Analysis of Metabolic Markers in Patients with Chronic Heart Failure before and after LVAD Implantation
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Laboratory Parameters
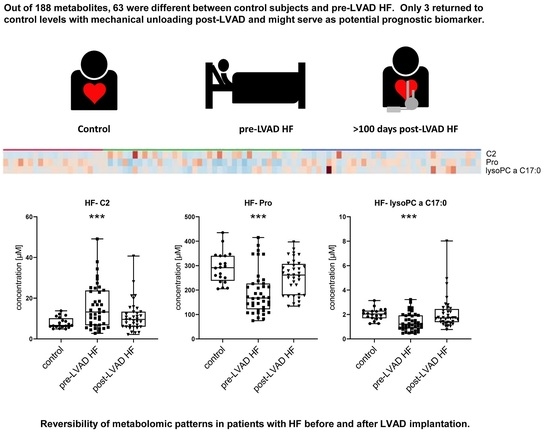
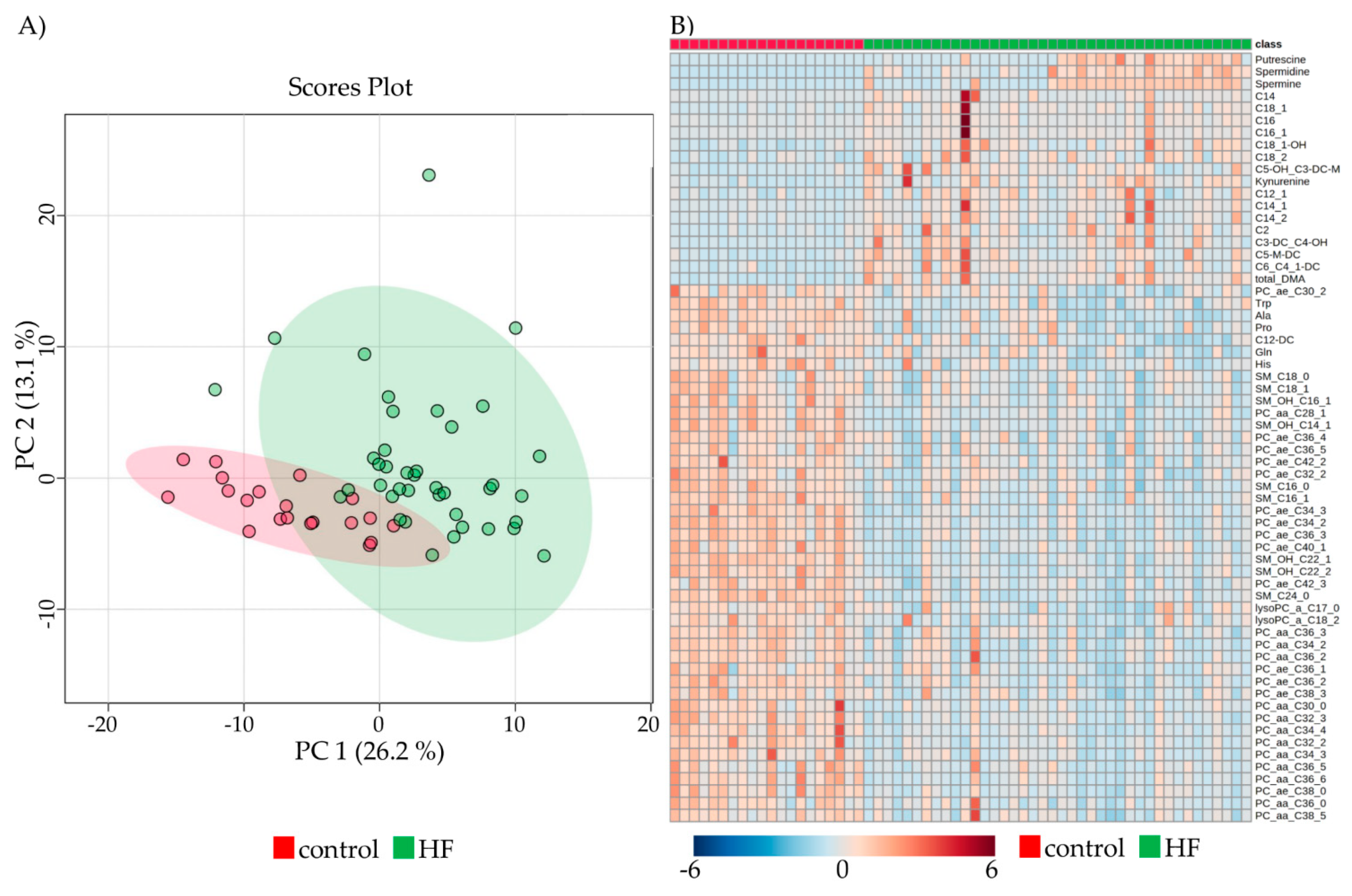
2.3. Metabolomic Profiles of Subjects with HF Pre-LVAD Compared to Controls
2.4. Comparison between DCM and ICM at Baseline and during Follow-Up
2.5. Correlations of Metabolites with Clinical and Standard Laboratory Parameters
3. Discussion
4. Material and Methods
4.1. Patient Cohort
4.2. Laboratory and Mass Spectrometric Analysis
4.3. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Timmis, A.; Townsend, N.; Gale, C.; Grobbee, R.; Maniadakis, N.; Flather, M.; Wilkins, E.; Wright, L.; Vos, R.; Bax, J.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur. Heart J. 2018, 39, 508–579. [Google Scholar] [CrossRef] [Green Version]
- Dal Ferro, M.; Severini, G.M.; Gigli, M.; Mestroni, L.; Sinagra, G. Genetics of Dilated Cardiomyopathy: Current Knowledge and Future Perspectives. In Dilated Cardiomyopathy: From Genetics to Clinical Management; Sinagra, G., Merlo, M., Pinamonti, B., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 45–69. [Google Scholar] [CrossRef] [Green Version]
- Krum, H. Guidelines for management of patients with chronic heart failure in Australia. Med. J. Aust. 2001, 174, 459–466. [Google Scholar] [PubMed]
- Franco-Cereceda, A.; Lockowandt, U.; Olsson, A.; Bredin, F.; Forssell, G.; Owall, A.; Runsio, M.; Liska, J. Early results with cardiac support device implant in patients with ischemic and non-ischemic cardiomyopathy. Scand. Cardiovasc. J. 2004, 38, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Zizola, C.; Schulze, P.C. Metabolic and structural impairment of skeletal muscle in heart failure. Heart Fail. Rev. 2013, 18, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Paneroni, M.; Pasini, E.; Comini, L.; Vitacca, M.; Schena, F.; Scalvini, S.; Venturelli, M. Skeletal Muscle Myopathy in Heart Failure: The Role of Ejection Fraction. Curr. Cardiol. Rep. 2018, 20, 116. [Google Scholar] [CrossRef]
- Bekfani, T.; Bekhite Elsaied, M.; Derlien, S.; Nisser, J.; Westermann, M.; Nietzsche, S.; Hamadanchi, A.; Frob, E.; Westphal, J.; Haase, D.; et al. Skeletal Muscle Function, Structure, and Metabolism in Patients with Heart Failure with Reduced Ejection Fraction and Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2020, 13, e007198. [Google Scholar] [CrossRef] [PubMed]
