Impact of pH and Ionic Molar Ratios on Phosphorous Forms Precipitation and Recovery from Different Wastewater Sludges
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Struvite
2.2. Sludge Characteristics
2.3. Equipment and Analytical Tools
2.4. Chemical Equilibrium Modeling for SI Calculation
2.5. Extended-Aeration Sludge Experimental Setup
3. Results
3.1. Synthetic Struvite
3.2. Analysis of Precipitates
3.2.1. Anaerobic Digestion Sludge
3.2.2. Denitrification Sludge
3.2.3. Extended Aeration Sludge
3.3. Effect of Different Reaction Parameters on Oxidation Sludge
3.3.1. Chemical Equilibrium Modeling
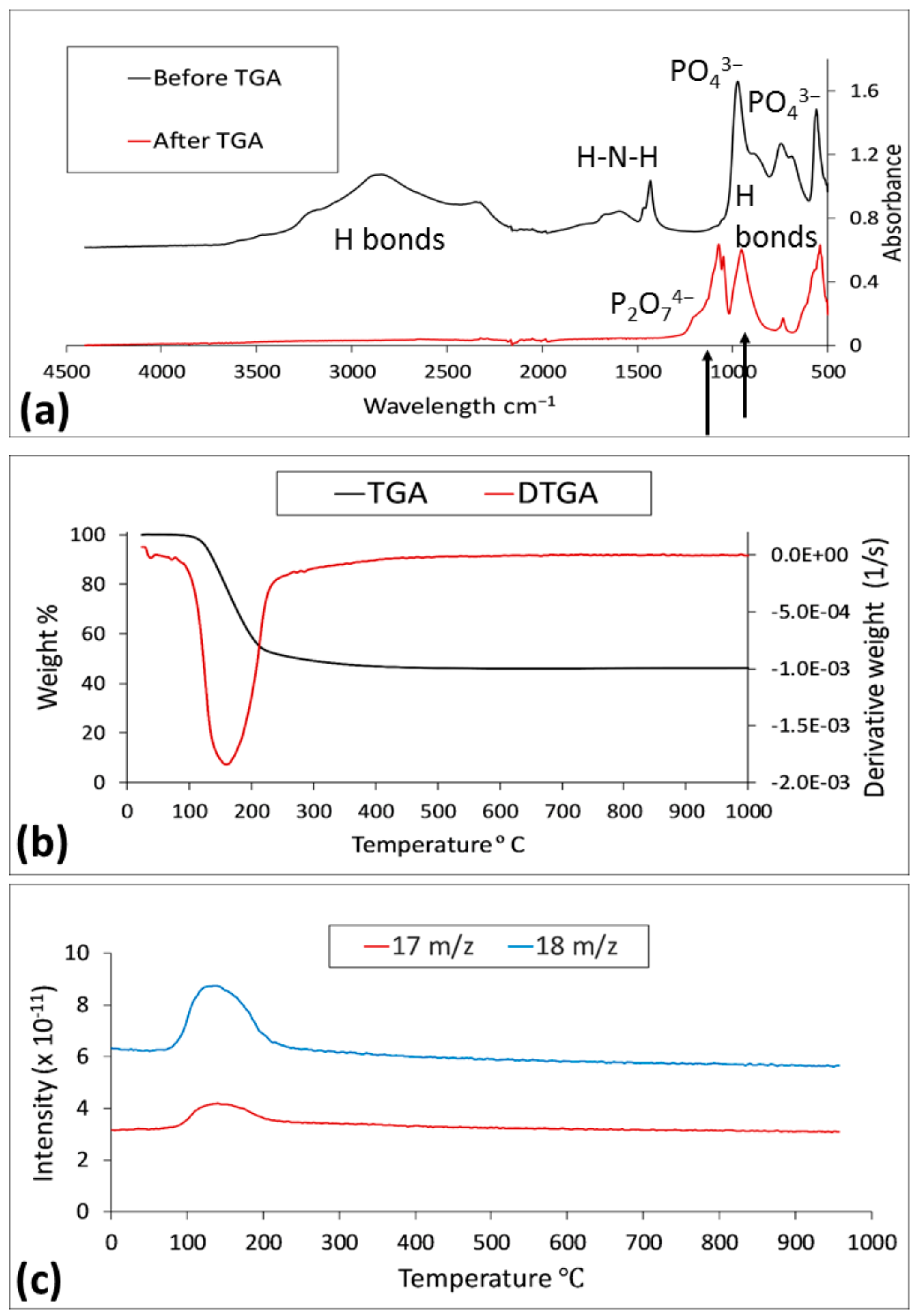
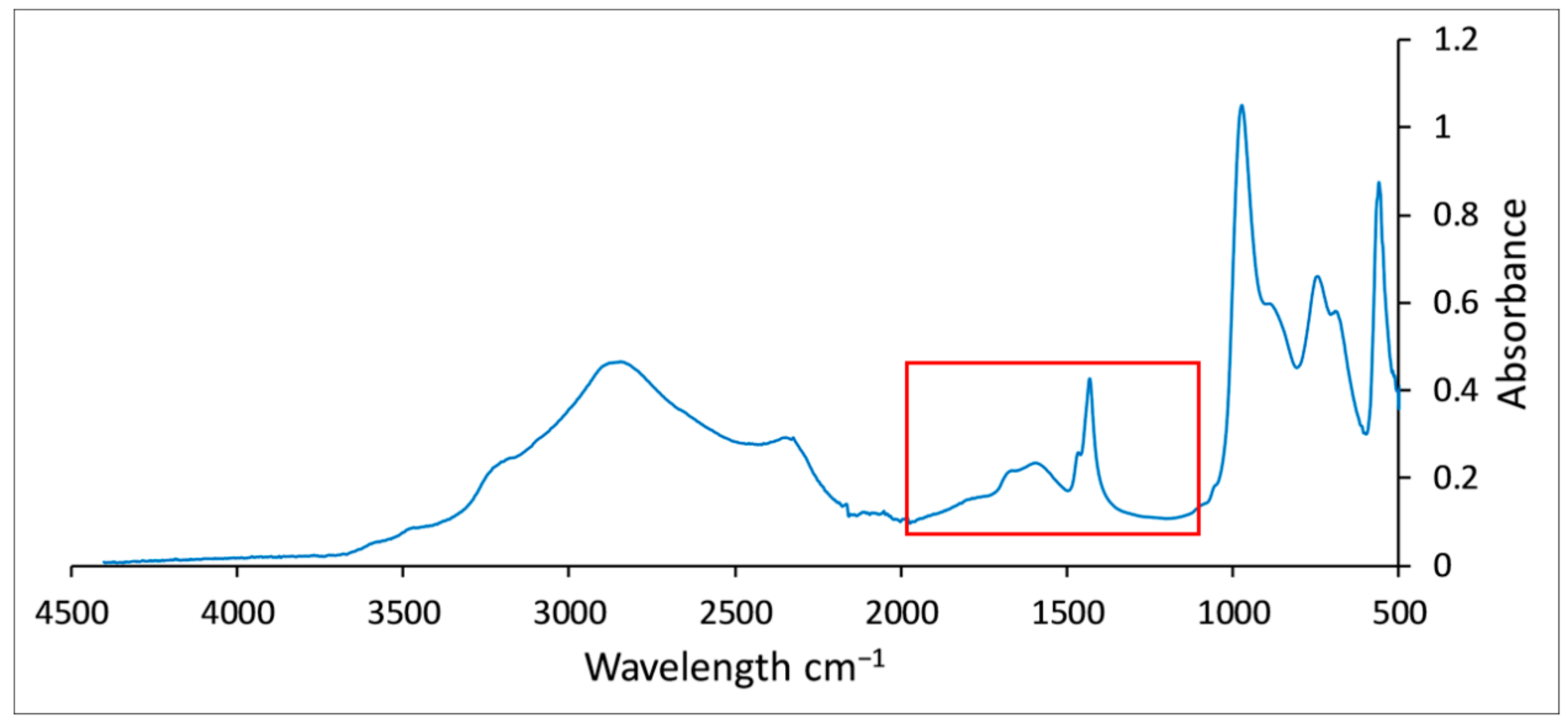
3.3.2. FTIR Analysis Results
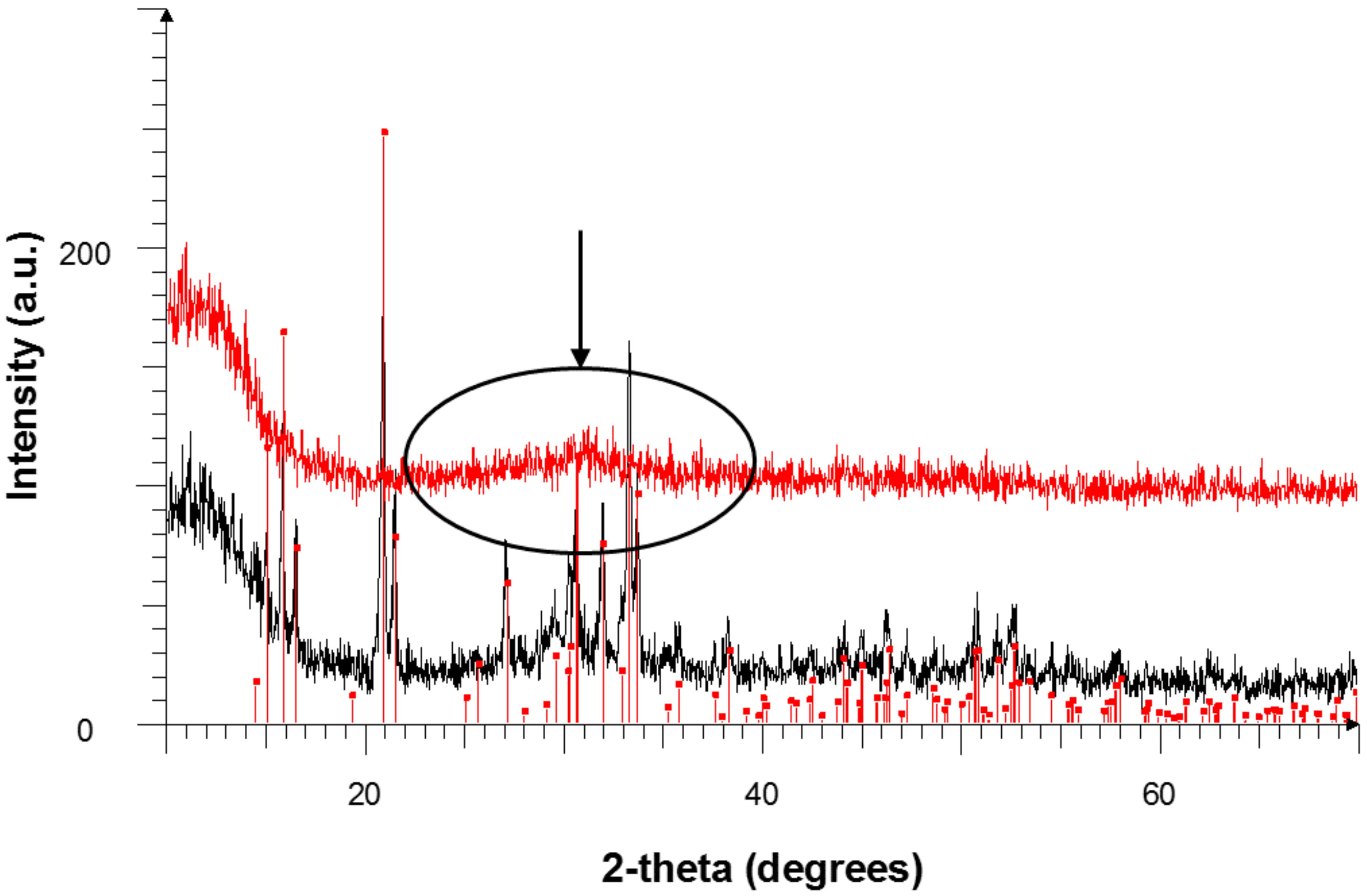
3.3.3. XRD Results
3.3.4. ICP-AES and Elemental Analysis Results
3.4. Using Ca(OH)2 as pH Adjustment Reagent
4. Discussion
4.1. Effect of pH
4.2. Effect of Ca:Mg and N:P Molar Ratios
4.3. Using Ca(OH)2 for pH Adjustment
4.4. Use of Final Precipitates as Fertilizers
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Daneshgar, S.; Callegari, A.; Capodaglio, A.G.; Vaccari, D. The Potential Phosphorus Crisis: Resource Conservation and Possible Escape Technologies: A Review. Resources 2018, 7, 37. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Cooper, J.; Lombardi, R.; Boardman, D.; Carliell-Marquet, C. The future distribution and production of global phosphate rock reserves. Resour. Conserv. Recycl. 2011, 57, 78–86. [Google Scholar] [CrossRef]
- Bendoricchio, G.; Di Luzio, M.; Baschieri, P.; Capodaglio, A.G. Diffuse pollution in the Lagoon of Venice. Water Sci. Technol. 1993, 28, 69–78. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Muraca, A.; Becchi, G. Accounting for water quality effects of future urbanization: Diffuse pollution loads estimates and control in Mantua’s Lakes (Italy). Water Sci. Technol. 