Textural Feature Analysis of Optical Coherence Tomography Phantoms
Abstract
:1. Introduction
2. Materials and Methods
2.1. OCT Device
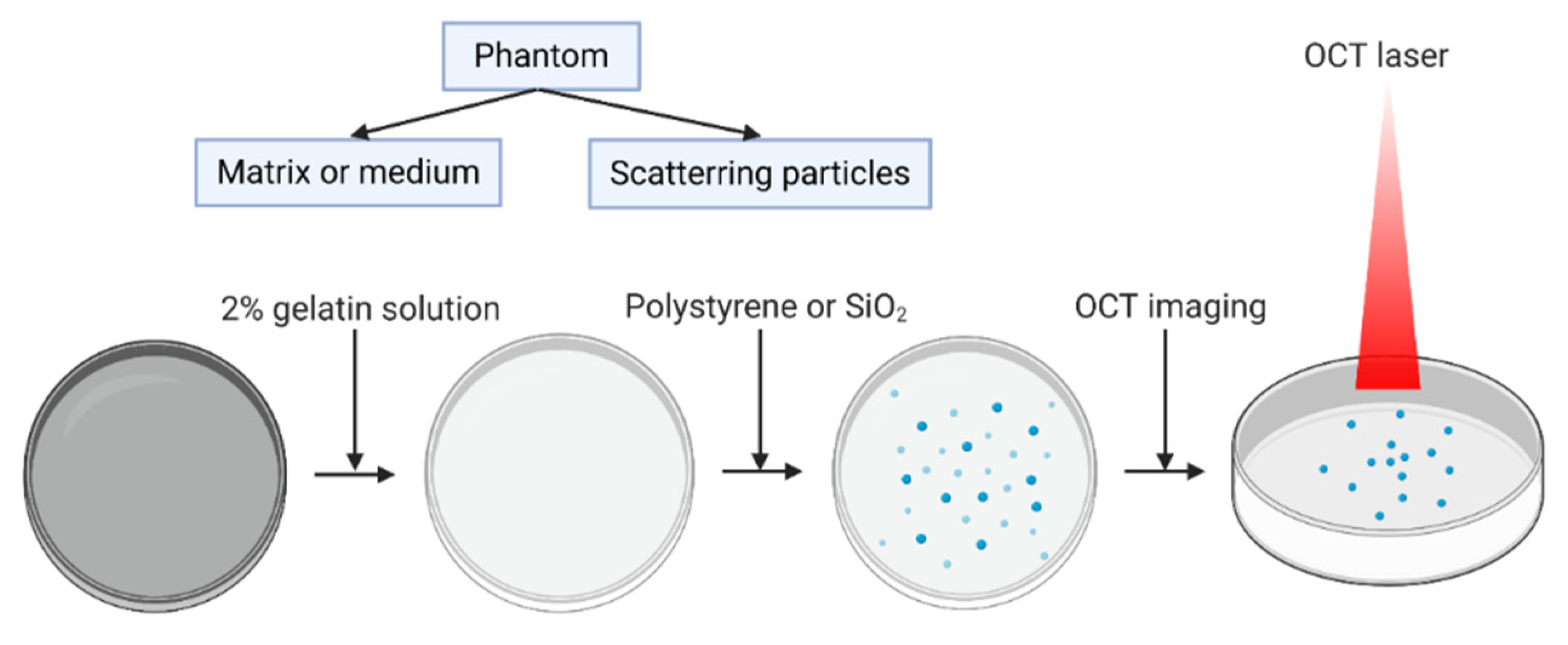
2.2. Phantom Preparation and Imaging
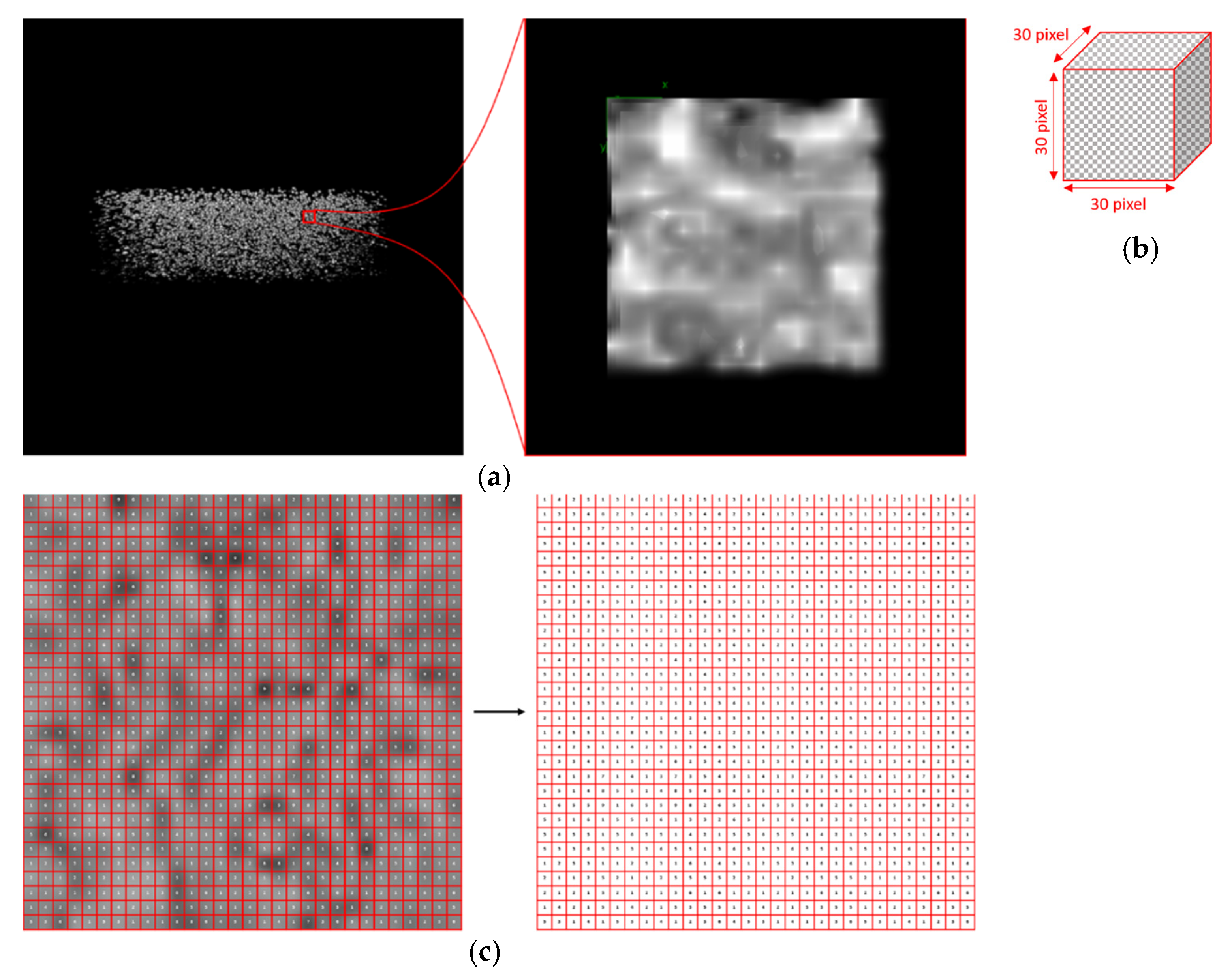
2.3. Texture Analysis
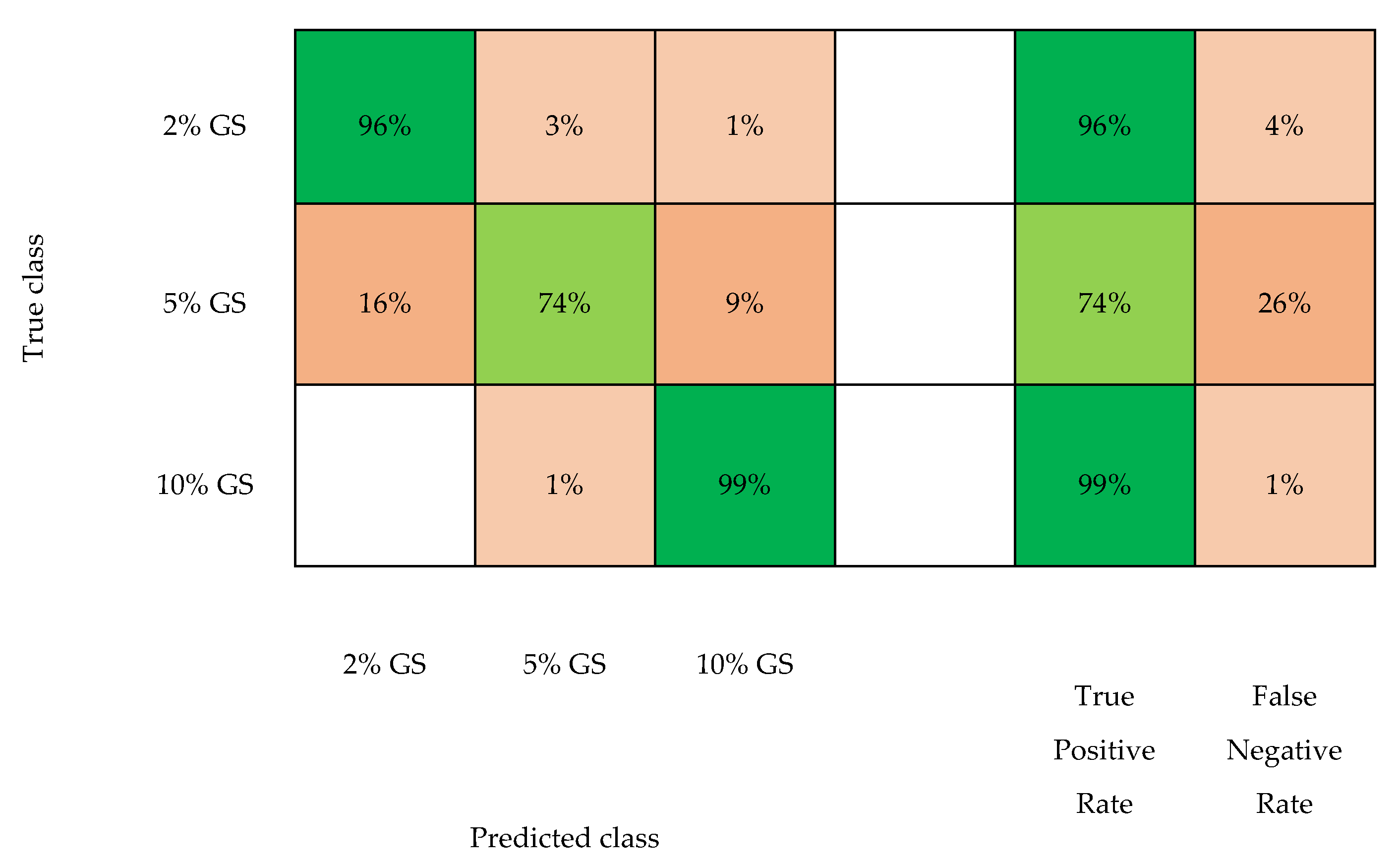
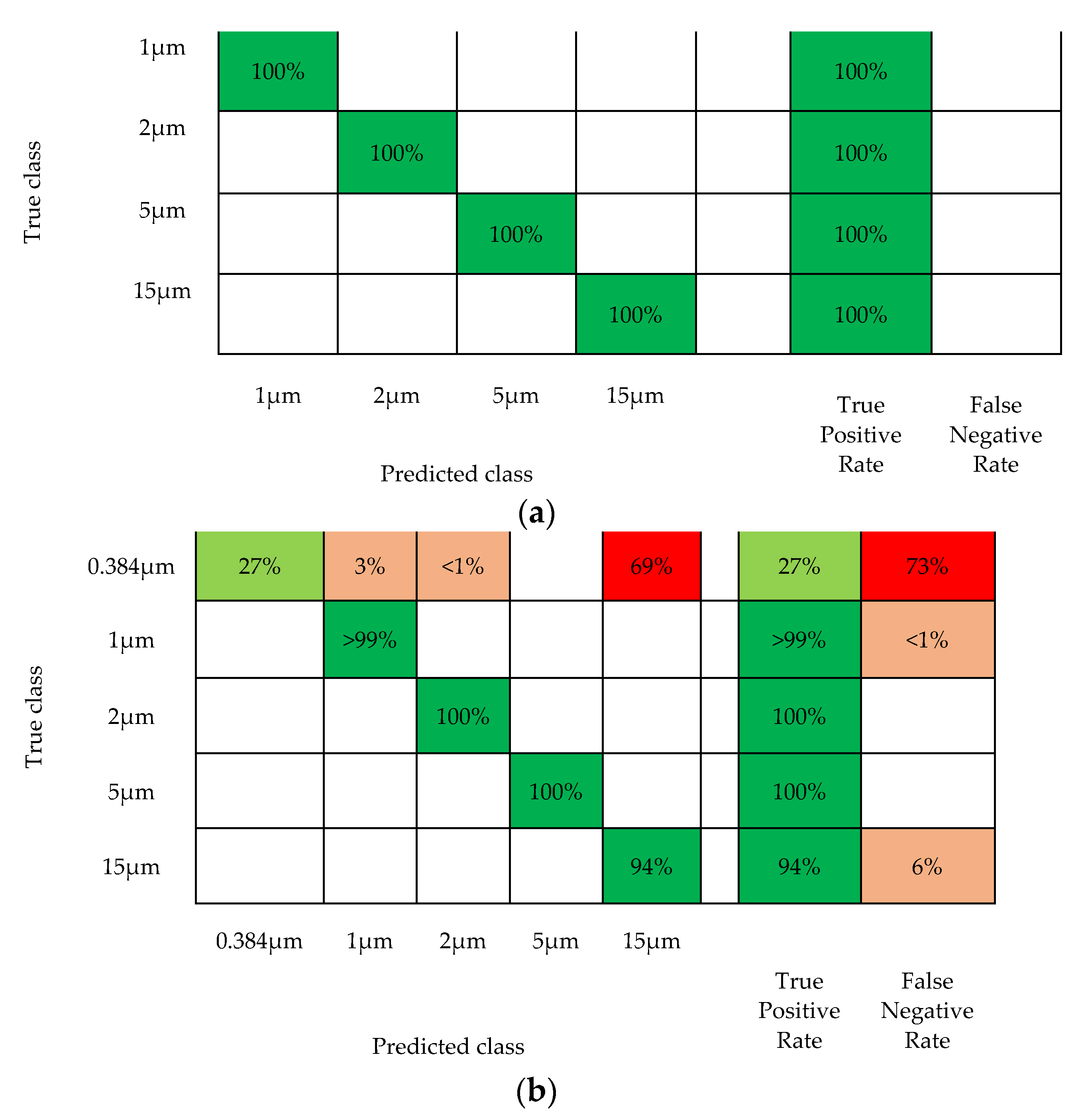
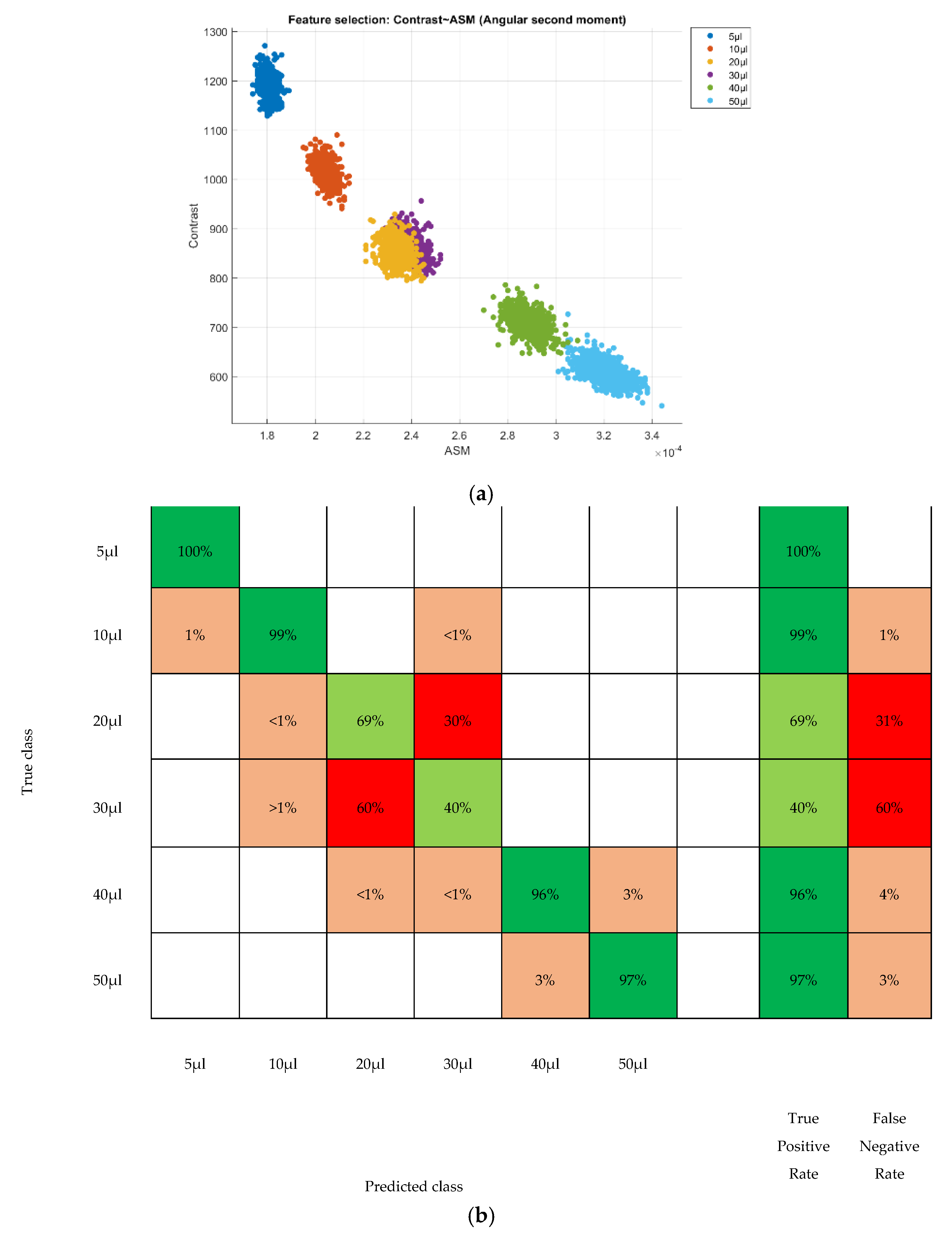
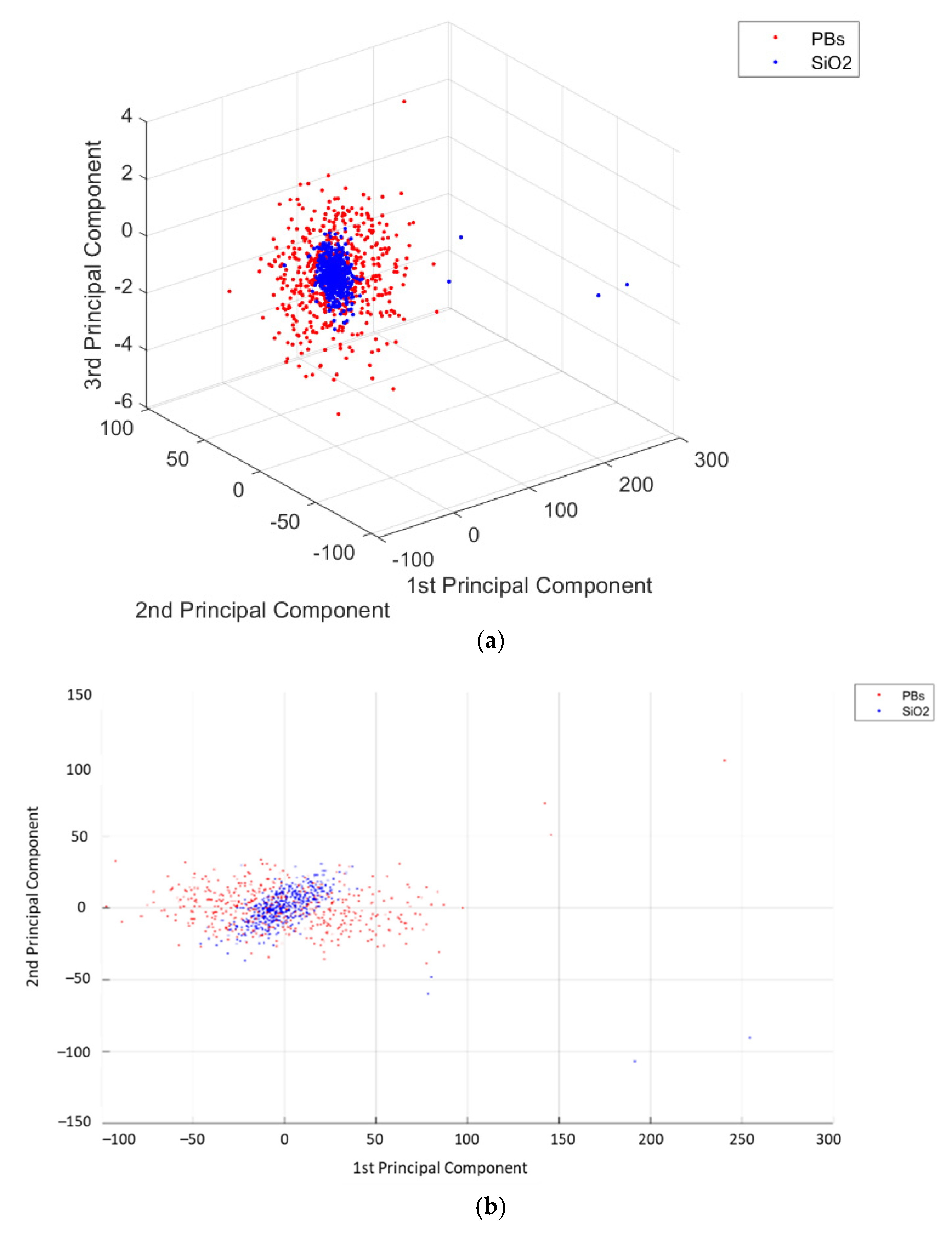
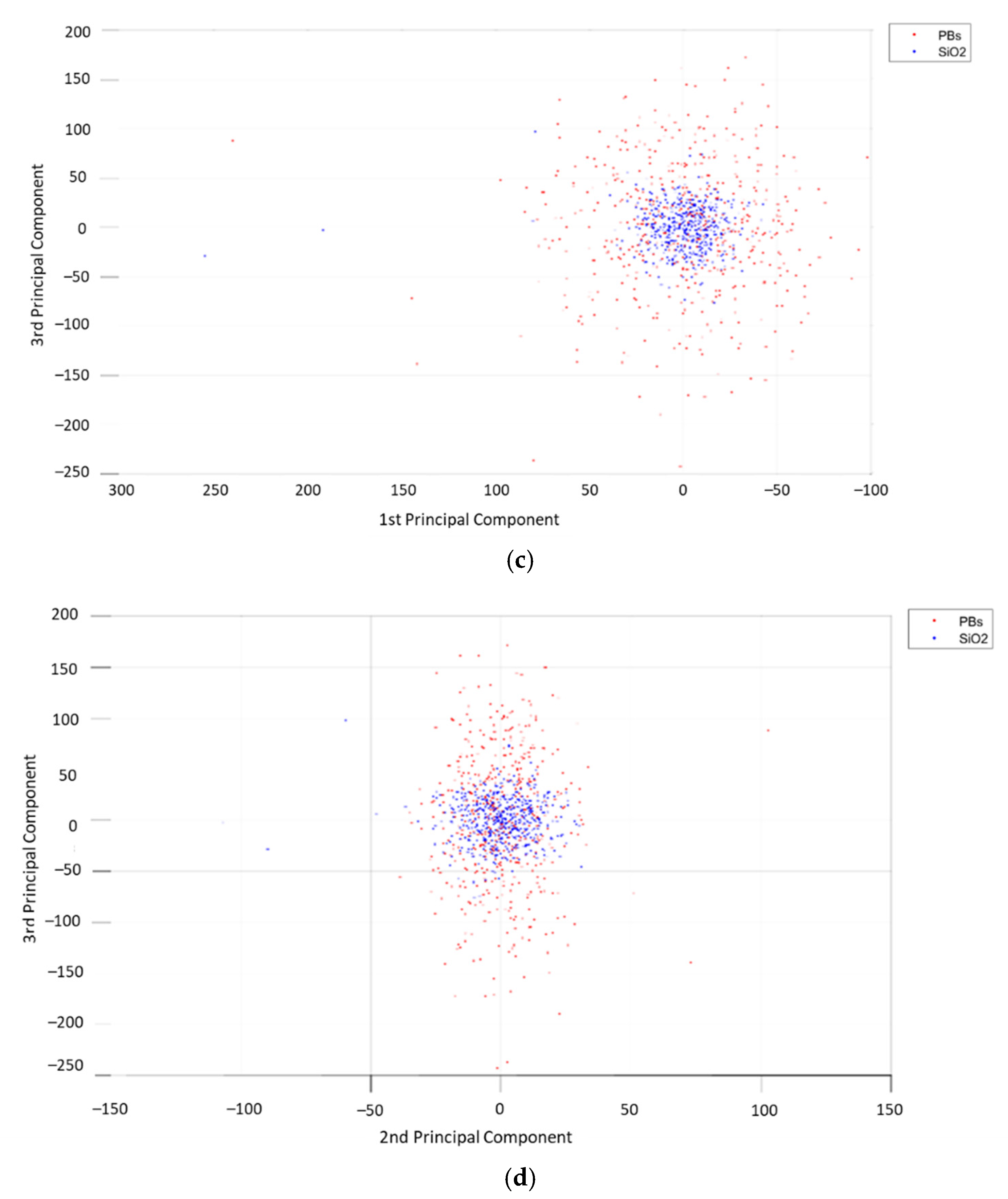
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Amorim Garcia Filho, C.A.; Yehoshua, Z.; Gregori, G.; Farah, M.E.; Feuer, W.; Rosenfeld, P.J. Optical Coherence Tomography. In Retina, 5th ed.; Wilkinson, C., Hinton, D., Sadda, S., Wiedemann, P., Ryan, S., Eds.; W.B. Saunders: London, UK, 2013; pp. 82–110. [Google Scholar] [CrossRef]
- Jonnal, R.S.; Kocaoglu, O.P.; Zawadzki, R.J.; Liu, Z.; Miller, D.T.; Werner, J.S. A Review of Adaptive Optics Optical Coherence Tomography: Technical Advances, Scientific Applications, and the Future. Investig. Ophthalmol Vis. Sci. 2016, 57, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, R.; Hitzenberger, C.K.; Fercher, A.F. Performance of fourier domain vs. time domain optical coherence tomography. Opt. Express 2003, 11, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Uttam, S.; Liu, Y. Fourier phase in Fourier-domain