Ascorbyl Tetraisopalmitate Inclusion into ?-Cyclodextrin and Mesoporous SBA-15: Preparation, Characterization and In Vitro Release Study
Abstract
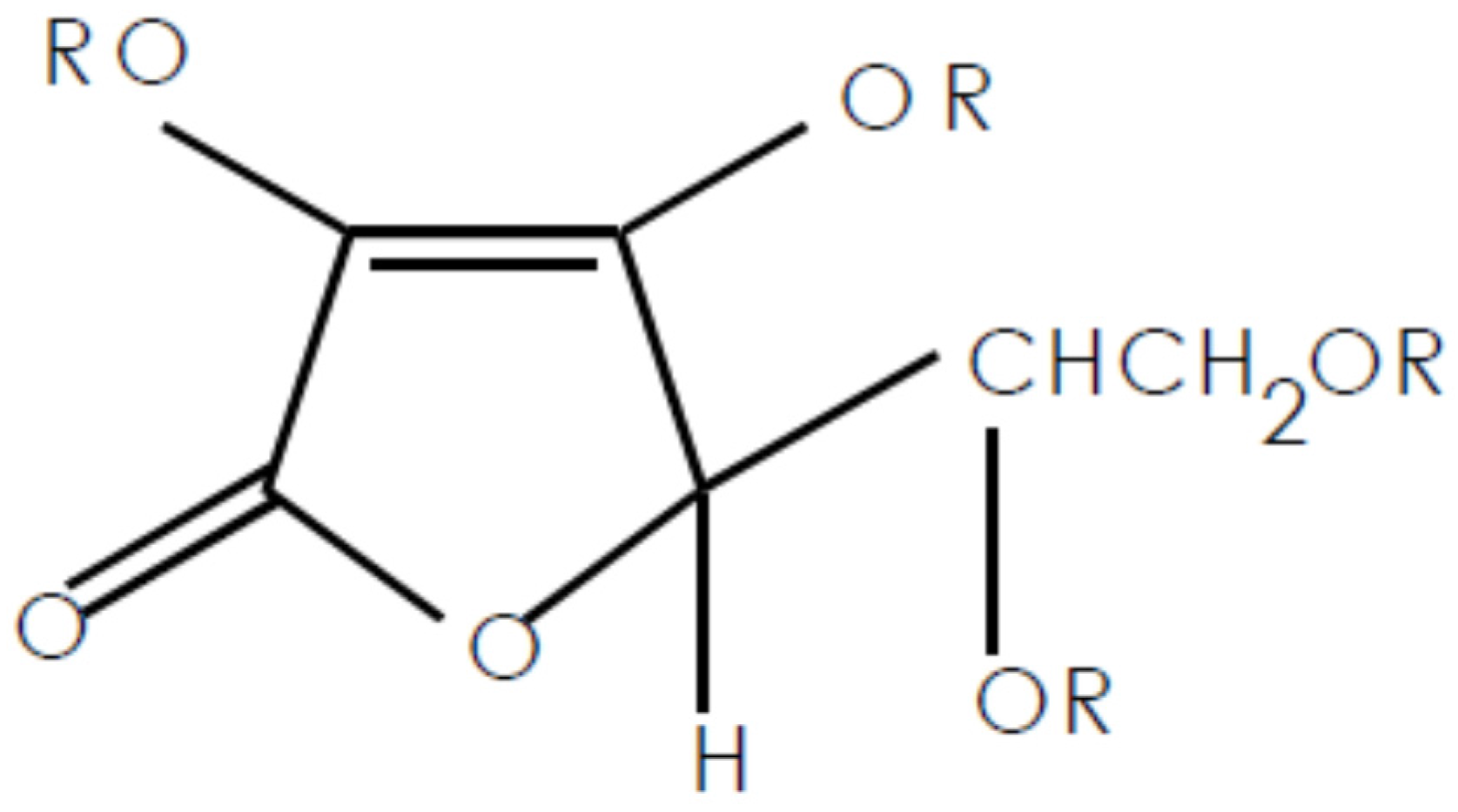
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvent
