Lipase-Catalyzed Synthesis of Kojic Acid Derivative in Bioreactors and the Analysis of Its Depigmenting and Antioxidant Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
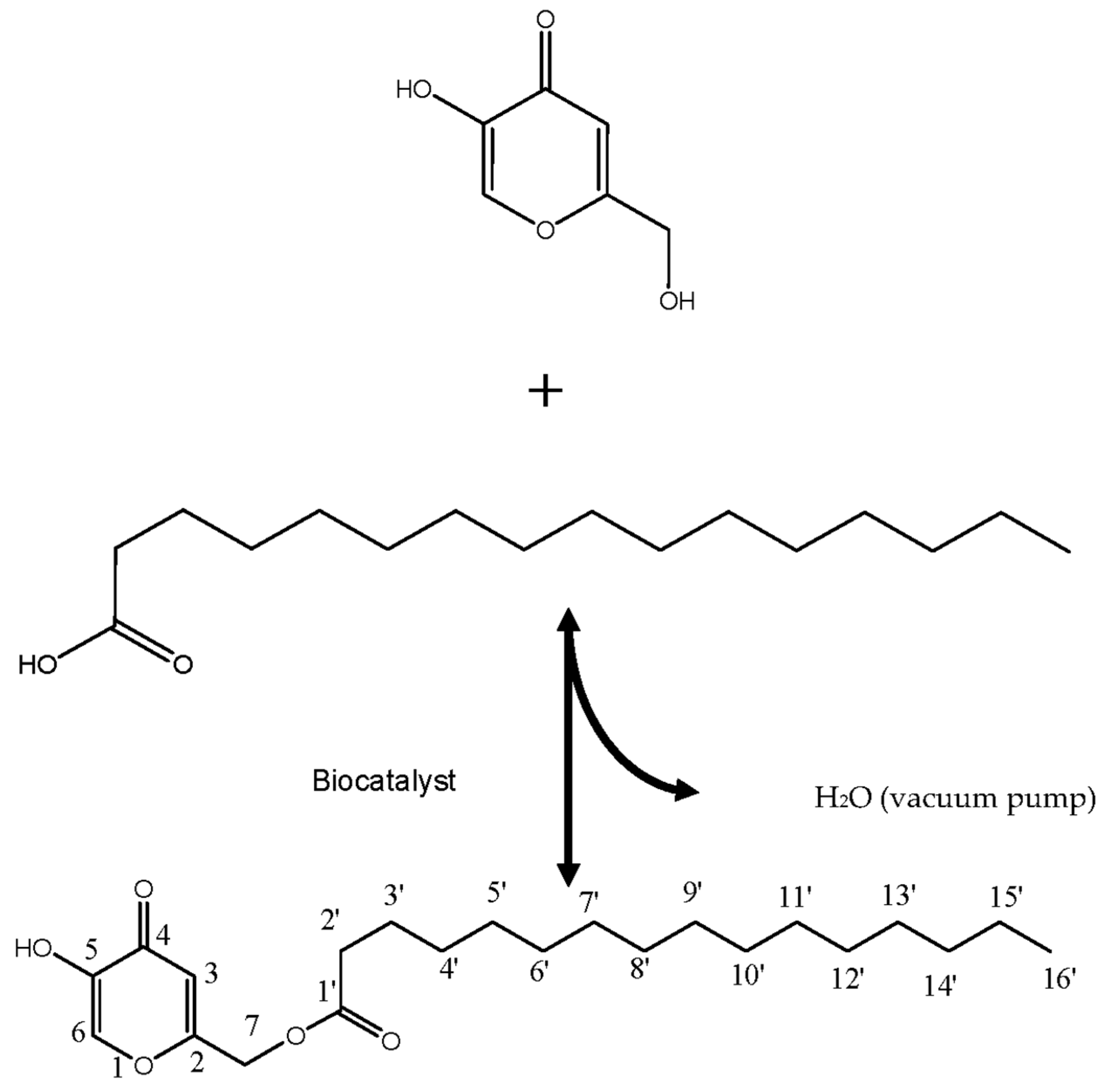
2.2. Enzymatic Synthesis of KAD in STR
2.3. Fluidized Tank Reactor (FTR)
2.4. Packed Bed Reactor (PBR)
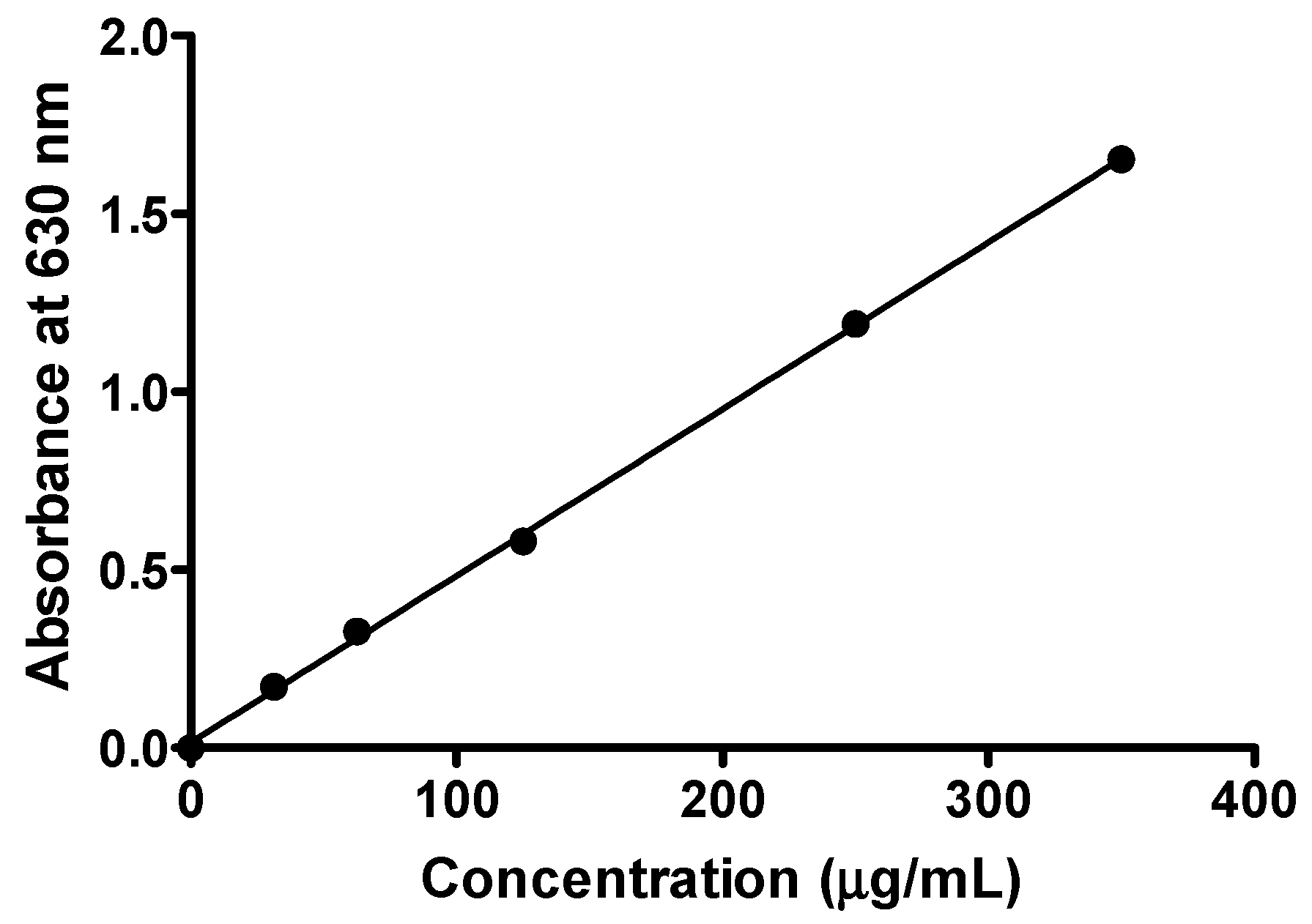
2.5. Conversion and Analysis
2.6. Purification and Molecular Structure
2.7. Rheological Behaviour
- K is the flow consistency index (SI units Pa·sn),
- γ is the shear rate or the velocity gradient perpendicular to the plane of shear (SI unit s−1), and
- n is the flow behavior index (dimensionless) represents an apparent or effective viscosity as a function of the shear rate (SI unit Pa·s).