optical coherence tomography. J. Opt. Soc. Am. 2015, 32, 2286–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Chen, X.; Zhu, W.; Cheng, X.; Xiang, D.; Shi, F. Speckle noise reduction in optical coherence tomography images based on edge-sensitive cGAN. Biomed. Opt. Express 2018, 9, 5129–5146. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.M.; Xiang, S.H.; Yung, K.M. Speckle in optical coherence tomography. J. Biomed. Opt. 1999, 4, 95–105. [Google Scholar] [CrossRef]
- Gossage, K.W.; Cynthia, M.S.; Elizabeth, M.K.; Lida, P.H.; Alice, L.S.; Jeffrey, J.R.; Williams, S.K.; Jennifer, K.B. Texture analysis of speckle in optical coherence tomography images of tissue phantoms. Phys. Med. Biol. 2006, 51, 1563–1575. [Google Scholar] [CrossRef] [Green Version]
- Oberholzer, M.; Östreicher, M.; Christen, H.; Brühlmann, M. Methods in quantitative image analysis. Histochem. Cell Biol. 1996, 105, 333–355. [Google Scholar] [CrossRef]
- Gossage, K.W.; Tkaczyk, T.S.; Rodriguez, J.J.; Barton, J.K. Texture analysis of optical coherence tomography images: Feasibility for tissue classification. J. Biomed. Opt. 2003, 8, 570–575. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Ashok, P.C.; Rao, K.D.; Majumder, S.K.; Verma, Y.; Gupta, P.K. Binary Tissue Classification Studies on Resected Human Breast Tissues Using Optical Coherence Tomography Images. J. Innov. Opt. Health Sci. 2011, 4, 59–66. [Google Scholar] [CrossRef]