- Mosterd, A.; Hoes, A.W. Clinical epidemiology of heart failure. Heart 2007, 93, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.J.; Ryan, R.; Nichols, L.; Gale, N.; Hobbs, F.R.; Marshall, T. Survival following a diagnosis of heart failure in primary care. Fam. Pract. 2017, 34, 161–168. [Google Scholar] [CrossRef]
- DeVore, A.D.; Patel, P.A.; Patel, C.B. Medical Management of Patients with a Left Ventricular Assist Device for the Non-Left Ventricular Assist Device Specialist. JACC Heart Fail. 2017, 5, 621–631. [Google Scholar] [CrossRef]
- Kadakia, S.; Moore, R.; Ambur, V.; Toyoda, Y. Current status of the implantable LVAD. Gen. Thorac. Cardiovasc. Surg. 2016, 64, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Kirklin, J.K.; Naftel, D.C.; Pagani, F.D.; Kormos, R.L.; Stevenson, L.W.; Blume, E.D.; Myers, S.L.; Miller, M.A.; Baldwin, J.T.; Young, J.B. Seventh INTERMACS annual report: 15,000 patients and counting. J. Heart Lung. Transpl. 2015, 34, 1495–1504. [Google Scholar] [CrossRef]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef]
- Severino, P.; Mather, P.J.; Pucci, M.; D’Amato, A.; Mariani, M.V.; Infusino, F.; Birtolo, L.I.; Maestrini, V.; Mancone, M.; Fedele, F. Advanced Heart Failure and End-Stage Heart Failure: Does a Difference Exist. Diagnostics 2019, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Rose, E.A.; Gelijns, A.C.; Moskowitz, A.J.; Heitjan, D.F.; Stevenson, L.W.; Dembitsky, W.; Long, J.W.; Ascheim, D.D.; Tierney, A.R.; Levitan, R.G.; et al. Long-term use of a left ventricular assist device for end-stage heart failure. N. Engl. J. Med. 2001, 345, 1435–1443. [Google Scholar] [CrossRef]
- Adams, E.E.; Wrightson, M.L. Quality of life with an LVAD: A misunderstood concept. Heart Lung. 2018, 47, 177–183. [Google Scholar] [CrossRef]
- Marcinkiewicz-Siemion, M.; Ciborowski, M.; Ptaszynska-Kopczynska, K.; Szpakowicz, A.; Lisowska, A.; Jasiewicz, M.; Waszkiewicz, E.; Kretowski, A.; Musial, W.J.; Kaminski, K.A. LC-MS-based serum fingerprinting reveals significant dysregulation of phospholipids in chronic heart failure. J. Pharm. Biomed. Anal. 2018, 154, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Wever-Pinzon, J.; Selzman, C.H.; Stoddard, G.; Wever-Pinzon, O.; Catino, A.; Kfoury, A.G.; Diakos, N.A.; Reid, B.B.; McKellar, S.; Bonios, M.; et al. Impact of Ischemic Heart Failure Etiology on Cardiac Recovery During Mechanical Unloading. J. Am. Coll. Cardiol. 2016, 68, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Haase, D.; Baz, L.; Bekfani, T.; Neugebauer, S.; Kiehntopf, M.; Mobius-Winkler, S.; Franz, M.; Schulze, P.C. Metabolomic profiling of patients with high gradient aortic stenosis undergoing transcatheter aortic valve replacement. Clin. Res. Cardiol. 