2.8. Cell Viability
2.9. Melanin Contents
2.10. Tyrosinase Activity
2.11. FRAP
2.12. Lipid Peroxidation
2.13. Statistics
3. Results
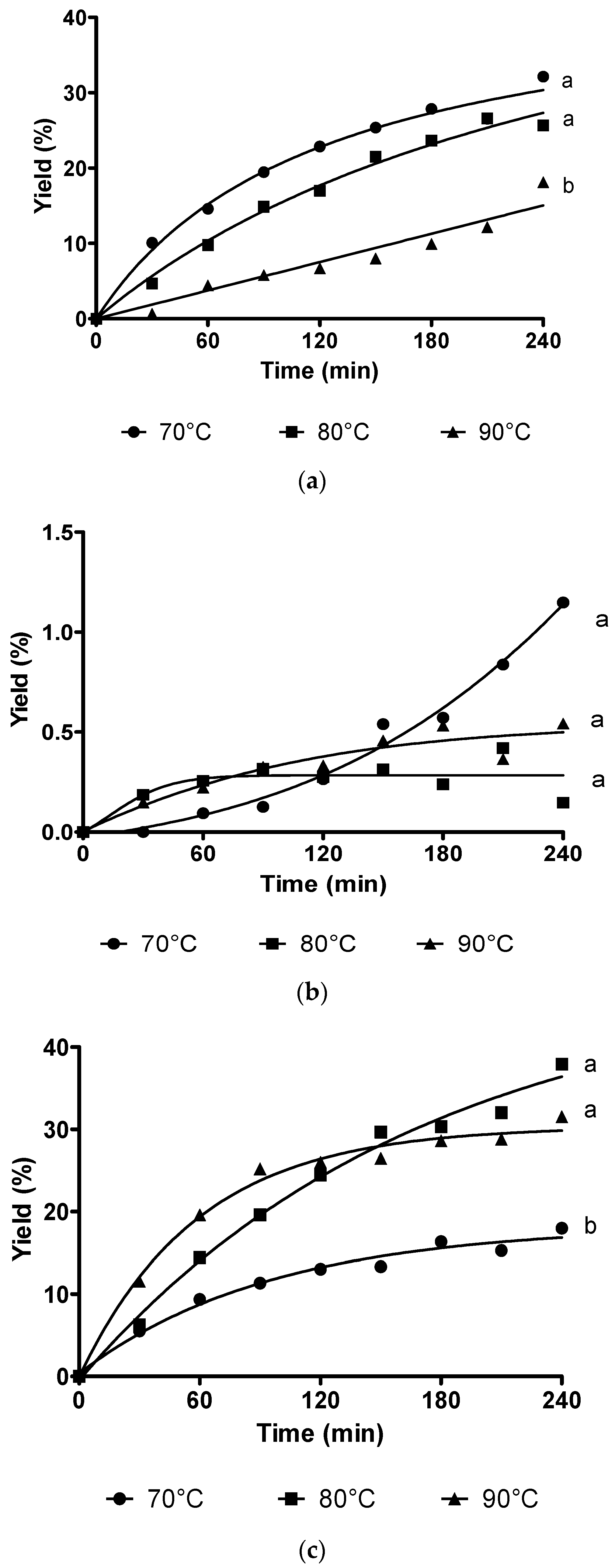
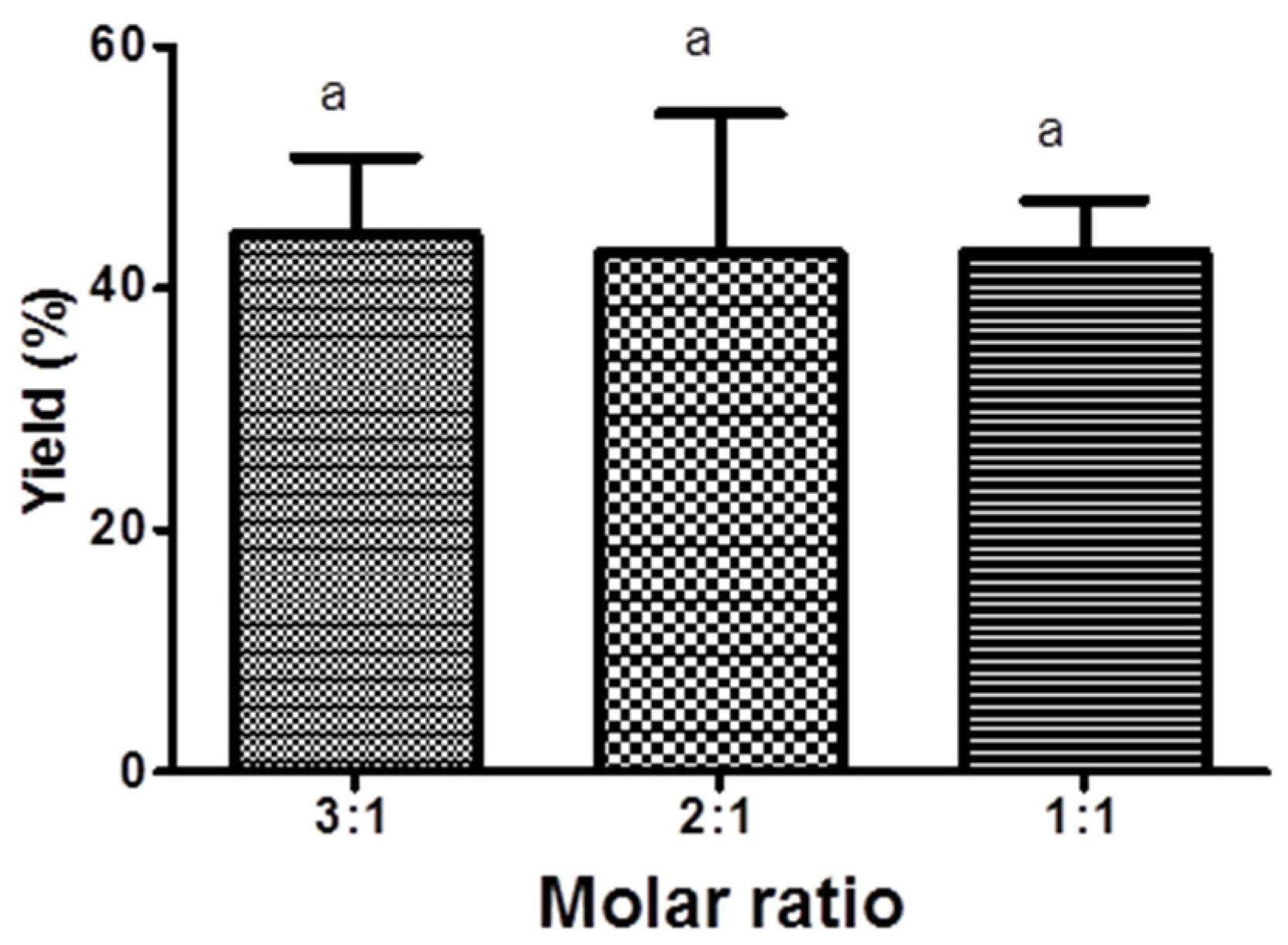
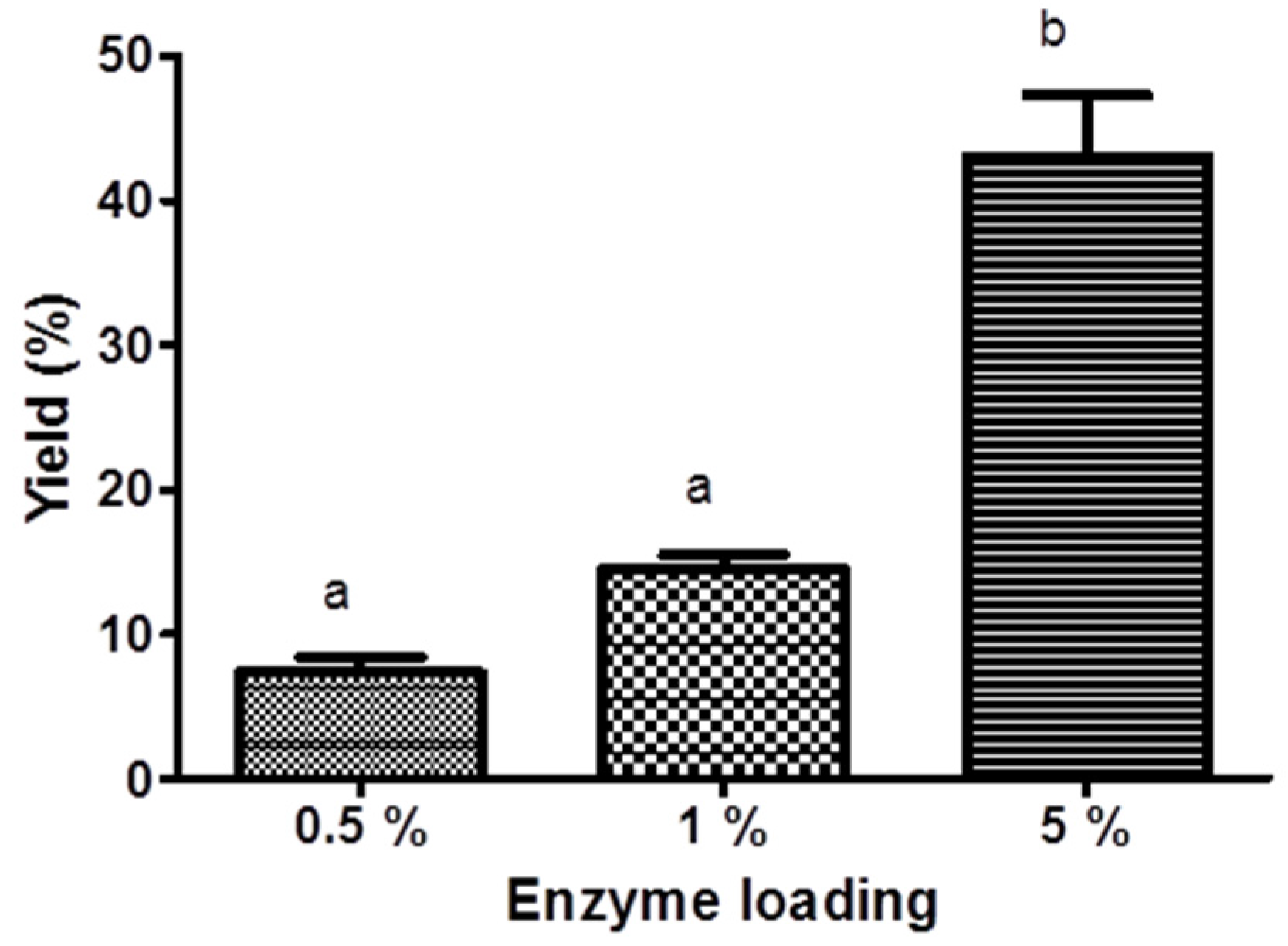
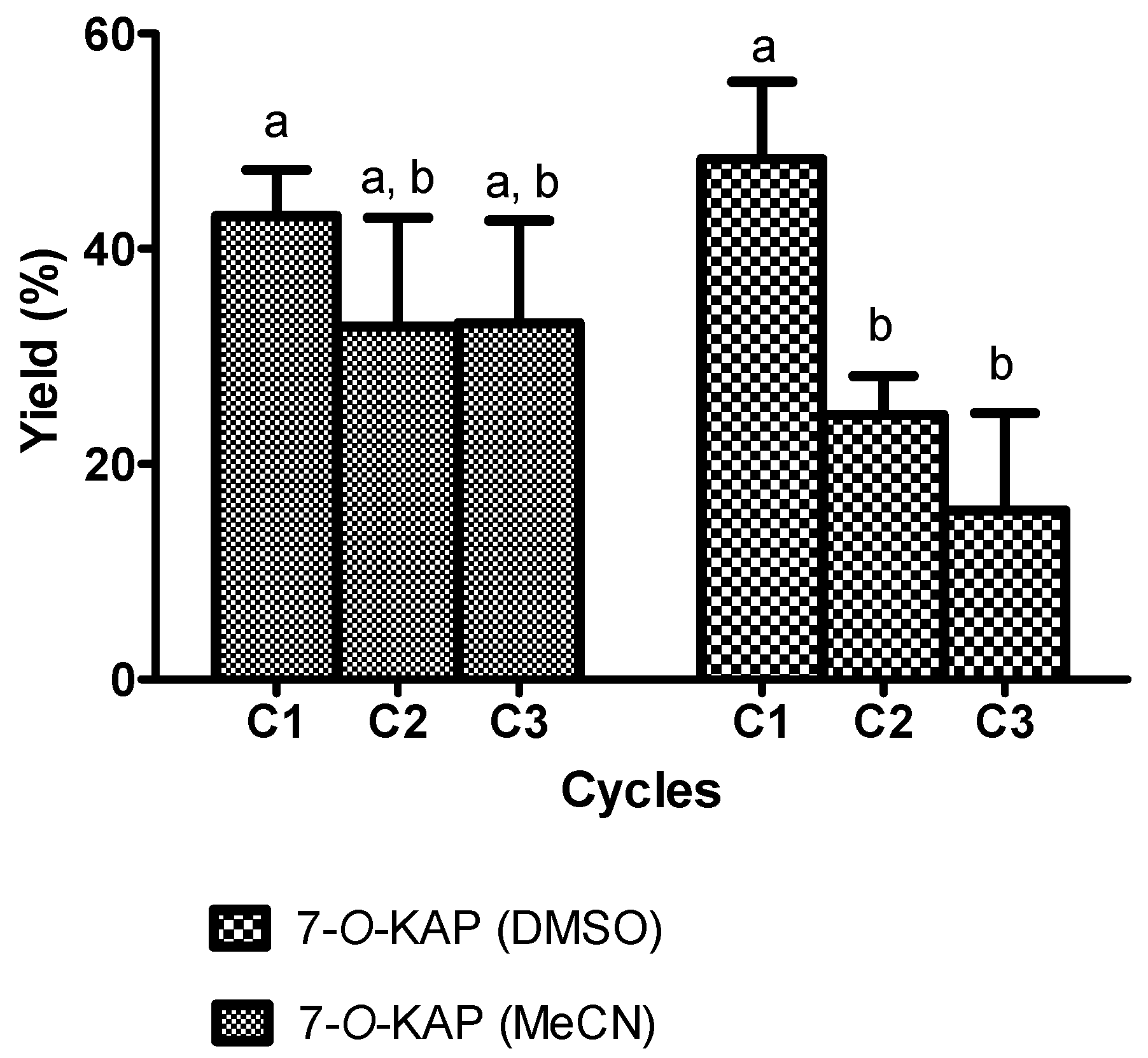
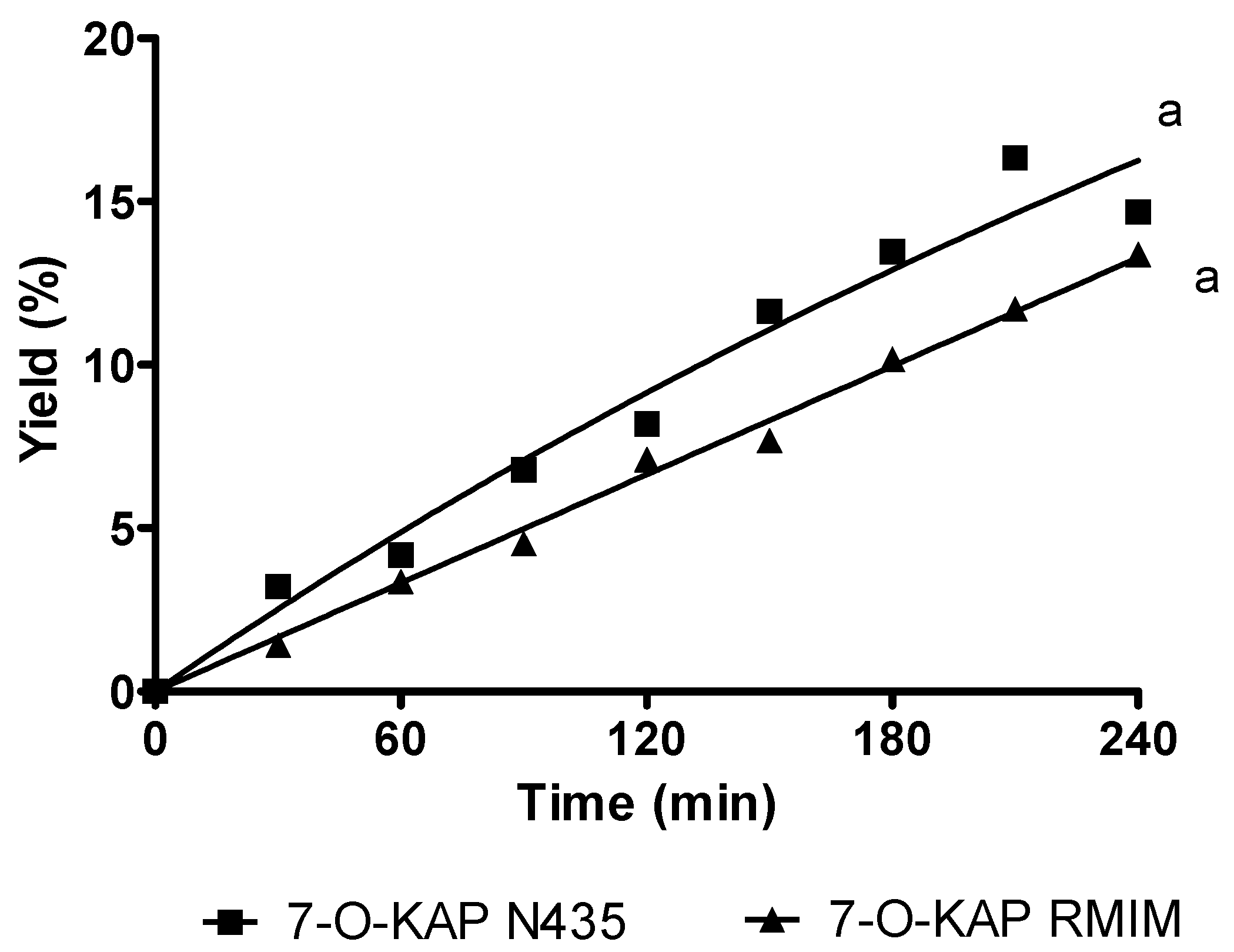
3.1. Bioreactors
3.1.1. Stirred Tank Reactor
3.1.2. Fluidized Tank Reactor and Packed Bed Reactor
3.2. Characterization of KAD
3.3. Rheological Behaviour
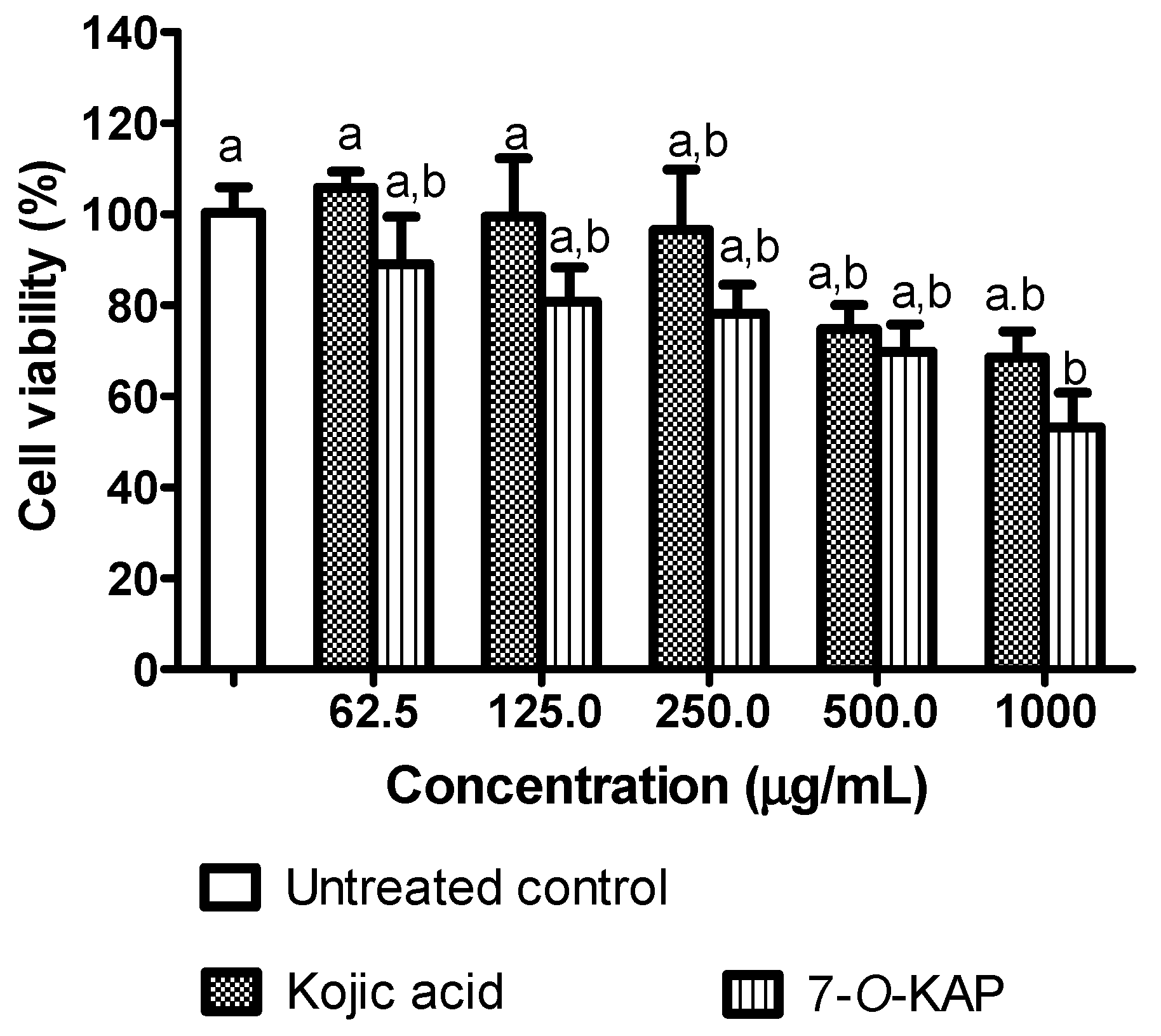
3.4. Cell Viability
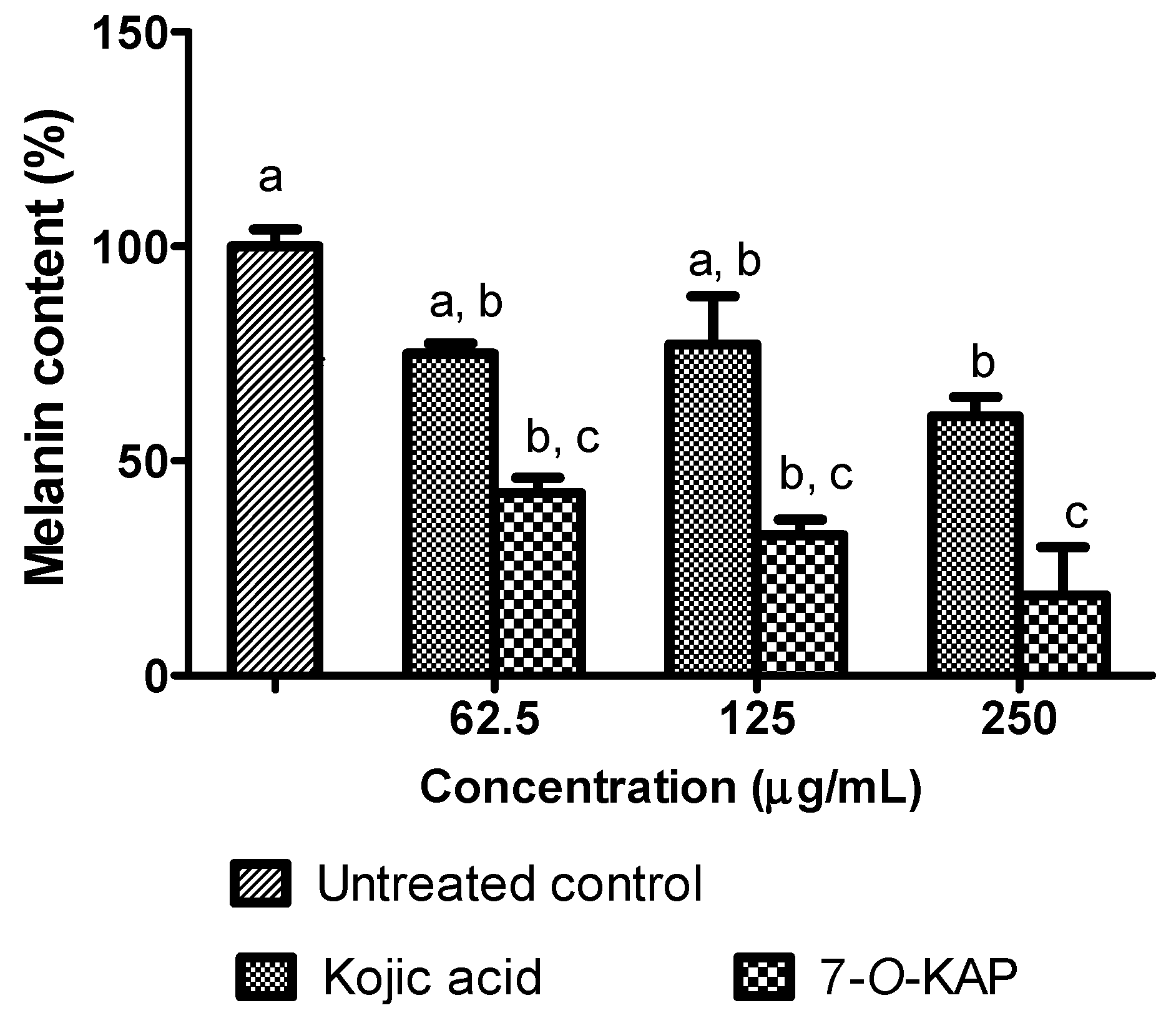
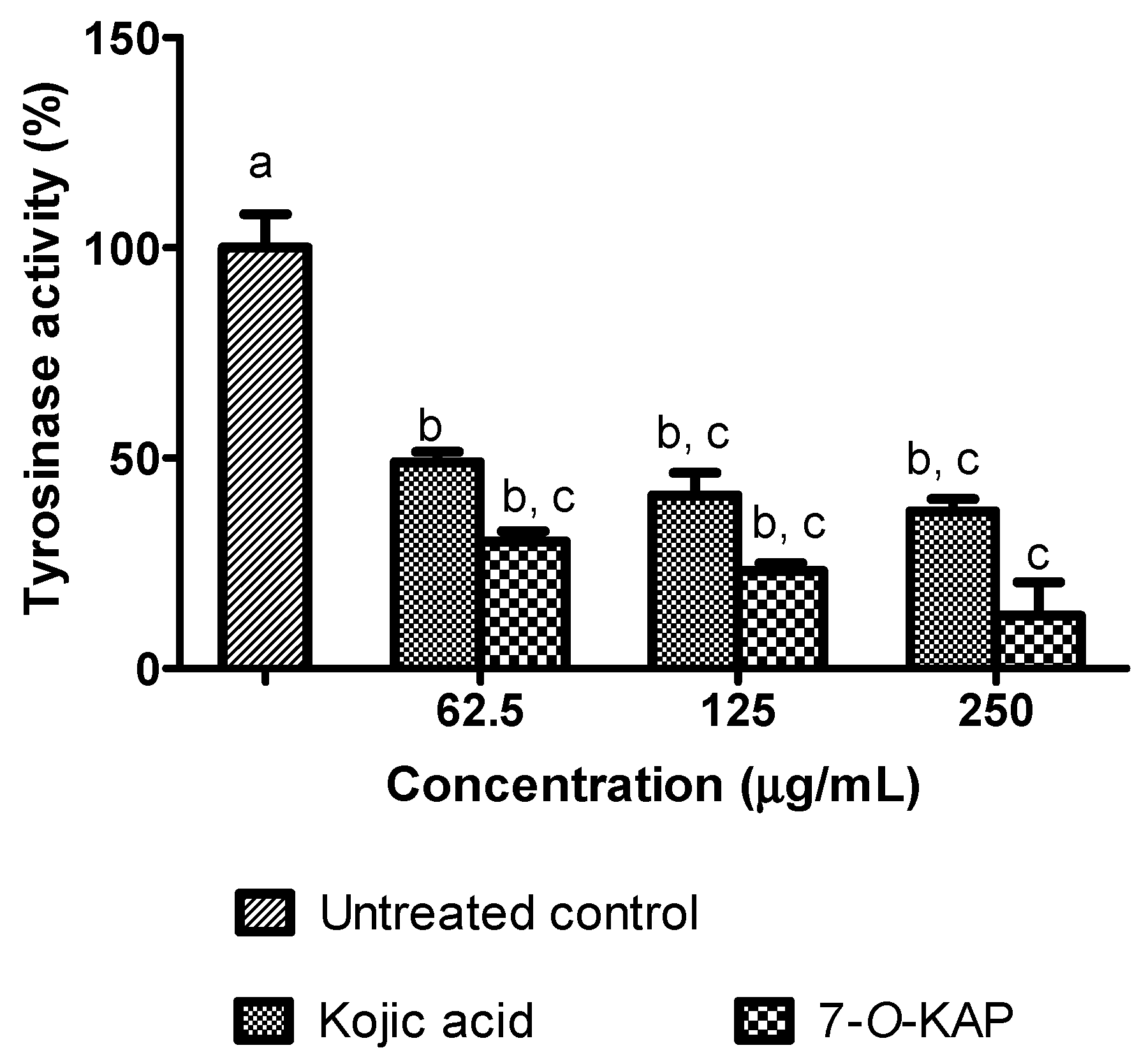
3.5. Depigmenting Activity
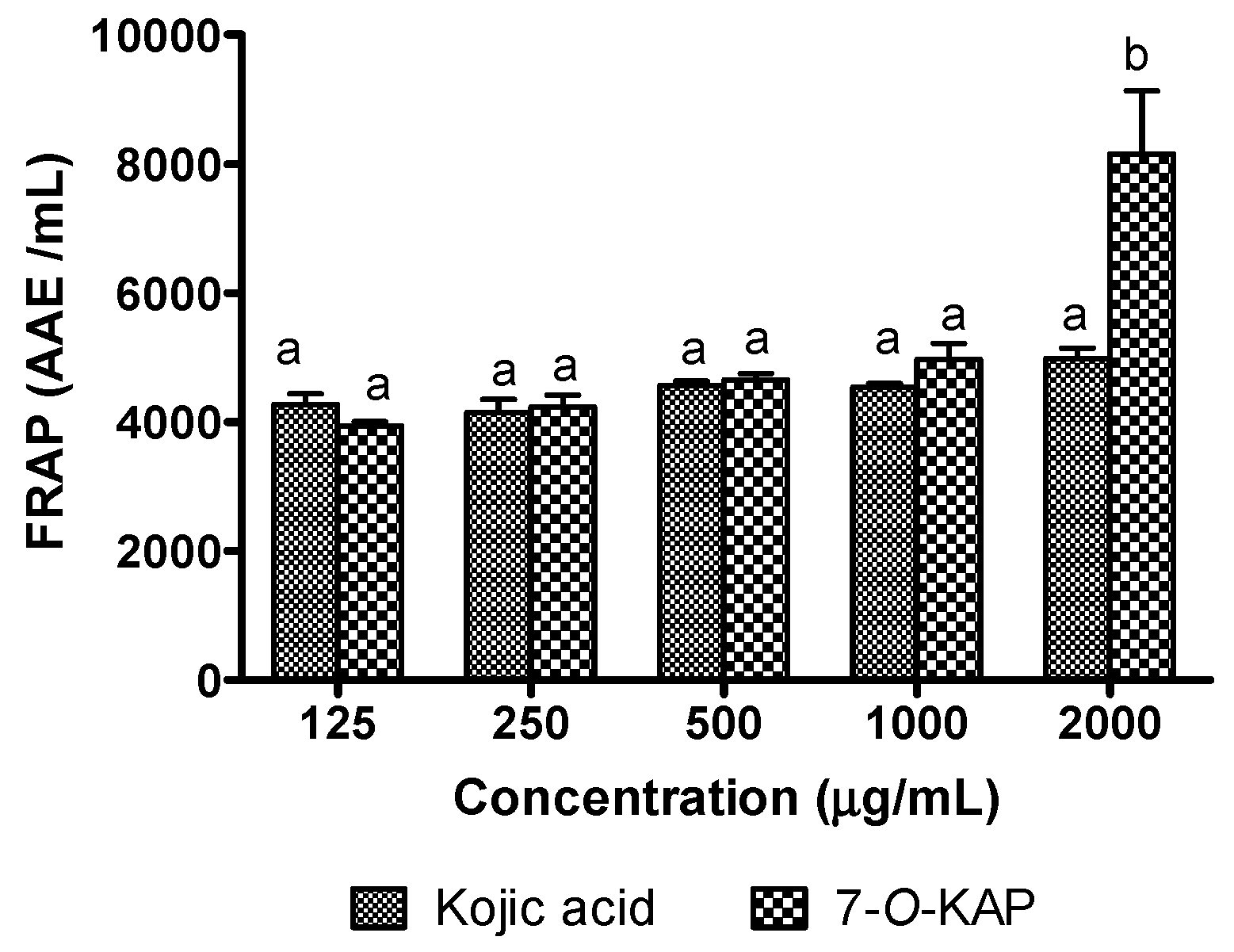
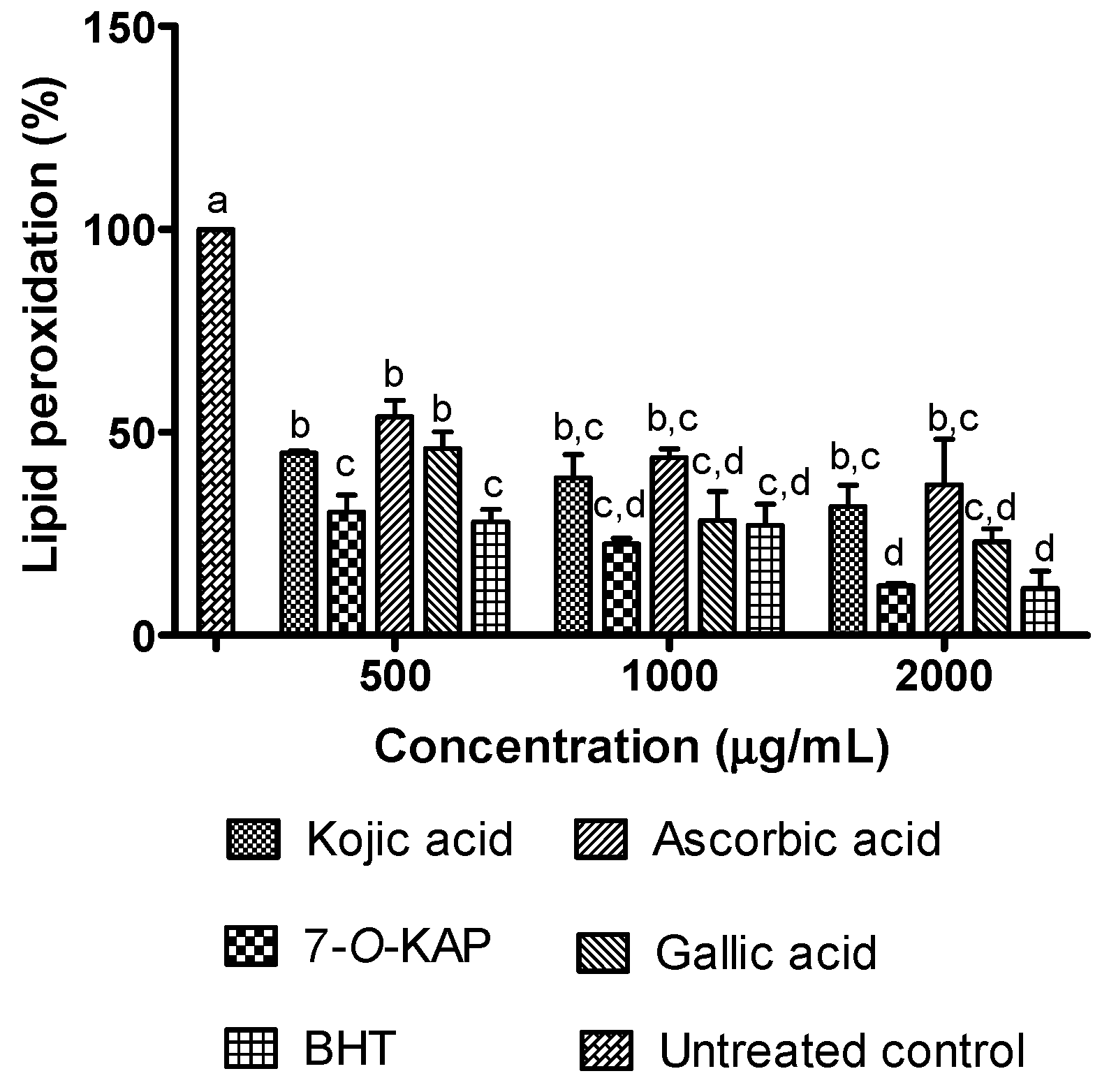
3.6. Antioxidant
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- El-Boulifi, N.; Ashari, S.E.; Serrano, M.; Aracil, J.; Martínez, M. Solvent-free lipase-catalyzed synthesis of a novel hydroxyl-fatty acid derivative of kojic acid. Enzym. Microb. Technol. 2014, 55, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Lajis, A.F.B.; Basri, M.; Mohamad, R.; Hamid, M.; Ashari, S.E.; Ishak, N.; Zookiflie, A.; Ariff, A.B. Enzymatic synthesis of kojic acid esters and their potential industrial applications. Chem. Pap. 2013, 67, 573–585. [Google Scholar]
- Adnani, A.; Basri, M.; Chaibakhsh, N.; Ahangar, H.A.; Salleh, A.B.; Rahman, R.N.Z.R.A.; Abdul-Rahman, M.B. Chemometric analysis of lipase-catalyzed synthesis of xylitol esters in a solvent-free system. Carbohydr. Res. 2011, 346, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Chaibakhsh, N.; Abdul-Rahman, M.B.; Vahabzadeh, F.; Abd-Aziz, S.; Basri, M.; Salleh, A.B. Optimization of operational conditions for adipate ester synthesis in a stirred tank reactor. Biotechnol. Bioprocess Eng. 2010, 15, 846–853. [Google Scholar] [CrossRef]