- Anantrasirichai, N.; Achim, A.; Morgan, J.E.; Erchova, I.; Nicholson, L. SVM-based texture classification in Optical Coherence Tomography. In Proceedings of the 2013 IEEE 10th International Symposium on Biomedical Imaging, San Francisco, CA, USA, 7–11 April 2013; pp. 1332–1335. [Google Scholar] [CrossRef] [Green Version]
- Kajić, V.; Považay, B.; Hermann, B.; Hofer, B.; Marshall, D.; Rosin, P.L.; Drexler, W. Robust segmentation of intraretinal layers in the normal human fovea using a novel statistical model based on texture and shape analysis. Opt. Express 2010, 18, 14730–14744. [Google Scholar] [CrossRef] [PubMed]
- González-López, A.; Remeseiro, B.; Ortega, M.; Penedo, M.; Charlón, P. A Texture-Based Method for Choroid Segmentation in Retinal EDI-OCT Images; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Costantini, R.; Sbaiz, L.; Susstrunk, S. Higher Order SVD Analysis for Dynamic Texture Synthesis. IEEE T. Image Process 2008, 17, 42–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxi, J.; Calhoun, W.; Sepah, Y.J.; Hammer, D.X.; Ilev, I.; Pfefer, T.J.; Nguyen, Q.D.; Agrawal, A. Retina-simulating phantom for optical coherence tomography. J. Biomed. Opt. 2014, 19, 21106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, B.F.; Loitsch, S.; McLaughlin, R.A.; Scolaro, L.; Rigby, P.; Sampson, D.D. Fibrin