenerators with Hierarchical Structure for Wearable Energy Harvesting and Sensing via Continuous Manufacturing. Nano Energy 2022, 91, 106672. [Google Scholar] [CrossRef]
- Arakawa, T.; Dao, D.V.; Mitsubayashi, K. Biosensors and Chemical Sensors for Healthcare Monitoring: A Review. IEEJ Trans. Electr. Electron. Eng. 2022, 17, 626–636. [Google Scholar] [CrossRef]
- Chen, X.; Xie, X.; Liu, Y.; Zhao, C.; Wen, M.; Wen, Z. Advances in Healthcare Electronics Enabled by Triboelectric Nanogenerators. Adv. Funct. Mater. 2020, 30, 2004673. [Google Scholar] [CrossRef]
- Mathew, A.A.; Vivekanandan, S. Design and Simulation of Single-Electrode Mode Triboelectric Nanogenerator-Based Pulse Sensor for Healthcare Applications Using COMSOL Multiphysics. Energy Technol. 2022, 10, 2101130. [Google Scholar] [CrossRef]
- Solanki, S.; Gupta, A.K.; Saha, U.; Krasnoslobodtsev, A.V.; Gupta, R.K.; Malhotra, B.D. Triboelectric Nanogenerator-Based Smart Biomedical Sensors for Healthcare. Sustain. Energy Technol. Assess. 2023, 57, 103233. [Google Scholar] [CrossRef]
- Xiao, X.; Xiao, X.; Nashalian, A.; Libanori, A.; Fang, Y.; Li, X.; Chen, J. Triboelectric Nanogenerators for Self-Powered Wound Healing. Adv. Healthc. Mater. 2021, 10, 2100975. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Xiao, X.; Nashalian, A.; Libanori, A.; Fang, Y.; Li, X.; Chen, J. Triboelectric Nanogenerators for Self-Powered Wound Healing (Adv. Healthcare Mater. 20/2021). Adv. Healthc. Mater. 2021, 10, 2170097. [Google Scholar] [CrossRef]
- Zhu, G.; Ren, P.; Yang, J.; Hu, J.; Dai, Z.; Chen, H.; Li, Y.; Li, Z. Self-Powered and Multi-Mode Flexible Sensing Film with Patterned Conductive Network for Wireless Monitoring in Healthcare. Nano Energy 2022, 98, 107327. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, Z.; Zhu, P.; Su, B.; Wei, C.; Tian, Y.; Zhang, Z.; Chai, H.; Liu, A.; Liang, L.; et al. A Multisensory-Feedback Tactile Glove with Dense Coverage of Sensing Arrays for Object Recognition. Chem. Eng. J. 2023, 455, 140890. [Google Scholar] [CrossRef]
- Mi, Y.; Lu, Y.; Shi, Y.; Zhao, Z.; Wang, X.; Meng, J.; Cao, X.; Wang, N. Biodegradable Polymers in Triboelectric Nanogenerators. Polymers 2023, 15, 222. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Hayakawa, J.; Iguchi, D.; Sakita, T.; Higuchi, T.; Takeyama, K.; Ohno, S.; Murata, M.; Usami, H. All-Monolithically Integrated Self-Scanning Addressable VCSEL Array for 3D Sensing. Photonics 2023, 10, 304. [Google Scholar] [CrossRef]
- Zhao, Z.; Lu, Y.; Mi, Y.; Meng, J.; Wang, X.; Cao, X.; Wang, N. Adaptive Triboelectric Nanogenerators for Long-Term Self-Treatment: A Review. Biosensors 2022, 12, 1127. [Google Scholar] [CrossRef]
- Liu, S.; Yang, W.; Liu, L.; Chen, H.; Liu, Y. Enhanced H 2 S Gas-Sensing Performance of Ni-Doped ZnO Nanowire Arrays. ACS Omega 2023, 8, 7595–7601. [Google Scholar] [CrossRef]
- Mo, M.; Yuan, H.; Zhang, J.; Wang, J.; Liu, Y.; Peng, J.; Zhao, L. Gold Nanocluster-Based Fluorescent Sensor Array for Antibiotic Sensing and Identification. Chemosensors 2023, 11, 330. [Google Scholar] [CrossRef]
- Qin, Q.; Cao, X.; Wang, N. Ball-Mill-Inspired Durable Triboelectric Nanogenerator for Wind Energy Collecting and Speed Monitoring. Nanomaterials 2023, 13, 939. [Google Scholar] [CrossRef]
- Xiang, H.; Yang, J.; Cao, X.; Wang, N. Flexible and Highly Sensitive Triboelectric Nanogenerator with Magnetic Nanocomposites for Cultural Heritage Conservation and Human Motion Monitoring. Nano Energy 2022, 101, 107570. [Google Scholar] [CrossRef]