- Saponjić, S.; Knežević-Jugović, Z.D.; Bezbradica, D.I.; Zuza, M.G.; Saied, O.A.; Bosković-Vragolović, N.; Mijin, D.Z. Use of Candida rugosa lipase immobilized on sepabeads for the amyl caprylate synthesis: Batch and fluidized bed reactor study. Electron. J. Biotechnol. 2010, 13, 1–15. [Google Scholar] [CrossRef]
- Skoronski, E.; Padoin, N.; Soares, C.; Furigo, A., Jr. Stability of immobilized Rhizomucor miehei lipase for the synthesis of pentyl octanoate in a continuous packed bed bioreactor. Braz. J. Chem. Eng. 2014, 31, 633–641. [Google Scholar] [CrossRef]
- Jakovetic, S.M.; Lukovic, N.D.; Boskovic-Vragolovic, N.M.; Bezbradica, D.I.; Picazo-Espinosa, R.; Knezevic-Jugovic, Z.D. Comparative study of batch and fluidized bed bioreactors for lipase-catalyzed ethyl cinnamate synthesis. Ind. Eng. Chem. Res. 2013, 52, 16689–16697. [Google Scholar] [CrossRef]
- Lajis, A.F.B.; Hamid, M.; Ariff, A.B. Depigmenting effect of Kojic acid esters in hyperpigmented B16F1 melanoma cells. J. Biomed. Biotechnol. 2012, 2012, 952452. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.C.H.; Ding, H.Y.; Kuo, S.Y.; Chin, L.W.; Wu, J.Y.; Chang, T.S. Evaluation of In Vitro and In Vivo depigmenting activity of raspberry ketone from Rheum officinale. Int. J. Mol. Sci. 2011, 12, 4819–4835. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Song, K.; Jung, P.; Kim, J.; Ro, H. Inhibitory effect of arctigenin from fructus arctii extract on melanin synthesis via repression of tyrosinase expression. Evidence-Based Complement. Altern. Med. 2013, 2013, 965312. [Google Scholar] [CrossRef] [PubMed]