phantom for use in optical coherence tomography. J. Biomed. Opt. 2010, 15, 030507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmonde-White, F.W.L.; Esmonde-White, K.A.; Kole, M.R.; Goldstein, S.A.; Roessler, B.J.; Morris, M.D. Biomedical tissue phantoms with controlled geometric and optical properties for Raman spectroscopy and tomography. Analyst 2011, 136, 4437–4446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, S.J.; Martinez, S.L.; DeCerce, J.; Romero, A.; Caldera, L.; Godavarty, A. Three-dimensional fluorescence tomography of human breast tissues in vivo using a hand-held optical imager. Phys. Med. Biol. 2013, 58, 1563. [Google Scholar] [CrossRef] [Green Version]
- Nivetha, K.B.; Sujatha, N. Development of thin skin mimicking bilayer solid tissue phantoms for optical spectroscopic studies. Biomed. Opt. Express 2017, 8, 3198–3212. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Birngruber, R.; Rosperich, J.; Engelhardt, R. Low-coherence optical tomography in turbid tissue: Theoretical analysis. Appl. Opt. 1995, 34, 6564–6574. [Google Scholar] [CrossRef]
- Schmitt, J.M.; Lee, S.L.; Yung, K.M. An optical coherence microscope with enhanced resolving power in thick tissue. Opt. Commun. 1997, 142, 203–207. [Google Scholar] [CrossRef]