- Liu, S.; Hu, X.; Lin, W.; Lu, Z.; Xi, S.; Gong, L.; Wang, X. Terahertz Compressed Sensing Imaging Based on Line Array Detection. Opt. Lasers Eng. 2023, 168, 107685. [Google Scholar] [CrossRef]
- Chen, X. Source Parameter Analysis Using Distributed Acoustic Sensing—An Example with the PoroTomo Array. Geophys. J. Int. 2023, 233, 2208–2214. [Google Scholar] [CrossRef]
- Li, T.; Zhu, X.; Hai, X.; Bi, S.; Zhang, X. Recent Progress in Sensor Arrays: From Construction Principles of Sensing Elements to Applications. ACS Sens. 2023, 8, 994–1016. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhang, J.A.; Huang, X.; Heath, R.W.; Guo, Y.J. Green Joint Communications and Sensing Employing Analog Multi-Beam Antenna Arrays. IEEE Commun. Mag. 2023, 61, 172–178. [Google Scholar] [CrossRef]
- Bai, G.; Zhong, X.; He, X.; Liao, C. A Compressed Sensing-based Diagnosis Method for Impaired Subarrayed Antenna Arrays. Microw. Opt. Technol. Lett. 2023, 65, 2632–2639. [Google Scholar] [CrossRef]
- Nan, X.; Xu, Z.; Cao, X.; Hao, J.; Wang, X.; Duan, Q.; Wu, G.; Hu, L.; Zhao, Y.; Yang, Z.; et al. A Review of Epidermal Flexible Pressure Sensing Arrays. Biosensors 2023, 13, 656. [Google Scholar] [CrossRef] [PubMed]
- Alzate-Carvajal, N.; Park, J.; Bargaoui, I.; Rautela, R.; Comeau, Z.J.; Scarfe, L.; Ménard, J.-M.; Darling, S.B.; Lessard, B.H.; Luican-Mayer, A. Arrays of Functionalized Graphene Chemiresistors for Selective Sensing of Volatile Organic Compounds. ACS Appl. Electron. Mater. 2023, 5, 1514–1520. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Bai, Y.; Yang, L.; Bai, J.; Sun, F.; Wang, Y.; Zhao, Y.; Li, T.; Zhang, T. Development of Flexible Tactile Sensing Arrays for Hardness Recognition. Sens. Actuators Phys. 2023, 359, 114478. [Google Scholar] [CrossRef]
- Zhao, Z.; Mi, Y.; Lu, Y.; Zhu, Q.; Cao, X.; Wang, N. From Biochemical Sensor to Wearable Device: The Key Role of the Conductive Polymer in the Triboelectric Nanogenerator. Biosensors 2023, 13, 604. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, T.; Wang, N. From Piezoelectric Nanogenerator to Non-Invasive Medical Sensor: A Review. Biosensors 2023, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Mi, Y.; Wu, T.; Cao, X.; Wang, N. From Triboelectric Nanogenerator to Polymer-Based Biosensor: A Review. Biosensors 2022, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Lu, Y.; Mi, Y.; Zhu, Q.; Meng, J.; Wang, X.; Cao, X.; Wang, N. Modular Design in Triboelectric Sensors: A Review on the Clinical Applications for Real-Time Diagnosis. Sensors 2023, 23, 4194. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Lu, Y.; Wang, X.; Zhao, Z.; Cao, X.; Wang, N. From Triboelectric Nanogenerator to Uninterrupted Power Supply System: The Key Role of Electrochemical Batteries and Supercapacitors. Batteries 2022, 8, 215. [Google Scholar] [CrossRef]
- Zhao, Z.; Lu, Y.; Mi, Y.; Meng, J.; Cao, X.; Wang, N. Structural Flexibility in Triboelectric Nanogenerators: A Review on the Adaptive Design for Self-Powered Systems. Micromachines 2022, 13, 1586. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Cao, X.; Wang, N. Triboelectric Nanogenerators in Sustainable Chemical Sensors. Chemosensors 2022, 10, 484. [Google Scholar] [CrossRef]
- Cao, S.; Zou, H.; Jiang, B.; Li, M.; Yuan, Q. Incorporation of ZnO Encapsulated MoS2 to Fabricate Flexible Piezoelectric Nanogenerator and Sensor. Nano Energy 2022, 102, 107635. [Google Scholar] [CrossRef]