- Jonfia-Essien, W.A.; West, G.; Alderson, P.G.; Tucker, G. Phenolic content and antioxidant capacity of hybrid variety cocoa beans. Food Chem. 2008, 108, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Kim, M.J.; Hayat, K.; Xia, S.; Feng, B.; Zhang, X. Characteristics and antioxidant activity of ultrafiltrated Maillard reaction products from a caseinglucose model system. Food Chem. 2009, 117, 48–54. [Google Scholar] [CrossRef]
- Sharma, S.; Kanwar, S.S. Organic solvent tolerant lipases and applications. Sci. World J. 2014, 2014, 625258. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ayub, M.A.Z. Effects of the combined use of Thermomyces lanuginosus and Rhizomucor miehei lipases for the transesterification and hydrolysis of soybean oil. Process Biochem. 2011, 46, 682–688. [Google Scholar] [CrossRef]
- Ray, S. Applications of extracellular microbial lipase—A review. Int. J. Res. Biotechnol. Biochem. 2015, 5, 6–12. [Google Scholar]
- Martins, A.B.; Graebin, N.G.; Lorenzoni, A.S.G.; Roberto, F.L.; Ayub, M.A.Z.; Rodriguesa, R.C. Rapid and high yields of synthesis of butyl acetate catalyzed by Novozym 435: Reaction optimization by response surface methodology. Process Biochem. 2011, 46, 2311–2316. [Google Scholar] [CrossRef]
- Hlavsov’a, K.; Wimmer, Z.; Xanthakis, E.; Bernasek, P.; Sovova, H.; Zarevuckae, M. Lipase activity enhancement by SC-CO2 treatment. Z. Naturforsch. 2008, 63, 779–784. [Google Scholar]
- Gandhi, N.N.; Vijayalakshmi, V.; Sawant, S.B.; Joshi, J.B. Immobilization of Mucor miehei lipase on ion exchange resins. Chem. Eng. J. 1996, 61, 149–156. [Google Scholar] [CrossRef]
- Ishak, N.; Lajis, A.F.B.; Mohamad, R.; Ariff, A.B.; Halim, M.; Wasoh, H. Kojic acid esters: Comparative review on its methods of synthesis. J. Biochem. Microbiol. Biotechnol. 2016, 4, 7–15. [Google Scholar]