2003, 47, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Lyu, T.; Mortimer, R. Comment: Closing phosphorus cycle from natural waters: Re-capturing phosphorus through an integrated water-energy-food strategy. J. Environ. Sci. 2018, 65, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Amann, A.; Zoboli, O.; Krampe, J.; Rechberger, H.; Zessner, M.; Egle, L. Environmental impacts of phosphorus recovery from municipal wastewater. Resour. Conserv. Recycl. 2018, 130, 127–139. [Google Scholar] [CrossRef]
- Egle, L.; Rechberger, H.; Krampe, J.; Zessner, M. Phosphorus recovery from municipal wastewater: An integrated comparative technological, environmental and economic assessment of P recovery technologies. Sci. Total Environ. 2016, 571, 522–542. [Google Scholar] [CrossRef] [PubMed]
- Desmidt, E.; Ghyselbrecht, K.; Zhang, Y.; Pinoy, L.; Van Der Bruggen, B.; Verstraete, W.; Meesschaert, B. Global phosphorus scarcity and full-scale P-recovery techniques: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 336–384. [Google Scholar] [CrossRef]
- Morse, G.K.; Brett, S.W.; Guy, J.A.; Lester, J.N. Phosphorus removal and recovery technologies. Sci. Total. Environ. 1998, 212, 69–81. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lal, R. Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kizito, S.; Luo, H.; Wu, S.; Ajmal, Z.; Lv, T.; Dong, R. Phosphate recovery from liquid fraction of anaerobic digestate using four slow pyrolyzed biochars: Dynamics of adsorption, desorption and regeneration. J. Environ. Manag. 2017, 201, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Daneshgar, S.; Buttafava, A.; Callegari, A.; Capodaglio, A.G. Simulations and laboratory tests for assessing phosphorus recovery efficiency from sewage sludge. Resources 2018, 7, 54. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Phosphorus Recovery from Wastewater by Struvite Crystallization: A Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef] [Green Version]