- Tang, W.; Fu, C.; Xia, L.; Su, L.; Lyu, P.; Fu, Z.; Gong, J.; Li, L.; Zhang, C.; Xu, W. Biomass-Derived Multifunctional 3D Film Framed by Carbonized Loofah toward Flexible Strain Sensors and Triboelectric Nanogenerators. Nano Energy 2023, 107, 108129. [Google Scholar] [CrossRef]
- Sun, J.; Chang, Y.; Liao, J.; Chang, S.; Dai, S.; Shang, Y.; Shan, C.-X.; Dong, L. Integrated, Self-Powered, and Omni-Transparent Flexible Electroluminescent Display System. Nano Energy 2022, 99, 107392. [Google Scholar] [CrossRef]
- Luo, Y.; Zhao, T.; Dai, Y.; Li, Q.; Fu, H. Flexible Nanosensors for Non-Invasive Creatinine Detection Based on Triboelectric Nanogenerator and Enzymatic Reaction. Sens. Actuators Phys. 2021, 320, 112585. [Google Scholar] [CrossRef]
- Kim, A.; Kumar, P.; Annamalai, P.K.; Patel, R. Recent Advances in the Nanomaterials, Design, Fabrication Approaches of Thermoelectric Nanogenerators for Various Applications. Adv. Mater. Inter. 2022, 9, 2201659. [Google Scholar] [CrossRef]
- Sapkal, S.; Kandasubramanian, B.; Panda, H.S. A Review of Piezoelectric Materials for Nanogenerator Applications. J. Mater. Sci. Mater. Electron. 2022, 33, 26633–26677. [Google Scholar] [CrossRef]
- Bagheri, M.H.; Khan, A.A.; Shahzadi, S.; Rana, M.M.; Hasan, M.S.; Ban, D. Advancements and Challenges in Molecular/Hybrid Perovskites for Piezoelectric Nanogenerator Application: A Comprehensive Review. Nano Energy 2024, 120, 109101. [Google Scholar] [CrossRef]
- Chen, W.; Yang, X.; Li, D.; Li, J. Model Calibration for Compressive Sensing Based Linear Antenna Array Fault Diagnosis. J. Electromagn. Waves Appl. 2023, 37, 453–473. [Google Scholar] [CrossRef]
- Sergeeva, K.A.; Tutov, M.V.; Zhizhchenko, A.Y.; Cherepakhin, A.B.; Leonov, A.A.; Chepak, A.K.; Mironenko, A.Y.; Sergeev, A.A. Ordered Photonic Nanojet Arrays for Luminescent Optical Sensing in Liquid and Gaseous Media. Sens. Actuators B Chem. 2023, 381, 133435. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, X.; Lu, Q.; Liu, M.; Li, H.; Zhang, Y.; Liu, Y.; Yao, S. Recognition Engineering-Mediated Multichannel Sensor Array for Gut Microbiota Sensing. Anal. Chem. 2023, 95, 5911–5919. [Google Scholar] [CrossRef]
- Hu, J.; Xiao, J.-Z.; Liao, W.-M.; Liu, S.; Li, J.; He, Y.; Yu, L.; Li, Q.; Xu, G.; He, J. Secondary Interaction-Manipulated Metal–Organic Crystalline Nanotube Array for Gas Sensing. J. Mater. Chem. A 2023, 11, 11463–11470. [Google Scholar] [CrossRef]
- Sun, W.; Liu, D.; Zou, Z.; Sun, W.; Chen, S.; Kang, Y. Sense: Model-Hardware Codesign for Accelerating Sparse CNNs on Systolic Arrays. IEEE Trans. Very Large Scale Integr. VLSI Syst. 2023, 31, 470–483. [Google Scholar] [CrossRef]
- Han, C.; Cao, Z.; Yuan, Z.; Zhang, Z.; Huo, X.; Zhang, L.; Wu, Z.; Wang, Z.L. Hybrid Triboelectric-Electromagnetic Nanogenerator with a Double-Sided Fluff and Double Halbach Array for Wave Energy Harvesting. Adv. Funct. Mater. 2022, 32, 2205011. [Google Scholar] [CrossRef]
- Wang, Z.; Bu, M.; Xiu, K.; Sun, J.; Hu, N.; Zhao, L.; Gao, L.; Kong, F.; Zhu, H.; Song, J.; et al. A Flexible, Stretchable and Triboelectric Smart Sensor Based on Graphene Oxide and Polyacrylamide Hydrogel for High Precision Gait Recognition in Parkinsonian and Hemiplegic Patients. Nano Energy 2022, 104, 107978. [Google Scholar] [CrossRef]