- Kuo, C.H.; Chen, H.H.; Chen, J.H.; Liu, Y.C.; Shieh, C.J. High yield of wax ester synthesized from cetyl alcohol and octanoic acid by lipozyme RMIM and Novozym 435. Int. J. Mol. Sci. 2012, 13, 11694–11704. [Google Scholar] [CrossRef] [PubMed]
- Bruno, L.M.; de Lima Filho, J.L.; de Castro, H.F. Comparative performance of microbial lipases immobilized on magnetic polysiloxane polyvinyl alcohol particles. Braz. Arch. Biol. Technol. 2008, 51, 889–896. [Google Scholar] [CrossRef]
- Sabeder, S.; Habulin, M.; Knez, Z. The lipase-catalyzed synthesis of fatty acid fructose esters in organic media and in supercritical carbon dioxide. Chem. Ind. Chem. Eng. Q. 2006, 12, 147–151. [Google Scholar] [CrossRef]
- Hilterhaus, L.; Minow, B.; Müller, J.; Berheide, M.; Quitmann, H.; Katzer, M.; Thum, O.; Antranikian, G.; Zeng, A.P.; Liese, A. Practical application of different enzymes immobilized on sepabeads. Bioprocess Biosyst. Eng. 2008, 31, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wang, X.J.; Nie, K.L.; Wang, F.; Liu, J.F.; Wang, P.; Tan, T.W. Synthesis of wax esters by lipase-catalyzed esterification with immobilized lipase from Candida sp. 99–125. Chin. J. Chem. Eng. 2011, 19, 978–982. [Google Scholar] [CrossRef]
- Keng, P.S.; Basri, M.; Ariff, A.B.; Abdul-Rahman, M.B.; Rahman, R.N.Z.A.; Salleh, A.B. Scale-up synthesis of lipase-catalyzed palm esters in stirred-tank reactor. Bioresour. Technol. 2008, 99, 6097–6104. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.S.; Deshpande, T.D.; Kulkarni, R.D.; Mahulikar, P.P. Synthesis of sucrose–coconut fatty acids esters: Reaction kinetics and rheological analysis. Ind. Eng. Chem. Res. 2013, 52, 5024–15033. [Google Scholar] [CrossRef]