- Rahaman, M.S.; Mavinic, D.S.; Meikleham, A.; Ellis, N. Modeling phosphorus removal and recovery from anaerobic digester supernatant through struvite crystallization in a fluidized bed reactor. Water Res. 2014, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.D.; Parsons, S.A. Struvite formation, control and recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef]
- Galbraith, S.C.; Schneider, P.A.; Flood, A.E. Model-driven experimental evaluation of struvite nucleation, growth and aggregation kinetics. Water Res. 2014, 56, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey Techniques and Methods; USGS: Denver, CO, USA, 2013; 497p. Available online: http://pubs.usgs.gov/tm/06/a43 (accessed on 30 October 2017).
- Çelen, I.; Buchanan, J.R.; Burns, R.T.; Bruce Robinson, R.; Raj Raman, D. Using a chemical equilibrium model to predict amendments required to precipitate phosphorus as struvite in liquid swine manure. Water Res. 2007, 41, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Musvoto, E.V.; Wentzel, M.C.M.; Ekama, G.A.M. Integrated Chemical—Physical Processes Modelling II. Simulating Aeration Treatment of Anaerobic Digester Supernatants. Water Res. 2000, 34, 1868–1880. [Google Scholar] [CrossRef]
- Mamais, D.; Pitt, P.A.; Cheng, Y.W.; Loiacono, J.; Jenkins, D.; Wen, Y. Digesters Determination to control in anaerobic of ferric chloride precipitation digesters dose struvite sludge. Water Environ. Res. 2012, 66, 912–918. [Google Scholar] [CrossRef]
- Abbona, F.; Madsen, H.E.L.; Boistelle, R. The initial phases of calcium and magnesium phosphates precipitated from solutions of high to medium concentrations. J. Cryst. Growth 1986, 74, 581–590. [Google Scholar] [CrossRef]
- Taylor, A.W.; Frazier, A.W.; Gurney, E.L. Solubility products of magnesium ammonium and magnesium potassium phosphates. Trans. Faraday Soc. 1963, 59, 1580. [Google Scholar] [CrossRef]