- Dan, X.; Cao, X.; Wang, Y.; Yang, J.; Wang, Z.L.; Sun, Q. A Stereoscopically Structured Triboelectric Nanogenerator for Bending Sensors and Hierarchical Interactive Systems. ACS Appl. Nano Mater. 2023, 6, 3590–3598. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, P.; Deng, L.; Zhang, W.; Liu, B.; Ren, D.; Yang, Z. Three-Dimensional Polypyrrole Nanoarrays for Wearable Triboelectric Nanogenerators. ACS Appl. Nano Mater. 2022, 5, 11219–11228. [Google Scholar] [CrossRef]
- Saqib, Q.M.; Shaukat, R.A.; Chougale, M.Y.; Khan, M.U.; Kim, J.; Bae, J. Particle Triboelectric Nanogenerator (P-TENG). Nano Energy 2022, 100, 107475. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Wu, M.; Yao, K.; Gao, Z.; Gao, Y.; Huang, X.; Wong, T.H.; Zhou, J.; Li, D.; et al. Thin, Soft, 3D Printing Enabled Crosstalk Minimized Triboelectric Nanogenerator Arrays for Tactile Sensing. Fundam. Res. 2023, 3, 111–117. [Google Scholar] [CrossRef]
- Chang, K.-B.; Parashar, P.; Shen, L.-C.; Chen, A.-R.; Huang, Y.-T.; Pal, A.; Lim, K.-C.; Wei, P.-H.; Kao, F.-C.; Hu, J.-J.; et al. A Triboelectric Nanogenerator-Based Tactile Sensor Array System for Monitoring Pressure Distribution inside Prosthetic Limb. Nano Energy 2023, 111, 108397. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, W.; Zhang, H. A High-Performance Textile-Based Triboelectric Nanogenerator Manufactured by a Novel Brush Method for Self-Powered Human Motion Pattern Detector. Sustain. Energy Technol. Assess. 2021, 46, 101290. [Google Scholar] [CrossRef]
- Li, M.; Xu, B.; Li, Z.; Gao, Y.; Yang, Y.; Huang, X. Toward 3D Double-Electrode Textile Triboelectric Nanogenerators for Wearable Biomechanical Energy Harvesting and Sensing. Chem. Eng. J. 2022, 450, 137491. [Google Scholar] [CrossRef]
- Dong, S.; Xu, F.; Sheng, Y.; Guo, Z.; Pu, X.; Liu, Y. Seamlessly Knitted Stretchable Comfortable Textile Triboelectric Nanogenerators for E-Textile Power Sources. Nano Energy 2020, 78, 105327. [Google Scholar] [CrossRef]
- Wu, Y.; Dai, X.; Sun, Z.; Zhu, S.; Xiong, L.; Liang, Q.; Wong, M.-C.; Huang, L.-B.; Qin, Q.; Hao, J. Highly Integrated, Scalable Manufacturing and Stretchable Conductive Core/Shell Fibers for Strain Sensing and Self-Powered Smart Textiles. Nano Energy 2022, 98, 107240. [Google Scholar] [CrossRef]
- Wu, F.; Lan, B.; Cheng, Y.; Zhou, Y.; Hossain, G.; Grabher, G.; Shi, L.; Wang, R.; Sun, J. A Stretchable and Helically Structured Fiber Nanogenerator for Multifunctional Electronic Textiles. Nano Energy 2022, 101, 107588. [Google Scholar] [CrossRef]
- Cao, Y.; Shao, H.; Wang, H.; Li, X.; Zhu, M.; Fang, J.; Cheng, T.; Lin, T. A Full-Textile Triboelectric Nanogenerator with Multisource Energy Harvesting Capability. Energy Convers. Manag. 2022, 267, 115910. [Google Scholar] [CrossRef]
- Kim, M.; Park, H.; Lee, M.H.; Bae, J.W.; Lee, K.Y.; Lee, J.H.; Lee, J.-H. Stretching-Insensitive Stretchable and Biocompatible Triboelectric Nanogenerators Using Plasticized PVC Gel and Graphene Electrode for Body-Integrated Touch Sensor. Nano Energy 2023, 107, 108159. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, X.; Huang, T.; Yu, H.; Zhu, M. Interconnected Array Design for Enhancing the Performance of an Enclosed Flexible Triboelectric Nanogenerator. Nano Energy 2021, 89, 106476. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, Y.; Wang, C.; Zhou, K.; Yan, C.; Zheng, G.; Huang, J.; Dai, K.; Liu, C.; Shen, C. Ultra-Stretchable, Durable and Conductive Hydrogel with Hybrid Double Network as High Performance Strain Sensor and Stretchable Triboelectric Nanogenerator. Nano Energy 2020, 76, 105035. [Google Scholar] [CrossRef]