- Faig, J.J.; Moretti, A.; Joseph, L.B.; Zhang, Y.; Nova, M.J.; Smith, K.; Uhrich, K.E. Biodegradable kojic acid-based polymers: Controlled delivery of bioactives for melanogenesis inhibition. Biomacromolecules 2017, 18, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Reece, J.B.; Urry, L.A.; Cain, M.L.; Wasserman, S.A.; Minorsky, P.V.; Jackson, R.B. Chapter 7: Membrane structure and function. In Campbell Biology, 9th ed.; Pearson: Boston, MA, USA, 2011; pp. 125–138. [Google Scholar]
- Rho, H.S.; Baek, H.S.; Ann, S.M.; Kim, D.H.; Chang, I.S. Synthesis of new melanogenic compounds containing two molecules of kojic acid. Bull. Korean Chem. Soc. 2008, 29, 1569–1571. [Google Scholar]
- Rho, H.S.; Baek, H.S.; You, J.W.; Kim, S.; Lee, J.Y.; Kim, D.H.; Chang, I.S. New 5-Hydroxy-2-(hydroxymethyl)-4H-pyran-4-one derivative has both tyrosinase inhibitory and antioxidant properties. Bull. Korean Chem. Soc. 2017, 28, 471–473. [Google Scholar] [CrossRef]
- Ismail, M.; Mariod, A.; Bagalkotkar, G.; Ling, H.S. Fatty acid composition and antioxidant activity of oils from two cultivars of Cantaloupe extracted by supercritical fluid extraction. Grasas Aceites 2009, 61, 37–44. [Google Scholar] [CrossRef]
- Ndhlala, R.; Moyo, M.; Staden, J.V. Natural antioxidants: Fascinating or mythical? Biomolecules 2010, 15, 6905–6930. [Google Scholar]
- Vavricka, J.; Christensen, B.M.; Li, J. Melanization in living organisms: A perspective of species evolution. Protein Cell 2010, 1, 830–841. [Google Scholar] [CrossRef] [PubMed]
| Acyl Donor | Length of the Packed Bed (cm) | Temperature (°C) | Solvent | Lipase | Conversion (%) |
|---|---|---|---|---|---|
| Palmitic acid | 2 | 60 | Acetonitrile | N435 | 0.00 ± 0.00 a |
| 4 | 0.23 ± 0.27 b | ||||
| 6 | 2.39 ± 0.96 c | ||||
| Palmitic acid | 2 | 60 | Acetonitrile | RMIM | 0.00 ± 0.00 a |