- Williams, D.R. Visual consequences of the foveal pit. Investig. Ophthalmol. Vis. Sci. 1980, 19, 653–667. [Google Scholar]
- Zhang, Q.; Zhong, L.; Tang, P.; Yuan, Y.; Liu, S.; Tian, J.; Lu, X. Quantitative refractive index distribution of single cell by combining phase-shifting interferometry and AFM imaging. Sci. Rep. UK 2017, 7, 2532. [Google Scholar] [CrossRef] [PubMed]
- Rappaz, B.; Marquet, P.; Cuche, E.; Emery, Y.; Depeursinge, C.; Magistretti, P.J. Measurement of the integral refractive index and dynamic cell morphometry of living cells with digital holographic microscopy. Opt. Express 2005, 13, 9361–9373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haseda, K.; Kanematsu, K.; Noguchi, K.; Saito, H.; Umeda, N.; Ohta, Y. Significant correlation between refractive index and activity of mitochondria: Single mitochondrion study. Biomed. Opt. Express 2015, 6, 859–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogowska, J.; Bryant, C.M.; Brezinski, M.E. Cartilage thickness measurements from optical coherence tomography. J. Opt. Soc. Am. A 2003, 20, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Chen, Y.-W. A Statistical Texture Model of the Liver Based on Generalized N-Dimensional Principal Component Analysis (GND-PCA) and 3D Shape Normalization. Int. J. Biomed. Imaging 2011, 