2.2. VC-IP@cyclodextrin Preparation
2.3. VC-IP@SBA-15 Preparation
2.4. Sample Characterization
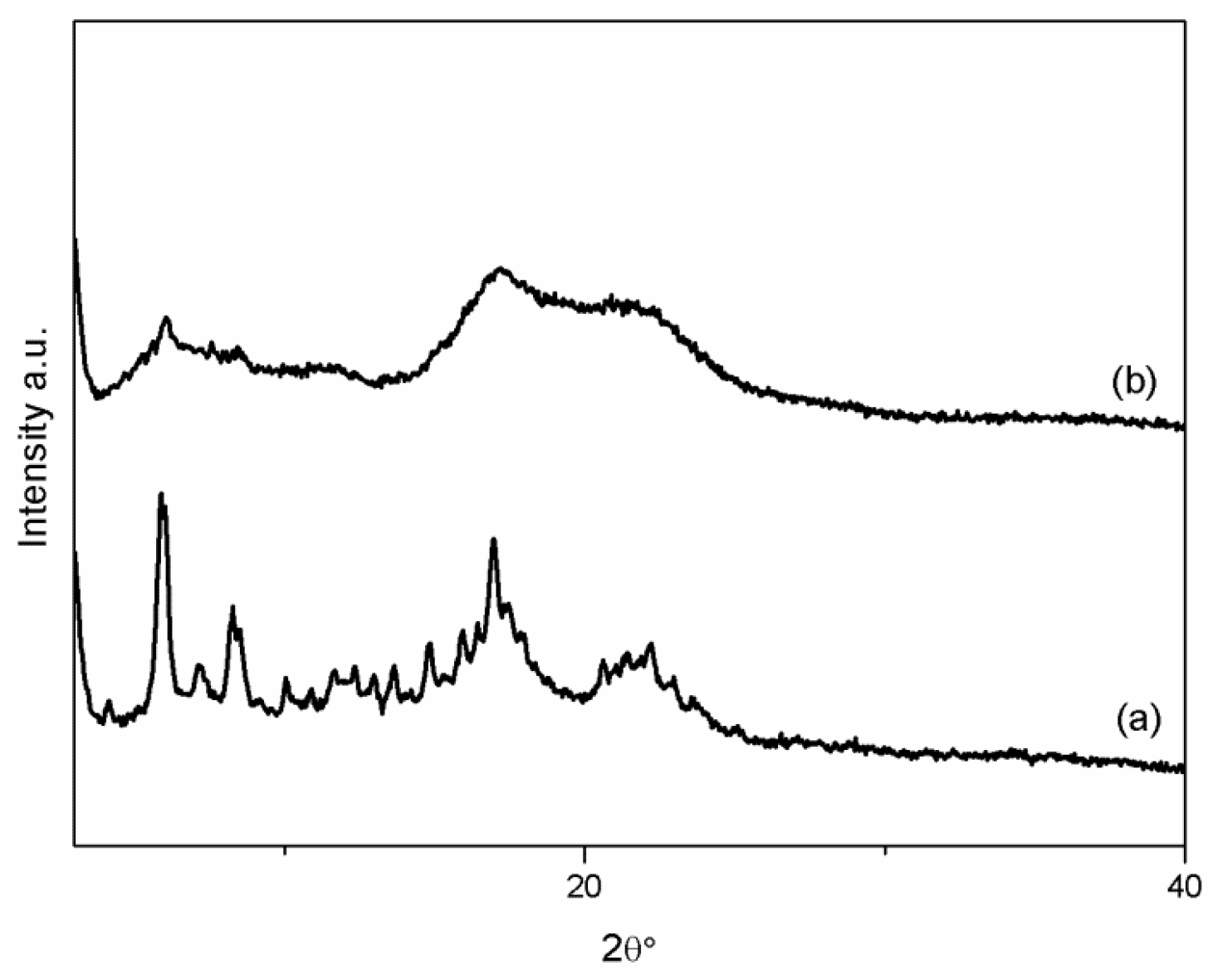
2.4.1. X-ray Powder Diffraction
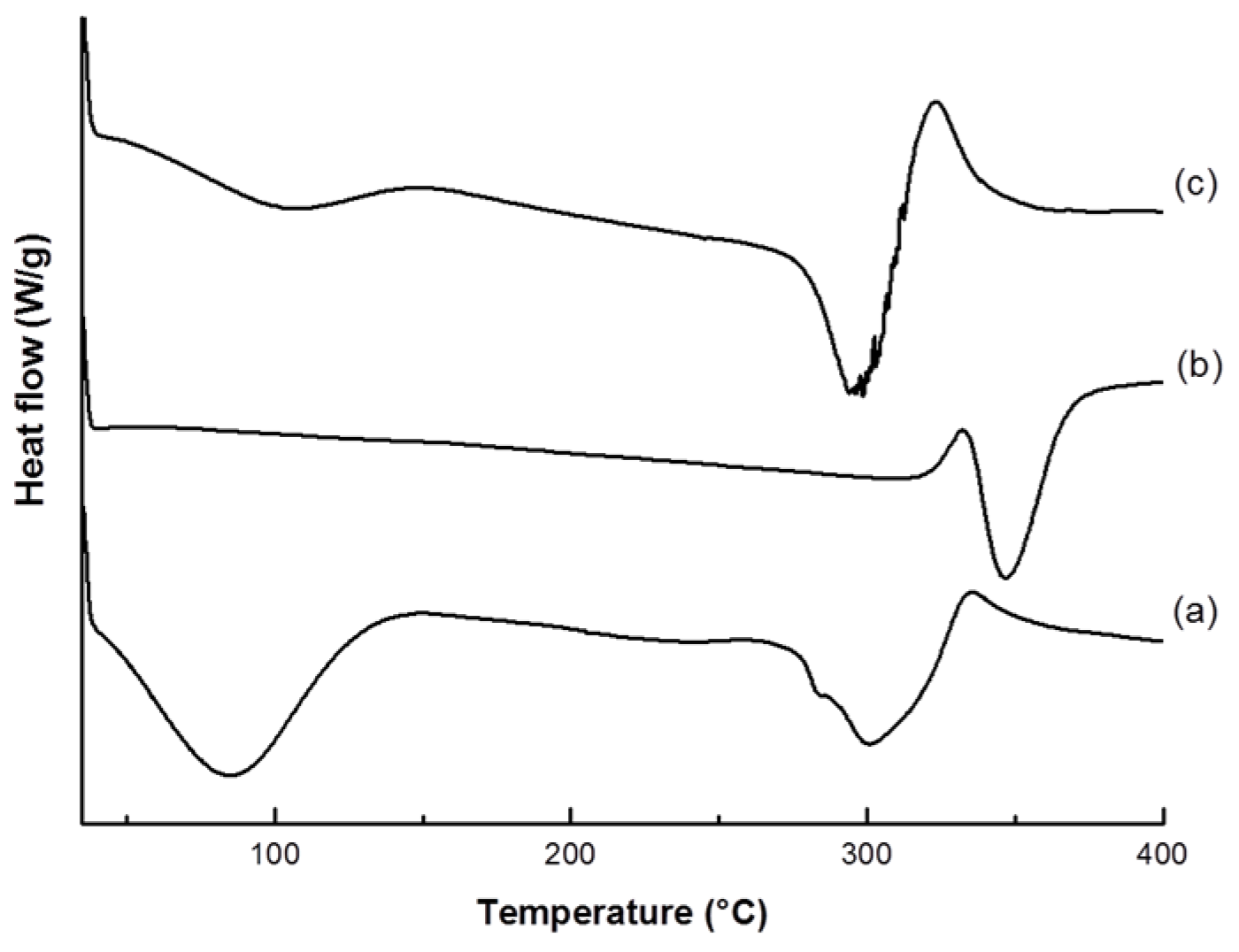