- Hao, Y.; Wen, J.; Gao, X.; Nan, D.; Pan, J.; Yang, Y.; Chen, B.; Wang, Z.L. Self-Rebound Cambered Triboelectric Nanogenerator Array for Self-Powered Sensing in Kinematic Analytics. ACS Nano 2022, 16, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Kou, H.; Wang, H.; Cheng, R.; Liao, Y.; Shi, X.; Luo, J.; Li, D.; Wang, Z.L. Smart Pillow Based on Flexible and Breathable Triboelectric Nanogenerator Arrays for Head Movement Monitoring during Sleep. ACS Appl. Mater. Interfaces 2022, 14, 23998–24007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, H.; Zhang, W.; Hu, Z.; Li, X.; Liu, J.; Xu, G.; Yang, C. Self-Powered Triboelectric Nanogenerator Driven Nanowires Electrode Array System for the Urine Sterilization. Nano Energy 2022, 96, 107111. [Google Scholar] [CrossRef]
- Fang, L.; Zheng, Q.; Hou, W.; Zheng, L.; Li, H. A Self-Powered Vibration Sensor Based on the Coupling of Triboelectric Nanogenerator and Electromagnetic Generator. Nano Energy 2022, 97, 107164. [Google Scholar] [CrossRef]
- Fang, H.; Guo, J.; Wu, H. Wearable Triboelectric Devices for Haptic Perception and VR/AR Applications. Nano Energy 2022, 96, 107112. [Google Scholar] [CrossRef]
- Fan, Y.-J.; Huang, M.-Z.; Hsiao, Y.-C.; Huang, Y.-W.; Deng, C.-Z.; Yeh, C.; Husain, R.A.; Lin, Z.-H. Enhancing the Sensitivity of Portable Biosensors Based on Self-Powered Ion Concentration Polarization and Electrical Kinetic Trapping. Nano Energy 2020, 69, 104407. [Google Scholar] [CrossRef]
- Fan, Y.; Li, S.; Tao, X.; Wang, Y.; Liu, Z.; Chen, H.; Wu, Z.; Zhang, J.; Ren, F.; Chen, X.; et al. Negative Triboelectric Polymers with Ultrahigh Charge Density Induced by Ion Implantation. Nano Energy 2021, 90, 106574. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhu, Q.; Lu, Y.; Mi, Y.; Cao, X.; Wang, N. Chemical Sensor Based on Piezoelectric/Triboelectric Nanogenerators: A Review of the Modular Design Strategy. Chemosensors 2023, 11, 304. [Google Scholar] [CrossRef]
- He, Y.; Tian, J.; Peng, W.; Huang, D.; Li, F.; He, Y. On the Contact Electrification Mechanism in Semiconductor–Semiconductor Case by Vertical Contact-Separation Triboelectric Nanogenerator. Nanotechnology 2023, 34, 295401. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, X.; Chen, P.; Hu, Y.; Li, L.; Sun, Z. On the Controlled Adhesive Contact and Electrical Performance of Vertical Contact-Separation Mode Triboelectric Nanogenerators with Micro-Grooved Surfaces. Nano Energy 2021, 85, 106037. [Google Scholar] [CrossRef]
- Ji, S.; Fu, T.; Hu, Y. Effect of Surface Texture on the Output Performance of Lateral Sliding-Mode Triboelectric Nanogenerator. J. Phys. Conf. Ser. 2020, 1549, 042095. [Google Scholar] [CrossRef]
- Saeed, A.; Liu, Y.; Shah, M.Z.; Wang, L.; Wang, Q.-G. Sliding Mode Lateral Guidance and Control of Finless Airship. J. Aerosp. Eng. 2022, 35, 04021131. [Google Scholar] [CrossRef]
- Manjari Padhan, A.; Hajra, S.; Sahu, M.; Nayak, S.; Joon Kim, H.; Alagarsamy, P. Single-Electrode Mode TENG Using Ferromagnetic NiO-Ti Based Nanocomposite for Effective Energy Harvesting. Mater. Lett. 2022, 312, 131644. [Google Scholar] [CrossRef]
- Seo, J.; Hajra, S.; Sahu, M.; Kim, H.J. Effect of Cilia Microstructure and Ion Injection upon Single-Electrode Triboelectric Nanogenerator for Effective Energy Harvesting. Mater. Lett. 2021, 304, 130674. [Google Scholar] [CrossRef]