2020, 110, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Tarasenko, T.N.; Cusmano-Ozog, K.; McGuire, P.J. Tissue acylcarnitine status in a mouse model of mitochondrial beta-oxidation deficiency during metabolic decompensation due to influenza virus infection. Mol. Genet. Metab. 2018, 125, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.; Labarthe, F.; Fortier, A.; Bouchard, B.; Thompson Legault, J.; Bolduc, V.; Rigal, O.; Chen, J.; Ducharme, A.; Crawford, P.A.; et al. Circulating acylcarnitine profile in human heart failure: A surrogate of fatty acid metabolic dysregulation in mitochondria and beyond. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H768–H781. [Google Scholar] [CrossRef] [Green Version]
- Quanico, J.; Hauberg-Lotte, L.; Devaux, S.; Laouby, Z.; Meriaux, C.; Raffo-Romero, A.; Rose, M.; Westerheide, L.; Vehmeyer, J.; Rodet, F.; et al. 3D MALDI mass spectrometry imaging reveals specific localization of long-chain acylcarnitines within a 10-day time window of spinal cord injury. Sci. Rep. 2018, 8, 16083. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, T.; Kelly, J.P.; McGarrah, R.W.; Hellkamp, A.S.; Fiuzat, M.; Testani, J.M.; Wang, T.S.; Verma, A.; Samsky, M.D.; Donahue, M.P.; et al. Prognostic Implications of Long-Chain Acylcarnitines in Heart Failure and Reversibility with Mechanical Circulatory Support. J. Am. Coll. Cardiol. 2016, 67, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, P.; Cowan, T.M.; Matern, D. Acylcarnitine profile analysis. Genet. Med. 2008, 10, 151–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuler, M.H.; Di Bartolomeo, F.; Martensson, C.U.; Daum, G.; Becker, T. Phosphatidylcholine Affects Inner Membrane Protein Translocases of Mitochondria. J. Biol. Chem. 2016, 291, 18718–18729. [Google Scholar] [CrossRef] [Green Version]
- Mueller-Hennessen, M.; Dungen, H.D.; Lutz, M.; Trippel, T.D.; Kreuter, M.; Sigl, J.; Muller, O.J.; Tahirovic, E.; Witt, H.; Ternes, P.; et al. A Novel Lipid Biomarker Panel for the Detection of Heart Failure with Reduced Ejection Fraction. Clin. Chem. 2017, 63, 267–277. [Google Scholar] [CrossRef]
- Li, X.; Fang, P.; Li, Y.; Kuo, Y.M.; Andrews, A.J.; Nanayakkara, G.; Johnson, C.; Fu, H.; Shan, H.; Du, F.; et al. Mitochondrial Reactive Oxygen Species Mediate Lysophosphatidylcholine-Induced Endothelial Cell Activation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1090–1100. [Google Scholar] [CrossRef] [Green Version]