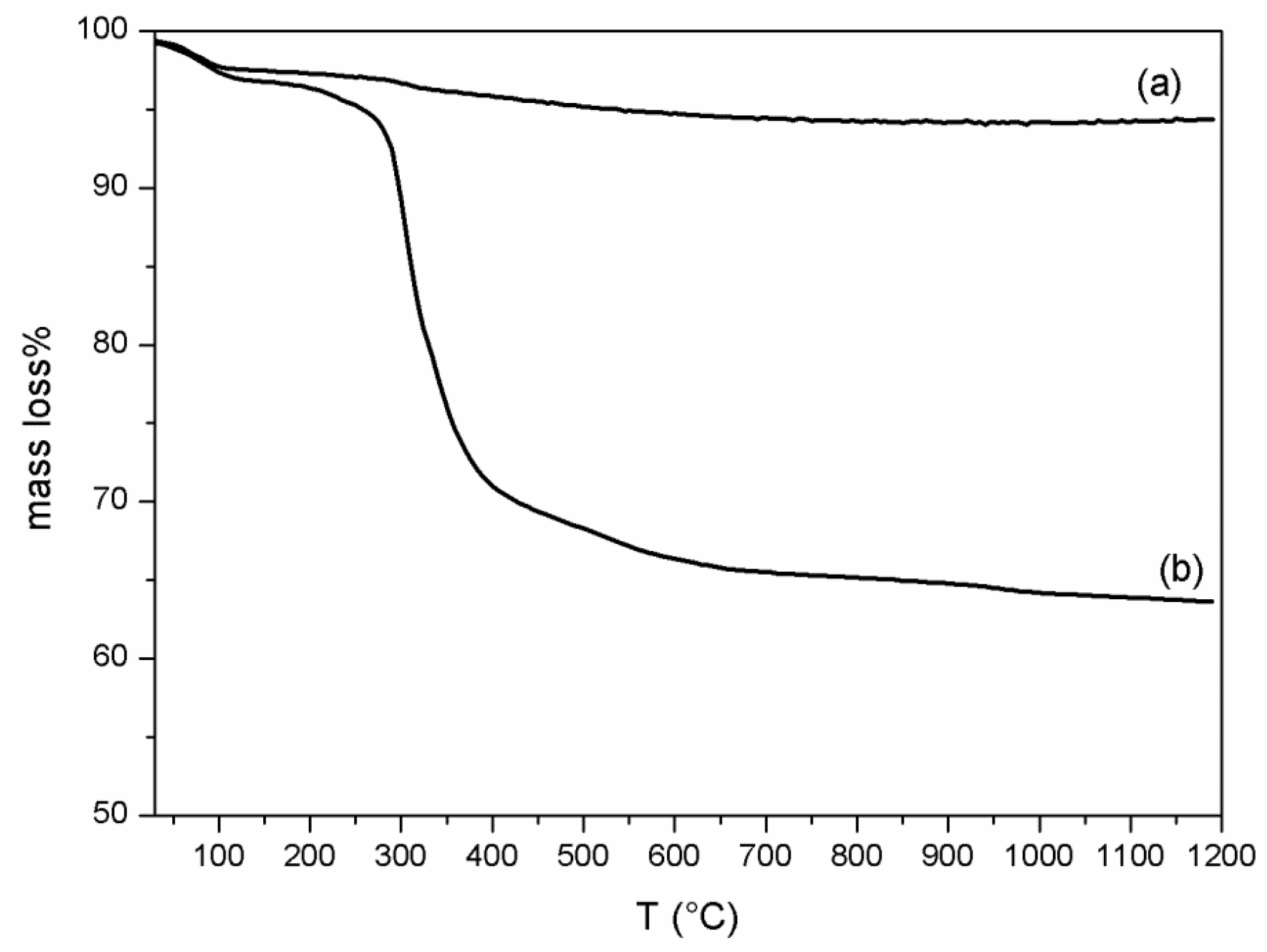
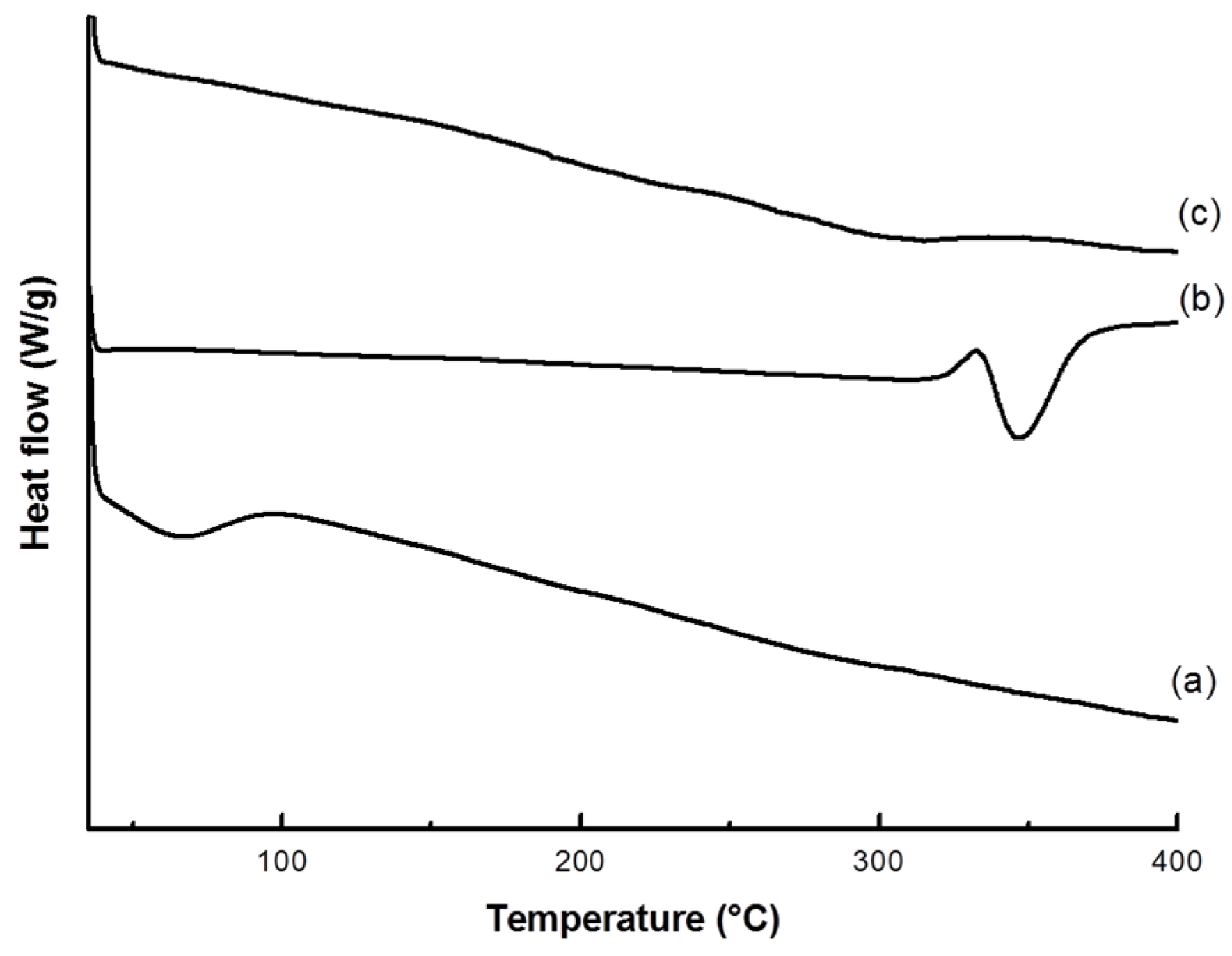
2.4.2. Thermal Analysis
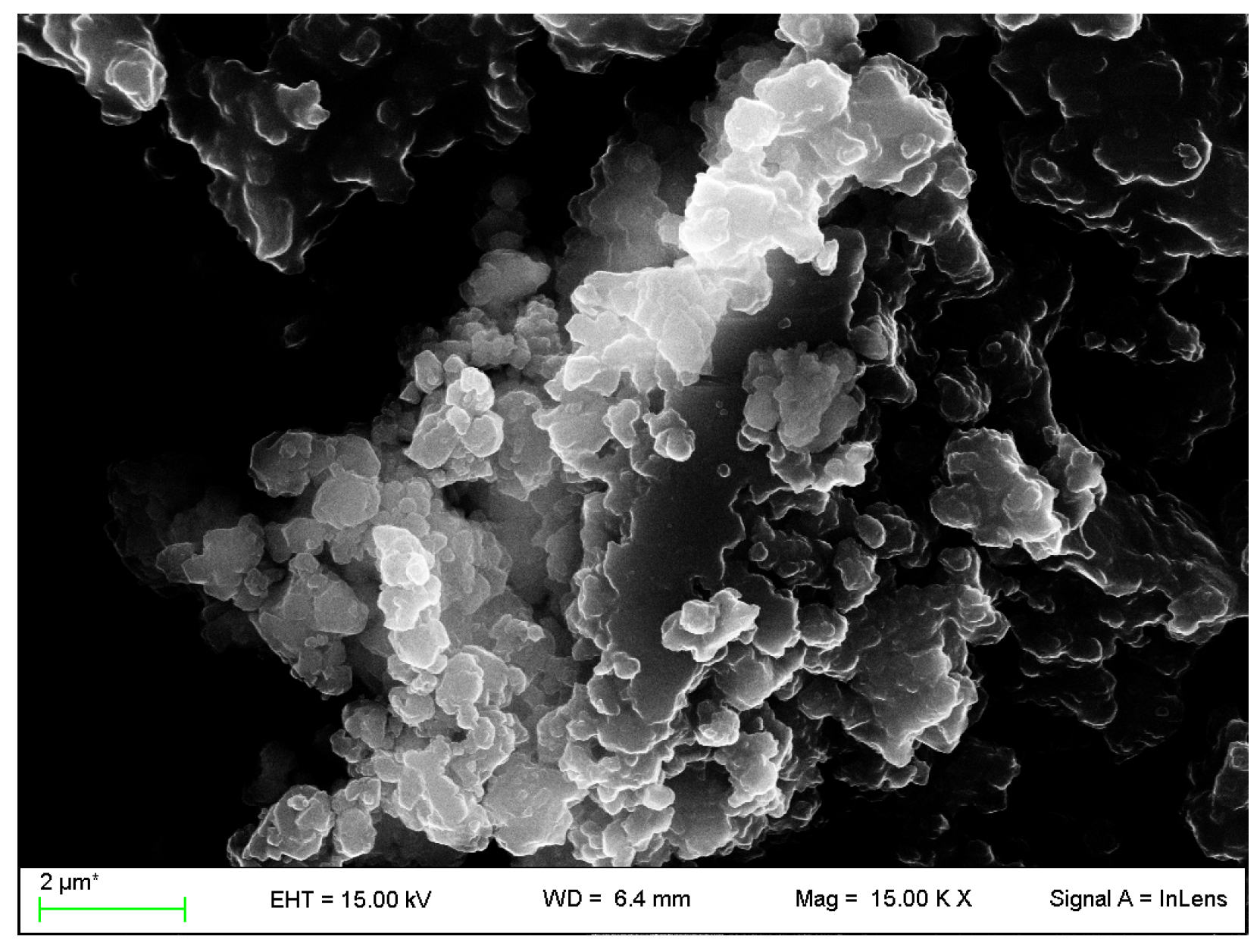
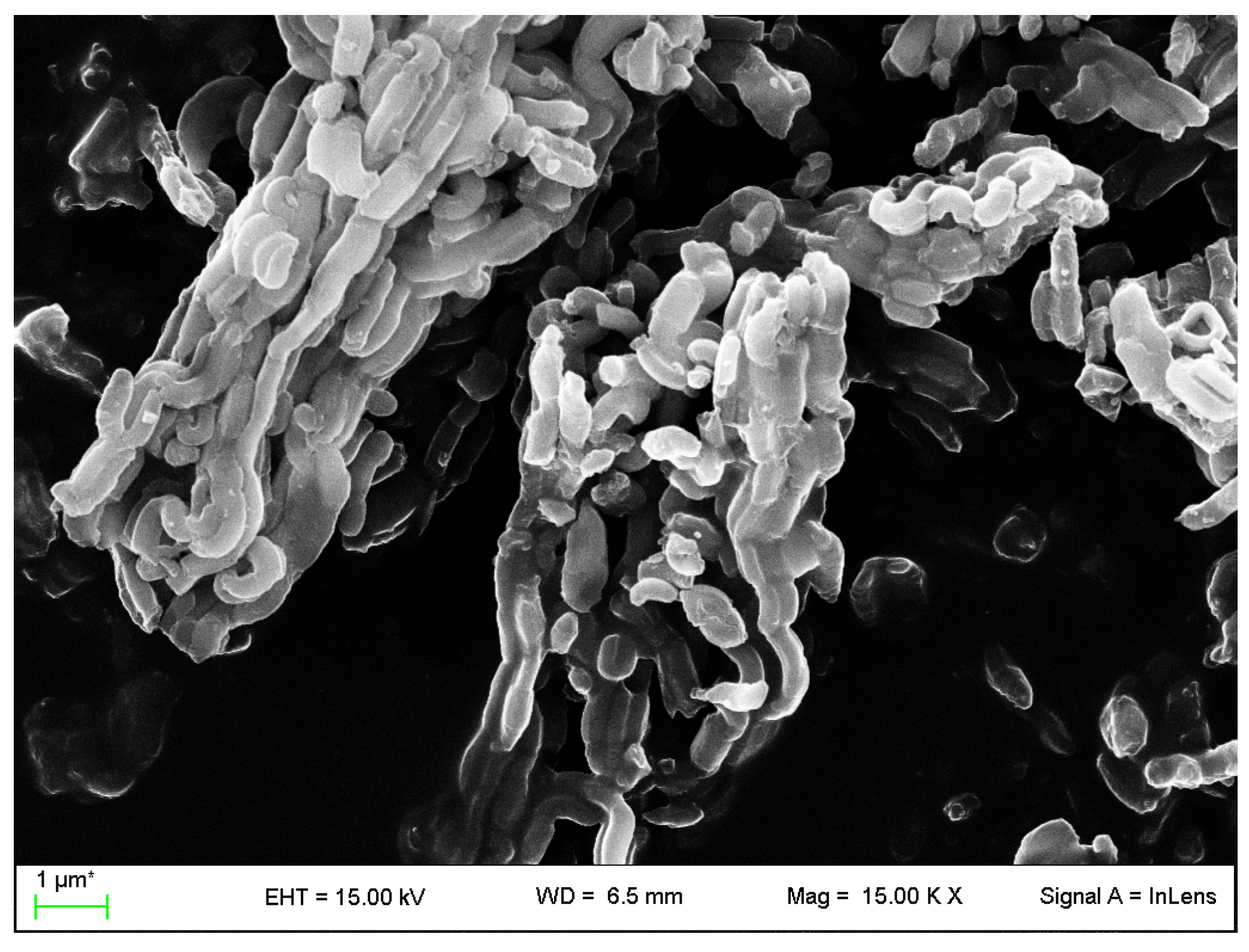
2.4.3. Field Emission Scanning Electron Microscopy
2.5. Extraction and HPLC Method for the Determination of VC-IP Content in the Hybrid Material
- Column: Zorbax eclipse XDB 4.6 × 150 mm 5 micron
- Mobile phase: methanol/isopropanol 25/75, isocratic
- Column temperature: 30 °C
- Sample volume: 20 μL
- Flux: 1 mL/min
- Detector: Variable Wavelength Detector (λ = 222 nm)
- Retention time: 5.9 min
- (1)
- A known amount of sample was dispersed by stirring in a proper volume of a methanol/isopropanol 50:50 solution.
- (2)
- The dispersion was treated for 30 min in an ultrasound bath.
- (3)
- Finally, the dispersion was filtered using a 0.20-μm pore size filter.