- Battistoni, P.; Paci, B.; Fatone, F.; Pavan, P. Phosphorus removal from supernatants at low concentration using packed and fluidized-bed reactors. Ind. Eng. Chem. Res. 2005, 44, 6701–6707. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoo, B.H.; Lim, S.J.; Kim, T.H.; Kim, S.K.; Kim, J.Y. Development and validation of an equilibrium model for struvite formation with calcium co-precipitation. J. Cryst. Growth 2013, 372, 129–137. [Google Scholar] [CrossRef]
- Lu, X.; Leng, Y. Theoretical analysis of calcium phosphate precipitation in simulated body fluid. Biomaterials 2005, 26, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, M.S.-A.; Nancollas, G.H. The Role of Brushite and Octacalcium Phosphate in Apatite Formation. Crit. Rev. Oral Boil. Med. 1992, 3, 61–82. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry; Wiley Interscience: New York, NY, USA, 1981. [Google Scholar]
- Nordstorm, D.K.; Plummer, N.L.; Langmuir, D.; Busenberg, E.; May, H.M.; Jones, B.F.; Parkhurst, D.L. Revised chemical and equilibrium data for major water-mineral reactions and their limitations. Chem. Model. Aqueous Syst. II 1990, 416, 398–413. [Google Scholar] [CrossRef]
- Murray, K.; May, P.M. Joint Expert Speciation System (JESS). An International Computer System for Determining Chemical Speciation and Non-Aqueous Environments; Supplied by Murdoch University, Murdoch 6150, Western Australia and the Division of Water Technology, CSIR, PO BOX 395; JESS: Pretoria, South Africa, 1996; Available online: http://jess.murdoch.edu.au/jess_home.htm (accessed on 5 November 2017).
- Levin, G.V.; Shapiro, J. Metabolic uptake of phosphorus by wastewater organism. J. Water Pollut. Control Fed. 1965, 37, 800–821. [Google Scholar]
- Barnard, J. A review of biological phosphorus removal in the activated sludge process. Water SA 1976, 2, 136–144. [Google Scholar]