2001, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kischkat, J.; Peters, S.; Gruska, B.; Semtsiv, M.; Chashnikova, M.; Klinkmüller, M.; Fedosenko, O.; Machulik, S.; Aleksandrova, A.; Monastyrskyi, G.; et al. Mid-infrared optical properties of thin films of aluminum oxide, titanium dioxide, silicon dioxide, aluminum nitride, and silicon nitride. Appl. Optics 2012, 51, 6789–6798. [Google Scholar] [CrossRef] [PubMed]
- Drexler, W.; Morgner, U.; Ghanta, R.K.; Kärtner, F.X.; Schuman, J.S.; Fujimoto, J.G. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat. Med. 2001, 7, 502. [Google Scholar] [CrossRef] [PubMed]
- Yogesan, K.; Jørgensen, T.; Albregtsen, F.; Tveter, K.J.; Danielsen, H.E. Entropy-based texture analysis of chromatin structure in advanced prostate cancer. Cytometry 1996, 24, 268–276. [Google Scholar] [CrossRef]
- Basset, O.; Sun, Z.; Mestas, J.L.; Gimenez, G. Texture Analysis of Ultrasonic Images of the Prostate by Means of Co-occurrence Matrices. Ultrason. Imaging 1993, 15, 218–237. [Google Scholar] [CrossRef]
- Conners, R.W.; Harlow, C.A. A theoretical comparison of texture algorithms. IEEE T. Pattern Anal. 1980, 2, 204–222. [Google Scholar] [CrossRef]
- Hongchuan, Y.; Bennamoun, M. 1D-PCA, 2D-PCA to nD-PCA. In Proceedings of the 18th International Conference on Pattern Recognition (ICPR’06), Hong Kong, China, 20–24 August 2006; pp. 181–184. [Google Scholar] [CrossRef]