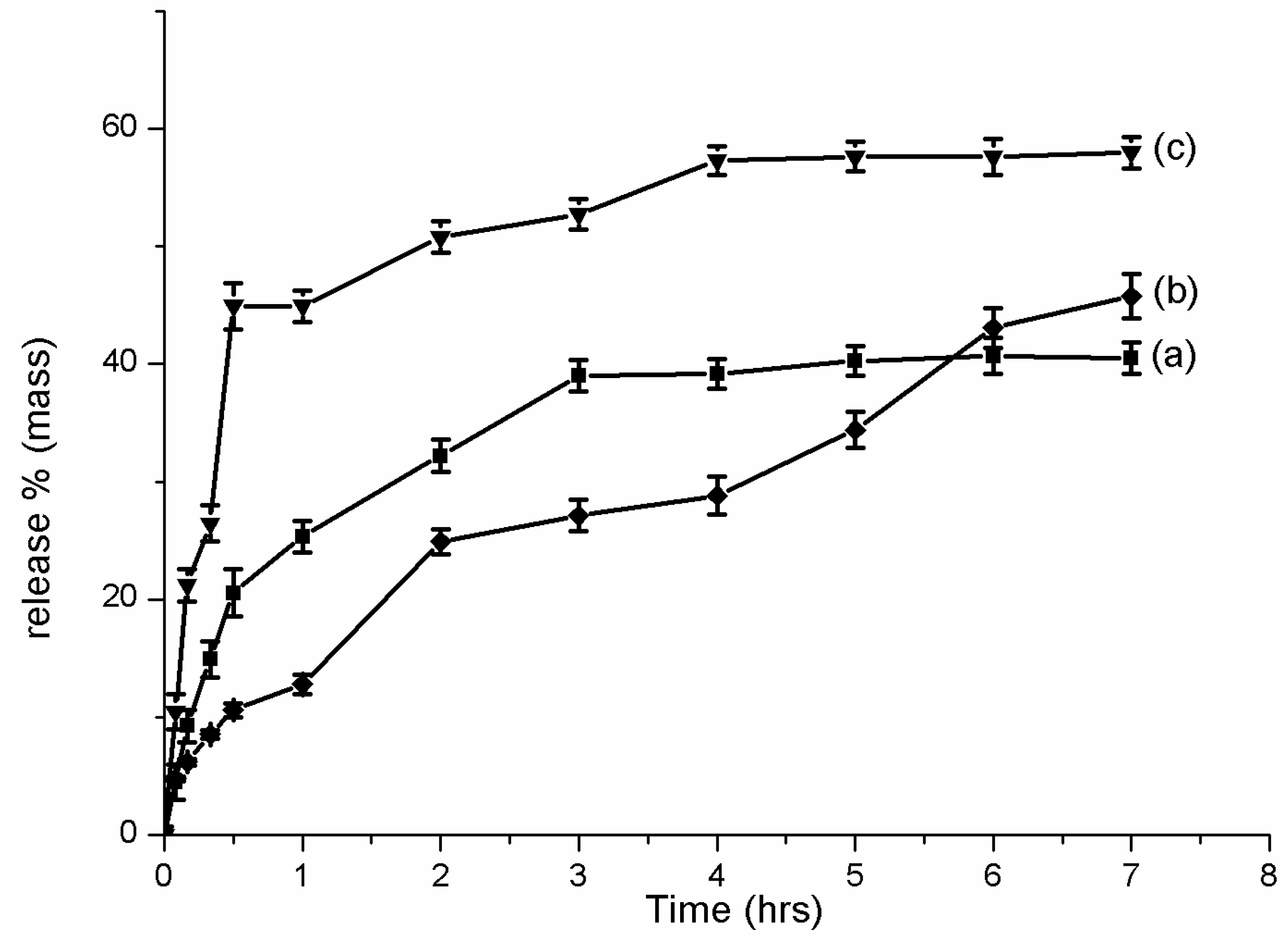
2.6. Franz Cell Release
3. Results and Discussion
3.1. VC-IP@cyclodextrin Characterization
3.2. VC-IP@SBA-15 Characterization
3.3. In Vitro Release Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maia, C.P.; Mara, S.G. Ascorbic acid and its derivatives in cosmetic formulations. Cosmet. Toilet. 2000, 115, 59–62. [Google Scholar]
- Darr, D.; Combs, S.; Dunston, S.; Manning, T.; Pinnell, S. Topical vitamin C protects porcine skin from ultraviolet radiation-induced damage. Br. J. Dermatol. 1992, 127, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Garg, A.; Krohn, R.; Bagchi, M.; Tran, M.X.; Stohs, S.J. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res. Commun. Mol. Pathol. Pharmacol. 1997, 95, 179–189. [Google Scholar] [PubMed]
- Linster, C.L.; Van Schaftingen, E. Vitamin C Biosynthesis, recycling and degradation in mammals. Febs J. 2007, 274, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Patricia, K.; Farris, M.D. Topical Vitamin C: A Useful Agent for Treating Photoaging and Other Dermatologic Conditions. Dermatol. Surg. 2005, 31, 814–818. [Google Scholar]
- Sheraz, M.A.; Ahmed, S.; Ahmad, I.; Shaikh, R.H.; Vaid, F.H.M.; Iqbal, K. Formulation and Stability of Ascorbic Acid in Topical Preparations. Syst. Rev. Pharm. 2011, 2, 86–90. [Google Scholar] [CrossRef]
- Gu, C.; Hu, C.; Ma, C.; Fang, Q.; Xing, T.; Xia, Q. Development and characterization of solid lipid microparticles containing vitamin C for topical and cosmetic use. Eur. J. Lipid Sci. Technol. 2016, 118, 1093–1103. [Google Scholar] [CrossRef]
- Ochiai, Y.; Kaburagi, S.; Obayashi, K.; Ujiie, N.; Hashimoto, S.; Okano, Y.; Masaki, H.; Ichihashi, M.; Sakurai, H. A new lipophilic pro-vitamin C, tetra isopalmitoyl ascorbic acid (VC-IP), prevents UV-induced skin pigmentation through its anti-oxidative properties. J. Dermatol. Sci. 2006, 44, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Maia Campos, P.M.B.G.; Gianeti, M.D.; Camargo, F.B., Jr.; Gaspar, L.R. Application of tetra-isopalmitoyl ascorbic acid in cosmetic formulations: Stability studies and in vivo efficacy. Eur. J. Pharm. Biopharm. 2012, 82, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Patravale, V.B.; Mandawgade, S.D. Novel cosmetic delivery systems: An application update. Int. J. Cosmet. Sci. 2008, 30, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. Ordered mesoporous materials for drug delivery. Microporous Mesoporous Mater. 2009, 117, 1–9. [Google Scholar] [CrossRef]