- United States Environmental Protection Agency (EPA). Method 365.3: Phosphorus, All Forms (Colorimetric, Ascorbic Acid, Two Reagent). 1978. Available online: https://www.epa.gov/sites/production/files/2015-08/.../method_365-3_1978.pdf (accessed on 15 February 2016).
- US Geological Survey. PHREEQC (Version 3)—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. Available online: https://wwwbrr.cr.usgs.gov/projects/GWC_coupled/phreeqc/) (accessed on 30 October 2017).
- Soptrajanov, B.; Stefov, V.; Lutz, H.D.; Engelen, B. Infrared and Raman Spectra of Magnesium Ammonium Phosphate Hexahydrate (struvite) and its Isomorphous Analogues. Spectrosc. Emerg. Mater. 2004, 689, 299–308. [Google Scholar]
- Stefov, V.; Abdija, Z.; Najdoski, M.; Koleva, V.; Petruševski, V.M.; Runčevski, T.; Soptrajanov, B. Infrared and Raman spectra of magnesium ammonium phosphate hexahydrate (struvite) and its isomorphous analogues. IX: Spectra of protiated and partially deuterated cubic magnesium cesium phosphate hexahydrate. Vib. Spectrosc. 2013, 68, 122–128. [Google Scholar] [CrossRef]
- Bhuiyan, M.I.H.; Mavinic, D.S.; Koch, F.A. Thermal decomposition of struvite and its phase transition. Chemosphere 2008, 70, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; McMurdie, H.F.; Evans, E.H.; Paretzkin, B.; Parker, H.S.; Wong-Ng, W.; Gladhill, D.M. Standard X-ray Diffraction Powder Patterns Section 21—Data for 92 Substances; International Center for Diffraction Data: Newtown Square, PA, USA, 1985. [Google Scholar]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. Infrared Spectrosc. Mater. Sci. Eng. Technol. 2012, 6, 123–149. [Google Scholar] [CrossRef]
- Dubberke, W.; Marks, V.J. Thermogravimetric Analysis of Carbonate Aggregate; Transportation Research Board: Washington, DC, USA, 1992; pp. 38–43. Available online: http://trid.trb.org/view.aspx?id=370975 (accessed on 20 March 2018).