- Schmitt, J.M.; Knüttel, A.; Yadlowsky, M.; Eckhaus, M.A. Optical-coherence tomography of a dense tissue: Statistics of attenuation and backscattering. Phys. Med. Biol. 1994, 39, 1705. [Google Scholar] [CrossRef] [PubMed]
- De Grand, A.M.; Lomnes, S.J.; Lee, D.S.; Pietrzykowski, M.; Ohnishi, S.; Morgan, T.G.; Gogbashian, A.; Laurence, R.G.; Frangioni, J.V. Tissue-like phantoms for near-infrared fluorescence imaging system assessment and the training of surgeons. J. Biomed. Opt. 2006, 11, 014007. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.M. OCT elastography: Imaging microscopic deformation and strain of tissue. Opt. Express 1998, 3, 199–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Phantom Groups | Phantom Types | Number of OCT Scans |
|---|---|---|
| Different concentrations of gelatin solution | 2% gelatin solution (2% GS) | 10 |
| 5% gelatin solution | 10 | |
| 10% gelatin solution | 10 | |
| Different size of scatterers | 2% GS + 384 nm 50 µL PBs | 10 |
| 2% GS + 1 µm 50 µL PBs | 10 | |
| 2% GS + 2 µm 50 µL PBs | 10 | |
| 2% GS + 5 µm 50 µL PBs | 10 | |
| 2% GS + 15 µm 50 µL PBs | 10 | |
| Different concentration of scatterers | 2% GS + 1 µm 5 µL PBs | 10 |
| 2% GS + 1 µm 10 µL PBs | 10 | |
| 2% GS + 1 µm 20 µL PBs | 10 | |
| 2% GS + 1 µm 30 µL PBs | 10 | |
| 2% GS + 1 µm 40 µL PBs | 10 | |
| 2% GS + 1 µm 50 µL PBs | 10 | |