- Chen, J.; Wang, P.; Li, J.; Wang, C.; Wang, J.; Zhang, D.; Peng, Y.; Wang, B.; Wu, Z. Self-Powered Antifouling UVC Pipeline Sterilizer Driven by the Discharge Stimuli Based on the Modified Freestanding Rotary Triboelectric Nanogenerator. Nano Energy 2022, 95, 106969. [Google Scholar] [CrossRef]
- Paosangthong, W.; Wagih, M.; Torah, R.; Beeby, S. Textile-Based Triboelectric Nanogenerator with Alternating Positive and Negative Freestanding Woven Structure for Harvesting Sliding Energy in All Directions. Nano Energy 2022, 92, 106739. [Google Scholar] [CrossRef]
- Du, S.; Suo, H.; Xie, G.; Lyu, Q.; Mo, M.; Xie, Z.; Zhou, N.; Zhang, L.; Tao, J.; Zhu, J. Self-Powered and Photothermal Electronic Skin Patches for Accelerating Wound Healing. Nano Energy 2022, 93, 106906. [Google Scholar] [CrossRef]
- Dong, L.; Wang, M.; Wu, J.; Zhu, C.; Shi, J.; Morikawa, H. Stretchable, Adhesive, Self-Healable, and Conductive Hydrogel-Based Deformable Triboelectric Nanogenerator for Energy Harvesting and Human Motion Sensing. ACS Appl. Mater. Interfaces 2022, 14, 9126–9137. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Chung, S.-H.; Lin, Z.-H.; Jin, Y.; Hong, J.; Lee, S. Dielectric Liquid-Based Self-Operating Switch Triboelectric Nanogenerator for Current Amplification via Regulating Air Breakdown. Nano Energy 2021, 88, 106292. [Google Scholar] [CrossRef]
- He, T.; Redoute, J.-M.; Lee, C.; Yuce, M.R. Flexible Forearm Triboelectric Sensors for Parkinson’s Disease Diagnosing and Monitoring. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 4909–4912. [Google Scholar]
- Abd El Hamid, M.M.; Shaheen, M.; Mabrouk, M.S.; Omar, Y.M.K. Machine learning for detecting epistasis interactions and its relevance to personalized medicine in alzheimer’s disease: Systematic review. Biomed. Eng. Appl. Basis Commun. 2021, 33, 2150047. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, Y.; Lang, J.; Li, L.; Zhang, Y. Triboelectric Nanogenerator and Artificial Intelligence to Promote Precision Medicine for Cancer. Nano Energy 2022, 92, 106783. [Google Scholar] [CrossRef]
- Cinquanta, L.; Infantino, M.; Bizzaro, N. Detecting Autoantibodies by Multiparametric Assays: Impact on Prevention, Diagnosis, Monitoring, and Personalized Therapy in Autoimmune Diseases. J. Appl. Lab. Med. 2022, 7, 137–150. [Google Scholar] [CrossRef]
- Di Meco, A.; Vassar, R. Early Detection and Personalized Medicine: Future Strategies against Alzheimer’s Disease. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2021; Volume 177, pp. 157–173. ISBN 978-0-12-824143-1. [Google Scholar]
- Qiu, H.-J.; Song, W.-Z.; Wang, X.-X.; Zhang, J.; Fan, Z.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. A Calibration-Free Self-Powered Sensor for Vital Sign Monitoring and Finger Tap Communication Based on Wearable Triboelectric Nanogenerator. Nano Energy 2019, 58, 536–542. [Google Scholar] [CrossRef]
- Huang, G.-W.; Li, N.; Xiao, H.-M.; Feng, Q.-P.; Fu, S.-Y. A Paper-Based Touch Sensor with an Embedded Micro-Probe Array Fabricated by Double-Sided Laser Printing. Nanoscale 2017, 9, 9598–9605. [Google Scholar] [CrossRef]
- Shlomy, I.; Divald, S.; Tadmor, K.; Leichtmann-Bardoogo, Y.; Arami, A.; Maoz, B.M. Restoring Tactile Sensation Using a Triboelectric Nanogenerator. ACS Nano 2021, 15, 11087–11098. [Google Scholar] [CrossRef]