- Destainville, A.; Rolo, A.; Champion, E.; Bernache-Assollant, D. Synthesis and characterization of beta tricalcium phosphate. Key Eng. Mater. 2003, 240–242, 489–492. [Google Scholar] [CrossRef]
- Mandel, S.; Tas, A.C. Brushite (CaHPO4·2H2O) to Octacalcium Phosphate (Ca8(HPO4)2(PO4)4·5H2O) Transformation in DMEM Solutions at 36.5 °C. Mater. Sci. Eng. 2010, 30, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Gu, Q.; Swedlund, P.J.; Marchesseau, S.; Hemar, Y. X-ray diffraction investigation of amorphous calcium phosphate and hydroxyapatite under ultra-high hydrostatic pressure. Int. J. Miner. Met. Mater. 2015, 22, 1225–1231. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Impact of calcium on struvite crystal size, shape and purity. J. Cryst. Growth 2005, 283, 514–522. [Google Scholar] [CrossRef] [Green Version]
- Crutchik, D.; Garrido, J.M. Struvite crystallization versus amorphous magnesium and calcium phosphate precipitation during the treatment of a saline industrial wastewater. Water Sci. Technol. 2011, 64, 2460–2467. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, O.; Hirooka, K. Removal and recovery of phosphorus as struvite from swine wastewater using microbial fuel cell. Bioresour. Technol. 2012, 114, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Capdevielle, A.; Sýkorová, E.; Biscans, B.; Béline, F.; Daumer, M.L. Optimization of struvite precipitation in synthetic biologically treated swine wastewater-Determination of the optimal process parameters. J. Hazard. Mater. 2013, 244–245, 357–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, J.D.; Philp, R.; Churchley, J.; Parsons, S.A. Analysis of struvite precipitation in real and synthetic liquors. Process. Saf. Environ. Prot. 2000, 78, 480–488. [Google Scholar] [CrossRef]
- Uysal, A.; Yilmazel, Y.D.; Demirer, G.N. The determination of fertilizer quality of the formed struvite from effluent of a sewage sludge anaerobic digester. J. Hazard. Mater. 2010, 181, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xu, C.; Zhang, W. Removal of nutrients from piggery wastewater using struvite precipitation and pyrogenation technology. Bioresour. Technol. 2011, 102, 2523–2528. [Google Scholar] [CrossRef] [PubMed]
- Muhmood, A.; Wu, S.; Lu, J.; Ajmal, Z.; Luo, H.; Dong, R. Nutrient recovery from anaerobically digested chicken slurry via struvite: Performance optimization and interactions with heavy metals and pathogens. Sci. Total Environ. 2018, 635, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, J.; Wang, S.; Jiang, Y.; Xiao, D.; Ding, L.; Gao, F. Nutrients removal from swine wastewater by struvite precipitation recycling technology with the use of Mg3(PO4)2 as active component. Ecol. Eng. 2016, 92, 111–118. [Google Scholar] [CrossRef]
- Song, Y.; Yuan, P.; Zheng, B.; Peng, J.; Yuan, F.; Gao, Y. Nutrients removal and recovery by crystallization of magnesium ammonium phosphate from synthetic swine wastewater. Chemosphere 2007, 69, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Shih, K. Effects of calcium and ferric ions on struvite precipitation: A new assessment based on quantitative X-ray diffraction analysis. Water Res. 2016, 95, 310–318. [Google Scholar] [CrossRef] [PubMed]