| 4 | 0.00 ± 0.00 a | ||||
| 6 | 0.37 ± 0.11 b | ||||
| Palmitic acid | 2 | 80 | Acetonitrile | N435 | 0.00 ± 0.00 a |
| 4 | 0.08 ± 0.04 a | ||||
| 6 | 0.49 ± 0.24 b | ||||
| Palmitic acid | 2 | 80 | Acetonitrile | RMIM | 0.00 ± 0.00 a |
| 4 | 0.07 ± 0.04 a | ||||
| 6 | 0.86 ± 0.31 c |
| Carbon No. | 1D NMR | 2D NMR (HSQC) H Atom Bind to Respective C Atom | 2D NMR (HMBC)Neighboring C Atom Near to Respective H Atom | ||||
|---|---|---|---|---|---|---|---|
| δ 1H (ppm) | δ 13C (ppm) | δ 1H (ppm) | δ 13C (ppm) | δ 13C (ppm) | δ 13C (ppm) | δ 13C (ppm) | |
| C-2 | - | 172 | - | - | - | - | - |
| C-3 | 6.61 | 110 | 6.61 | 110 | 168 (C-4) | 146 (C-5) | - |
| C-4 | - | 168 | - | - | - | - | - |
| C-5 | - | 146 | - | - | - | - | - |
| C-6 | 7.85 | 138.5 | 7.85 | 138.5 | 174.5 (C-2) | 168 (C-4) | 146 (C-5) |
| C-7 | 4.93 | 61.13 | 4.93 | 61.13 | 172 (C-2) | 163 (C-1’) | 111 (C-3) |
| C-1’ | - | 163 | - | - | - | - | - |
| C-2’ | 2.40 | 34 | 2.40 | 34 | - | - | - |
| C-3’ | 1.66 | 24 | 1.66 | 24 | - | - | - |
| C-4’-C-14’ | 1.29 | 27–32 | 1.29 | 27–32 | - | - | - |
| C-15’ | 1.30 | 22 | 1.30 | 22 | - | - | - |
| C-16’ | 0.88 | 14 | 0.88 | 14 | - | - | - |
| Compound | Temperature (°C) | Ratio | Power law (Ostwald–de Waele) | Herschel-Bulkley’s Consistency Index | Yield Stress | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Consistency Index | Flow Behaviour Index | |||||||||
| K (Pa·sn) | n (–) | K (Pa·sn) | τo (Pa) | |||||||
| Palmitic acid | 70 | - | 0.0182 | ±0.0005 | 0.8774 | ±0.0043 | 0.0168 | ±0.0005 | 0.3203 | ±0.0243 |
| Palmitic acid | 90 | - | 0.0152 | ±0.0007 | 0.8527 | ±0.0129 | 0.0138 | ±0.0006 | 0.2087 | ±0.0187 |
| PA:KA | 90 | 1:1 | 0.5586 | ±0.2002 | 0.6025 | ±0.0569 | 0.3476 | ±0.1389 | 5.9336 | ±0.6091 |