| Different refractive index of scatterers | 2% GS + 1 µm 50 µL PBs | 10 |
| 2% GS + 1 µm 50 µL SiO2 | 10 |
| Phantom Groups | Accuracy of SVM | AUC | ||
|---|---|---|---|---|
| with PCA | without PCA | with PCA | without PCA | |
| Different concentrations of gelatin solution | 91.9% | 100% | 0.99 | 1.00 |
| Different size of scatterers | 100% | 100% | 1.00 | 1.00 |
| Different size of scatterers with submicron particles | 82.5% | 100% | 1.00 | 1.00 |
| Different concentration of scatterers | 86.7% | 99.9% | 1.00 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulmaganbetov, M.; Bevan, R.J.; Anantrasirichai, N.; Achim, A.; Erchova, I.; White, N.; Albon, J.; Morgan, J.E. Textural Feature Analysis of Optical Coherence Tomography Phantoms. Electronics 2022, 11, 669. https://doi.org/10.3390/electronics11040669
Kulmaganbetov M, Bevan RJ, Anantrasirichai N, Achim A, Erchova I, White N, Albon J, Morgan JE. Textural Feature Analysis of Optical Coherence Tomography Phantoms. Electronics. 2022; 11(4):669. https://doi.org/10.3390/electronics11040669
Chicago/Turabian StyleKulmaganbetov, Mukhit, Ryan J. Bevan, Nantheera Anantrasirichai, Alin Achim, Irina Erchova, Nick White, Julie Albon, and James E. Morgan. 2022. "Textural Feature Analysis of Optical Coherence Tomography Phantoms" Electronics 11, no. 4: 669. https://doi.org/10.3390/electronics11040669