- LopezValero, I.; GomezLorente, C.; Boistelle, R. Effects of sodium and ammonium ions on occurrence, evolution and crystallinity of calcium phosphates. J. Cryst. Growth 1992, 121, 297–304. [Google Scholar] [CrossRef]
- Szögi, A.A.; Vanotti, M.B. Removal of phosphorus from livestock effluents. J. Environ. Qual. 2009, 38, 576. [Google Scholar] [CrossRef] [PubMed]
- Vanotti, M.B.; Szogi, A.A.; Hunt, P. Extraction of soluble phosphorus from swine wastewater. Trans. ASAE 2003, 46, 1665–1674. [Google Scholar] [CrossRef]
- Hosni, K.; Ben Moussa, S.; Chachi, M.; Ben Amor, M. The removal of PO43− by calcium hydroxide from synthetic wastewater: Optimization of the operating conditions. Desalination 2008, 223, 337–343. [Google Scholar] [CrossRef]
- Da Cruz, S.G.; Monte, D.M.; Dutra, A.J.B. Electroflotation of precipitated phosphate from synthetic solution. Braz. J. Chem. Eng. 2017, 34, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Muster, T.H.; Douglas, G.B.; Sherman, N.; Seeber, A.; Wright, N.; Guzukara, Y. Towards effective phosphorus recycling from wastewater: Quantity and quality. Chemosphere 2013, 91, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, Y.; Clark, T.A.; Pearce, P.; Parsons, S.A. Potential phosphorus recovery by struvite formation. Water Res. 2002, 36, 1834–1842. [Google Scholar] [CrossRef]
- Dockhorn, T. About the economy of phosphorus recovery. In International Conference on Nutrient Recovery from Wastewater Streams, Vancouver, Canada; IWA Publishing: London, UK, 2009; pp. 145–158. ISBN 9781843392323. [Google Scholar]
- Johnston, A.E.; Richards, I.R. Effectiveness of different precipitated phosphate as phosphorus source for plants. Soil Use Manag. 2003, 19, 45–49. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Bashan, Y. Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res. 2004, 38, 4222–4246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.C.; Chang, C.J. Precipitation flotation of phosphate from water. Colloids Surf. A Physicochem. Eng. Asp. 2009, 34, 215–219. [Google Scholar] [CrossRef]
- Forrest, A.L.; Fattah, K.P.; Mavinic, D.S.; Koch, F.A. Optimizing struvite production for phosphate recovery in WWTP. J. Environ. Eng. 2008, 134, 395–402. [Google Scholar] [CrossRef]
- Woods, N.C.; Sock, S.M.; Diagger, G.T. Phosphorus recovery technology modeling and feasibility evaluation for municipal wastewater treatment plants. Environ. Technol. 1999, 20, 743–748. [Google Scholar] [CrossRef]
- Mayer, B.K.; Baker, L.; Boyer, T.H.; Drechsel, P.; Gifford, M.; Hanjra, M.A.; Parameswaran, P.; Stoltzfus, J.; Westerhoff, P.; Rittmann, B.E. Total value of phosphorus recovery. Environ. Sci. Technol. 2016, 50, 6606–6620. [Google Scholar] [CrossRef] [PubMed]











| Solid Phase | Reaction | Condition | pKsp (25 °C) | Reference |
|---|---|---|---|---|
| Struvite | Mg2+ + NH4+ + PO43− + 6H2O ↔ MgNH4PO4·6H2O | 7 < pH < 11 | 13.26 | [24] |
| Newberyite | Mg2+ + HPO42− +3H2O ↔ MgHPO4·3H2O | High Mg2+/P, pH < 6 | 5.8 | [21] |
| Bobierrite | 3Mg2+ + 2PO43− + 8H2O ↔ Mg3(PO4)2·8H2O | Days to precipitate | 25.2 | [21] |
| Hydroxyapatite (HAP) | 10Ca2+ + 6PO43− +2OH− ↔ Ca10(PO4)6(OH)2 | Slow formation from ACP, DCPD | 58.62 | [25,26] |
| Tricalcium phosphate (TCP) | 3Ca2+ + 2PO43− ↔ Ca3(PO4)2 | Slow formation from ACP, DCPD | 32.63 | [21] |