- Fratta Pasini, A.; Stranieri, C.; Pasini, A.; Vallerio, P.; Mozzini, C.; Solani, E.; Cominacini, M.; Cominacini, L.; Garbin, U. Lysophosphatidylcholine and carotid intima-media thickness in young smokers: A role for oxidized LDL-induced expression of PBMC lipoprotein-associated phospholipase A2? PLoS ONE 2013, 8, e83092. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, I.; Edsfeldt, A.; Ko, N.Y.; Grufman, H.; Berg, K.; Bjorkbacka, H.; Nitulescu, M.; Persson, A.; Nilsson, M.; Prehn, C.; et al. Evidence supporting a key role of Lp-PLA2-generated lysophosphatidylcholine in human atherosclerotic plaque inflammation. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- Lavi, S.; McConnell, J.P.; Rihal, C.S.; Prasad, A.; Mathew, V.; Lerman, L.O.; Lerman, A. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: Association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation 2007, 115, 2715–2721. [Google Scholar] [CrossRef] [Green Version]
- Mannheim, D.; Herrmann, J.; Versari, D.; Gossl, M.; Meyer, F.B.; McConnell, J.P.; Lerman, L.O.; Lerman, A. Enhanced expression of Lp-PLA2 and lysophosphatidylcholine in symptomatic carotid atherosclerotic plaques. Stroke 2008, 39, 1448–1455. [Google Scholar] [CrossRef] [Green Version]
- Law, S.H.; Chan, M.L.; Marathe, G.K.; Parveen, F.; Chen, C.H.; Ke, L.Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.K.; Lee, D.H.; Kim, J.K.; Park, M.J.; Yan, J.J.; Song, D.K.; Vaziri, N.D.; Noh, J.W. Lysophosphatidylcholine, oxidized low-density lipoprotein and cardiovascular disease in Korean hemodialysis patients: Analysis at 5 years of follow-up. J. Korean Med. Sci. 2013, 28, 268–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stegemann, C.; Pechlaner, R.; Willeit, P.; Langley, S.R.; Mangino, M.; Mayr, U.; Menni, C.; Moayyeri, A.; Santer, P.; Rungger, G.; et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation 2014, 129, 1821–1831. [Google Scholar] [CrossRef] [Green Version]
- Ganna, A.; Salihovic, S.; Sundstrom, J.; Broeckling, C.D.; Hedman, A.K.; Magnusson, P.K.; Pedersen, N.L.; Larsson, A.; Siegbahn, A.; Zilmer, M.; et al. Large-scale metabolomic profiling identifies novel biomarkers for incident coronary heart disease. PLoS Genet. 2014, 10, e1004801. [Google Scholar] [CrossRef]
- Ward-Caviness, C.K.; Xu, T.; Aspelund, T.; Thorand, B.; Montrone, C.; Meisinger, C.; Dunger-Kaltenbach, I.; Zierer, A.; Yu, Z.; Helgadottir, I.R.; et al. Improvement of myocardial infarction risk prediction via inflammation-associated metabolite biomarkers. Heart 2017, 103, 1278–1285. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; Sung, M.M.; Ezekowitz, J.; Mandal, R.; Han, B.; Bjorndahl, T.C.; Bouatra, S.; Anderson, T.; Oudit, G.Y.; Wishart, D.S.; et al. Metabolomic fingerprint of heart failure with preserved ejection fraction. PLoS ONE 2015, 10, e0124844. [Google Scholar] [CrossRef]