| PA:KA | 90 | 3:1 | 0.0259 | ±0.0016 | 0.8774 | ±0.0043 | 0.0220 | ±0.0005 | 0.9142 | ±0.3039 |
| 7-O-KAP | 90 | - | 0.0342 | ±0.0041 | 0.7492 | ±0.0044 | 0.0269 | ±0.0032 | 0.7720 | ±0.0923 |
| Linear Regression Analysis | Values/Description | |
|---|---|---|
| Best-Fit Values | Slope | 0.004683 ± 0.00005271 |
| Y-intercept when X = 0.0 | 0.01512 ± 0.009754 | |
| X-intercept when Y = 0.0 | −3.229 | |
| 1/slope | 213.5 | |
| 95% Confidence Intervals | Slope | 0.004572 to 0.004795 |
| Y-intercept when X = 0.0 | −0.005557 to 0.03580 | |
| X-intercept when Y = 0.0 | −9.459 to 2.842 | |
| Goodness of Fit | r² | 0.9980 |
| Sy.x | 0.02796 | |
| Is slope significantly non-zero? | F | 7896 |
| DFn, DFd | 1.000, 16.00 | |
| P value | <0.0001 | |
| Deviation from zero? | Significant | |
| Data | Number of X values | 6 |
| Maximum number of Y replicates | 3 | |
| Total number of values | 18 | |
| Number of missing values | 0 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lajis, A.F.B.; Hamid, M.; Ahmad, S.; Ariff, A.B. Lipase-Catalyzed Synthesis of Kojic Acid Derivative in Bioreactors and the Analysis of Its Depigmenting and Antioxidant Activities. Cosmetics 2017, 4, 22. https://doi.org/10.3390/cosmetics4030022
Lajis AFB, Hamid M, Ahmad S, Ariff AB. Lipase-Catalyzed Synthesis of Kojic Acid Derivative in Bioreactors and the Analysis of Its Depigmenting and Antioxidant Activities. Cosmetics. 2017; 4(3):22. https://doi.org/10.3390/cosmetics4030022
Chicago/Turabian StyleLajis, Ahmad Firdaus B., Muhajir Hamid, Syahida Ahmad, and Arbakariya B. Ariff. 2017. "Lipase-Catalyzed Synthesis of Kojic Acid Derivative in Bioreactors and the Analysis of Its Depigmenting and Antioxidant Activities" Cosmetics 4, no. 3: 22. https://doi.org/10.3390/cosmetics4030022