- Zou, X.H. Advances in Pathogen Diagnosis of Respiratory Infection Diseases in 2021. Chin. J. Tuberc. Respir. Dis. 2022, 45, 78–82. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Dong, Z.; Wang, Z.; Yang, Z.; Yu, X.; Chang, L. Micro/Nano Biomedical Devices for Point-of-Care Diagnosis of Infectious Respiratory Diseases. Med. Nov. Technol. Devices 2022, 14, 100116. [Google Scholar] [CrossRef] [PubMed]
- Vera Anaya, D.; Yuce, M.R. Stretchable Triboelectric Sensor for Measurement of the Forearm Muscles Movements and Fingers Motion for Parkinson’s Disease Assessment and Assisting Technologies. Med. Devices Sens. 2021, 4, e10154. [Google Scholar] [CrossRef]
- Vásquez, V.; Orozco, J. Detection of COVID-19-Related Biomarkers by Electrochemical Biosensors and Potential for Diagnosis, Prognosis, and Prediction of the Course of the Disease in the Context of Personalized Medicine. Anal. Bioanal. Chem. 2022, 415, 1003–1031. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Alveal, C.; Olsen, T.R.; Worgall, T.S. Personalized Immunoglobulin Aptamers for Detection of Multiple Myeloma Minimal Residual Disease in Serum. Commun. Biol. 2020, 3, 781. [Google Scholar] [CrossRef]
- Lorenzo, E.; Evangelista, L.S. Disparities in Cardiovascular Disease: Examining the Social Determinants of Health. Eur. J. Cardiovasc. Nurs. 2022, 21, 187–189. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Wang, Y.; Wang, H.; Shen, X.; Zhang, M.; Lu, H.; He, M.; Zhang, Y. Bioinspired Fluffy Fabric with In Situ Grown Carbon Nanotubes for Ultrasensitive Wearable Airflow Sensor. Adv. Mater. 2020, 32, 1908214. [Google Scholar] [CrossRef]
- Wang, R.; Mu, L.; Bao, Y.; Lin, H.; Ji, T.; Shi, Y.; Zhu, J.; Wu, W. Holistically Engineered Polymer–Polymer and Polymer–Ion Interactions in Biocompatible Polyvinyl Alcohol Blends for High-Performance Triboelectric Devices in Self-Powered Wearable Cardiovascular Monitorings. Adv. Mater. 2020, 32, 2002878. [Google Scholar] [CrossRef]
- Wang, S.; Liu, S.; Zhou, J.; Li, F.; Li, J.; Cao, X.; Li, Z.; Zhang, J.; Li, B.; Wang, Y.; et al. Advanced Triboelectric Nanogenerator with Multi-Mode Energy Harvesting and Anti-Impact Properties for Smart Glove and Wearable e-Textile. Nano Energy 2020, 78, 105291. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, D.; Tang, M.; Zhang, H.; Chen, F.; Wang, T.; Li, Z.; Zhao, P. Rotating Triboelectric-Electromagnetic Nanogenerator Driven by Tires for Self-Powered MXene-Based Flexible Wearable Electronics. Chem. Eng. J. 2022, 446, 136914. [Google Scholar] [CrossRef]
- Su, C.; Huang, X.; Zhang, L.; Zhang, Y.; Yu, Z.; Chen, C.; Ye, Y.; Guo, S. Robust Superhydrophobic Wearable Piezoelectric Nanogenerators for Self-Powered Body Motion Sensors. Nano Energy 2023, 107, 108095. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Gao, Z.; Liang, L.; Wang, X.; Liu, X.; Wu, Y.; Zheng, H. Modular Design and Fully Packed Triboelectric Nanogenerator Based on Escapement Mechanism for Harvesting High Entropy Energy in Harsh Environments. Nano Energy 2023, 109, 108266. [Google Scholar] [CrossRef]
- Rodrigues, C.; Kumar, M.; Proenca, M.P.; Gutierrez, J.; Melo, R.; Pereira, A.; Ventura, J. Triboelectric Energy Harvesting in Harsh Conditions: Temperature and Pressure Effects in Methane and Crude Oil Environments. Nano Energy 2020, 72, 104682. [Google Scholar] [CrossRef]
- Zhao, L.; Li, H.; Meng, J.; Li, Z. The Recent Advances in Self-powered Medical Information Sensors. InfoMat 2020, 2, 212–234. [Google Scholar] [CrossRef]