- Casanova, F.; Santos, L. Encapsulation of cosmetic active ingredients for topical application—A review. J. Microencapsul. 2016, 33, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regi, M.; Balas, F.; Arcos, D. Mesoporous Materials for Drug Delivery. Angew. Chem. Int. Ed. Engl. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.M.; Alves, J.M.P.; Patto, D.C.S.; Lima, C.R.R.C.; Quenca-Guillen, J.S.; Santoro, M.I.R.M.; Kedor-Hackmann, E.R.M. Determination of tocopheryl acetate and ascorbyl tetraisopalmitate in cosmetic formulations by HPLC. Int. J. Cosmet. Sci. 2009, 31, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Anitha, R.; Ghosh, S.; Nanda, A.; Kumria, R. Complexation of celecoxib with β-cyclodextrin: Characterization of the interaction in solution and in solid state. J. Pharm. Sci. 2005, 94, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Han, Y.-H.; Song, T.W.; Sung, J.H.; Kwon, O.-S.; Song, S.; Chung, Y.-B. Physicochemical characteristics and bioavailability of a novel intestinal metabolite of ginseng saponin (IH901) complexed with gamma-cyclodextrin. Int. J. Pharm. 2006, 316, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Mandelli de Almeida, M.; Ribeiro de Castro Lima, C.R.; Quenca-Guillen, J.S.; Filho, E.M.; Mercuri, L.P.; Rocha Miritello Santoro, M.I.; Kedor-Hackmann, E.R.M. Stability evaluation of tocopheryl acetate and ascorbyl tetraisopalmitate in isolation and incorporated in cosmetic formulations using thermal analysis. Braz. J. Pharm. Sci. 2010, 46, 129–134. [Google Scholar] [CrossRef]
- Singh, R.; Bharti, N.; Madan, J.; Hiremath, S.N. Characterization of Cyclodextrin Inclusion Complexes—A Review. J. Pharm. Sci. Technol. 2010, 2, 171–183. [Google Scholar]
- Martin Del Valle, E.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Davis, E.M.; Brewster, E.M. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastianini, M.; Sisani, M.; Petracci, A. Ascorbyl Tetraisopalmitate Inclusion into ?-Cyclodextrin and Mesoporous SBA-15: Preparation, Characterization and In Vitro Release Study. Cosmetics 2017, 4, 21. https://doi.org/10.3390/cosmetics4030021
Bastianini M, Sisani M, Petracci A. Ascorbyl Tetraisopalmitate Inclusion into ?-Cyclodextrin and Mesoporous SBA-15: Preparation, Characterization and In Vitro Release Study. Cosmetics. 2017; 4(3):21. https://doi.org/10.3390/cosmetics4030021
Chicago/Turabian StyleBastianini, Maria, Michele Sisani, and Annarita Petracci. 2017. "Ascorbyl Tetraisopalmitate Inclusion into ?-Cyclodextrin and Mesoporous SBA-15: Preparation, Characterization and In Vitro Release Study" Cosmetics 4, no. 3: 21. https://doi.org/10.3390/cosmetics4030021
APA StyleBastianini, M., Sisani, M., & Petracci, A. (2017). Ascorbyl Tetraisopalmitate Inclusion into ?-Cyclodextrin and Mesoporous SBA-15: Preparation, Characterization and In Vitro Release Study. Cosmetics, 4(3), 21. https://doi.org/10.3390/cosmetics4030021