- Polzin, A.; Piayda, K.; Keul, P.; Dannenberg, L.; Mohring, A.; Graler, M.; Zeus, T.; Kelm, M.; Levkau, B. Plasma sphingosine-1-phosphate concentrations are associated with systolic heart failure in patients with ischemic heart disease. J. Mol. Cell. Cardiol. 2017, 110, 35–37. [Google Scholar] [CrossRef]
- Sun, H.; Olson, K.C.; Gao, C.; Prosdocimo, D.A.; Zhou, M.; Wang, Z.; Jeyaraj, D.; Youn, J.Y.; Ren, S.; Liu, Y.; et al. Catabolic Defect of Branched-Chain Amino Acids Promotes Heart Failure. Circulation 2016, 133, 2038–2049. [Google Scholar] [CrossRef] [PubMed]
- Uddin, G.M.; Zhang, L.; Shah, S.; Fukushima, A.; Wagg, C.S.; Gopal, K.; Al Batran, R.; Pherwani, S.; Ho, K.L.; Boisvenue, J.; et al. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc. Diabetol. 2019, 18, 86. [Google Scholar] [CrossRef]
- Kennel, P.J.; Givens, R.; Brunjes, D.; Chen, E.; Castillero, E.; Takayama, H.; Naka, Y.; George, I.; Mancini, D.; Schulze, P.C. Serum-Derived Exosomal Proteome Analysis of Patients with Heart Failure and After Heart Transplantation. J. Heart Lung Transplant. 2015, 34, S301. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Z.; Li, X.; Li, C.; Hao, J.; Luo, Y.; Lei, M.; Li, J.; Liu, C.; He, K. Macitentan Attenuates Chronic Mountain Sickness in Rats by Regulating Arginine and Purine Metabolism. J. Proteome Res. 2020, 19, 3302–3314. [Google Scholar] [CrossRef]
- Sansbury, B.E.; DeMartino, A.M.; Xie, Z.; Brooks, A.C.; Brainard, R.E.; Watson, L.J.; DeFilippis, A.P.; Cummins, T.D.; Harbeson, M.A.; Brittian, K.R.; et al. Metabolomic analysis of pressure-overloaded and infarcted mouse hearts. Circ. Heart Fail. 2014, 7, 634–642. [Google Scholar] [CrossRef] [Green Version]
- Nagano, T.; Nakashima, A.; Onishi, K.; Kawai, K.; Awai, Y.; Kinugasa, M.; Iwasaki, T.; Kikkawa, U.; Kamada, S. Proline dehydrogenase promotes senescence through the generation of reactive oxygen species. J. Cell Sci. 2017, 130, 1413–1420. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.A.; Donald, S.P.; Yu, J.; Lin, W.W.; Liu, Z.; Steel, G.; Obie, C.; Valle, D.; Phang, J.M. Overexpression of proline oxidase induces proline-dependent and mitochondria-mediated apoptosis. Mol. Cell Biochem. 2007, 295, 85–92. [Google Scholar] [CrossRef]