- Yokota, T.; Fukuda, K.; Someya, T. Recent Progress of Flexible Image Sensors for Biomedical Applications. Adv. Mater. 2021, 33, 2004416. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Zou, Y.; Rao, W.; Gai, Y.; Xue, J.; Wu, L.; Qu, X.; Liu, Y.; Xu, G.; et al. A Multi-Mode Triboelectric Nanogenerator for Energy Harvesting and Biomedical Monitoring. Nano Energy 2022, 92, 106715. [Google Scholar] [CrossRef]
- Baro, B.; Khimhun, S.; Das, U.; Bayan, S. ZnO Based Triboelectric Nanogenerator on Textile Platform for Wearable Sweat Sensing Application. Nano Energy 2023, 108, 108212. [Google Scholar] [CrossRef]
- Zhong, X.; Song, T.; Dong, H.; Jiang, S.; Wei, R. Ionogel Based Triboelectric Nanogenerator Textiles for High-Precision Human Fall Recognition. Chem. Eng. J. 2023, 474, 145686. [Google Scholar] [CrossRef]





| Data | Size | Energy Sources | Outputs | Applications | Working Modes |
|---|---|---|---|---|---|
| 2022 [49] | None | Vibration | 16.96 W m−3 | Wave Energy Collection | Lateral sliding |
| 2022 [50] | 5 cm × 5 cm | Movement | 26 mW | Gait Recognition | Contact–separation |
| 2023 [51] | None | Movement | 6 nA. | Finger Bending Sensing | Contact–separation |
| 2022 [52] | 2 cm × 2 cm | Movement | 3 μA | Body Motion Sensing | Contact–separation |
| 2022 [53] | None | Vibration | 85 V | Energy Collection | Contact–separation |
| 2023 [54] | 7.5 cm × 7.5 cm | Vibration | 0.11 V/kPa | Pressure Sensing | Contact–separation |
| 2023 [55] | 1 cm × 1 cm | Movement | 15 nA | Tactile Sensing | Contact–separation |
| 2021 [56] | 2 cm × 2 cm | Vibration | 51.2 V | Body Motion Sensing | Contact–separation |
| 2022 [57] | 2.8 cm × 3 cm | Vibration | 200.93 mW/m2 | Energy Collection | Contact–separation |
| 2020 [58] | 8 cm × 8 cm | Vibration | 7531 μW/m2 | Energy Collection | Contact–separation |
| 2022 [59] | 4 cm × 4 cm | Movement | 469 mW/m2 | Energy Collection | Contact–separation |
| 2022 [60] | None | Movement | 52 V | Energy Collection | Contact–separation |
| 2022 [61] | 8 cm × 8 cm | Movement | 138.55 mW/m2 | Body Motion Sensing | Contact–separation |
| 2023 [62] | 2 cm × 2 cm | Movement | 48 V | Body Energy Collection | Contact–separation |
| 2021 [63] | 3 cm × 3 cm | Vibration | 12 μA | Energy Collection | Contact–separation |
| 2020 [64] | None | Movement | 26.9 μA | Energy Collection | Contact–separation |
| 2022 [65] | None | Movement | 1.25 mW/m2 | Dangerous Motion Sensing | Contact–separation |
| 2022 [66] | 2 cm × 2 cm | Vibration | 64 V | Sleep State Sensing | Contact–separation |
| 2022 [67] | None | Vibration | 230 V | Sterilization | Contact–separation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Zhu, Q.; Wang, Y.; Shoaib, M.; Cao, X.; Wang, N. Array-Designed Triboelectric Nanogenerator for Healthcare Diagnostics: Current Progress and Future Perspectives. J. Low Power Electron. Appl. 2024, 14, 7. https://doi.org/10.3390/jlpea14010007
Zhao Z, Zhu Q, Wang Y, Shoaib M, Cao X, Wang N. Array-Designed Triboelectric Nanogenerator for Healthcare Diagnostics: Current Progress and Future Perspectives. Journal of Low Power Electronics and Applications. 2024; 14(1):7. https://doi.org/10.3390/jlpea14010007
Chicago/Turabian StyleZhao, Zequan, Qiliang Zhu, Yifei Wang, Muhammad Shoaib, Xia Cao, and Ning Wang. 2024. "Array-Designed Triboelectric Nanogenerator for Healthcare Diagnostics: Current Progress and Future Perspectives" Journal of Low Power Electronics and Applications 14, no. 1: 7. https://doi.org/10.3390/jlpea14010007