| Octacalcium phosphate (OCP) | 8Ca2+ + 2HPO42− + 4PO43− ↔ Ca8(HPO4)2(PO4)4 | Hydrolysis of DCPD at pH = 5–6 | 36.48 | [27] |
| Monetite (DCP) | Ca2+ + HPO42− ↔ CaHPO4 | Fast formation from ACP, DCPD | 6.81 | [28] |
| Brushite (DCPD) | Ca2+ + HPO42− + 2H2O ↔ CaHPO4·2H2O | pH < 7 | 6.6 | [29] |
| Amorphous calcium phosphate (ACP) | 3Ca2+ + 2PO43− + xH2O ↔ Ca3(PO4)2·xH2O | pH > 6 | 25.46 | [21] |
| Calcite | Ca2+ + CO32− ↔ CaCO3 | Stable at 25 °C and atmospheric P | 8.42–8.22–8.48 | [21,29,30,31] |
| Magnesite | Mg2+ + CO32− ↔ MgCO3 | Stable at pH < 10.7 | 7.46–8.2 | [29,31] |
| Brucite | Mg2+ + 2OH− ↔ Mg(OH)2 | pH > 9.5 | 11.16 | [29,30] |
| Ca(OH)2 | Ca2+ + 2OH− ↔ Ca(OH)2 | pH > 9.5 | 5.2 | [21,30] |
| Constituent Ions | Extended Oxidation | After Anaerobic Digestion | Denitrification Pond |
|---|---|---|---|
| (mg/L) | (mg/L) | (mg/L) | |
| Ca2+ | 101 | 30.9 | 66.4 |
| Mg2+ | 26.4 | 17.0 | 24.2 |
| P | 37 | 8.1 ± 1.5 | 1.66 ± 0.3 |
| NH4+ | 32.6 | 1510 ± 340 | 1.70 ± 0.38 |
| Mg2+:NH4+:P | ||
|---|---|---|
| 3:3:1 | 3:1:1 | 1:3:1 |
| 5:5:1 | 5:1:1 | 1:5:1 |
| Mg:NH4:P | ||||||
|---|---|---|---|---|---|---|
| 5:5:1–3:3:1 | 5:1:1–3:1:1 | 1:5:1–1:3:1 | ||||
| pH | P rem.% | FTIR 1200–2000 cm−1 | P rem.% | FTIR 1200–2000 cm−1 | P rem.% | FTIR 1200–2000 cm−1 |
| 8.5 | 65.1 |  | 53.4 |  | 51.2 |  |
| 49.7 | 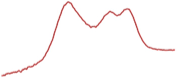 | 52.3 |  | 41.2 |  | |
| 9.0 | 85.8 | 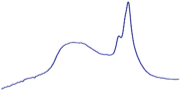 | 81.0 | 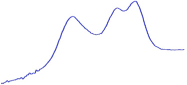 | 76.2 |  |
| 78.5 |  | 79.7 | 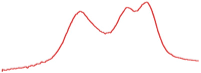 | 76.9 | 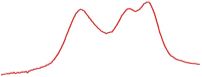 | |
| 9.5 | 93.2 | 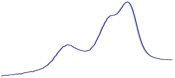 | 94.6 |  | 90.2 | 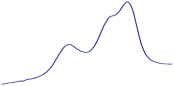 |
| 91.8 | 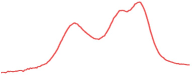 | 91.1 | 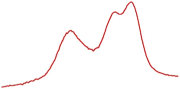 | 86.3 | 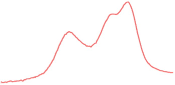 | |
| Mg:NH4:P | ||
|---|---|---|
| Without Additives | ||
| pH | P Removal % | FTIR 1200–2000 cm−1 |
| 8.5 | 54.4 |  |
| 9.0 | 76.7 | 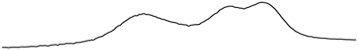 |
| 9.5 | 94.5 |  |
| Using Ca(OH)2 | ||||
|---|---|---|---|---|
| Mg:NH4:P | ||||
| 5:5:1 | 3:3:1 | |||
| pH | P Removal % | FTIR 1200–2000 cm−1 | P Removal % | FTIR 1200–2000 cm−1 |
| 8.5 | 68.1 |  | 71.7 |  |
| 9.0 | 89.5 | 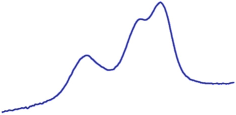 | 88.4 | 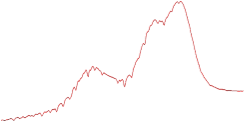 |
| 9.5 | 99.2 |  | 99.6 | 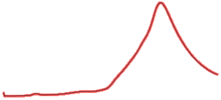 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daneshgar, S.; Buttafava, A.; Capsoni, D.; Callegari, A.; Capodaglio, A.G. Impact of pH and Ionic Molar Ratios on Phosphorous Forms Precipitation and Recovery from Different Wastewater Sludges. Resources 2018, 7, 71. https://doi.org/10.3390/resources7040071
Daneshgar S, Buttafava A, Capsoni D, Callegari A, Capodaglio AG. Impact of pH and Ionic Molar Ratios on Phosphorous Forms Precipitation and Recovery from Different Wastewater Sludges. Resources. 2018; 7(4):71. https://doi.org/10.3390/resources7040071
Chicago/Turabian StyleDaneshgar, Saba, Armando Buttafava, Doretta Capsoni, Arianna Callegari, and Andrea G. Capodaglio. 2018. "Impact of pH and Ionic Molar Ratios on Phosphorous Forms Precipitation and Recovery from Different Wastewater Sludges" Resources 7, no. 4: 71. https://doi.org/10.3390/resources7040071