- Phang, J.M. Proline Metabolism in Cell Regulation and Cancer Biology: Recent Advances and Hypotheses. Antioxid. Redox Signal. 2019, 30, 635–649. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xue, Z.; Lin, J.; Wang, Y.; Ying, H.; Lv, Q.; Hua, C.; Wang, M.; Chen, S.; Zhou, B. Proline improves cardiac remodeling following myocardial infarction and attenuates cardiomyocyte apoptosis via redox regulation. Biochem. Pharmacol. 2020, 178, 114065. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, Y.; Chen, Y.; Zhao, Y.J.; Liu, L.W.; Li, J.; Wang, S.L.; Alolga, R.N.; Yin, Y.; Wang, X.M.; et al. Comprehensive Metabolomic Characterization of Coronary Artery Diseases. J. Am. Coll. Cardiol. 2016, 68, 1281–1293. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Li, L.; Chai, N.; Chen, Y.; Wu, F.; Zhang, W.; Wang, L.; Shi, S.; Zhang, L.; et al. Spermine and spermidine reversed age-related cardiac deterioration in rats. Oncotarget 2017, 8, 64793–64808. [Google Scholar] [CrossRef] [Green Version]
- Hunter, W.G.; Kelly, J.P.; McGarrah, R.W., 3rd; Khouri, M.G.; Craig, D.; Haynes, C.; Ilkayeva, O.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.J.; et al. Metabolomic Profiling Identifies Novel Circulating Biomarkers of Mitochondrial Dysfunction Differentially Elevated in Heart Failure with Preserved Versus Reduced Ejection Fraction: Evidence for Shared Metabolic Impairments in Clinical Heart Failure. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [Green Version]




| Controls (n = 20) | DCM (n = 20) | ICM (n = 20) | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Female/male (w%) | 13/7 (65%) | 4/16 (20%) | 6/14 (30%) | 0.010 |
| Age (years) | 65.5 (9) | 58.0 (19) | 60.5 (16) | 0.062 |
| BMI (kg/m2) | 25.2 (4.8) | 27.4 (5.3) | 24.6 (6.2) | 0.091 |
| LV characteristics | ||||
| LV-EF (%) (after LVAD) | 61.0 (7) | 20.0 (7) * 27.0 (7) ** | 24.0 (5) * 25.0 (8) ** | <0.0001 0.443 |
| LVEDD (mm) (after LVAD) | 43.0 (26.3) | 71.5 (26.3) * 57.0 (22) ** | 67.0 (9.8) 58.0 (16.8) ** | <0.0001 0.667 |
| CV risk factors | ||||
| Type 2 diabetes | 2 | 5 | 1 | 0.246 |
| Arterial Hypertension (≥140/90 mmHg) | 10 | 8 | 11 | 0.726 |
| Overweight (BMI > 25) | 11 | 16 | 10 | 0.127 |
| Controls | Heart Failure | ||||
|---|---|---|---|---|---|
| Pre-LVAD | 30 Days Post-LVAD | >100 Days Post-LVAD | p Value | ||
| Leucocytes (109/L) | 6.1 (1.9) | 8.5 (5.4) °,% | 9.6 (4.6) § | 7.4 (4.0) | <0.0001 * |
| Cholesterol (mg/dL) | 232.4 (60.7) | 162.0 (82) ° | 169.0 (72) | 198.5 (72) | <0.001 * |
| Triglycerides (mg/dL) | 118.6 (71.3) | 97.5 (78.8) #,% | 113.5 (82.8) | 138.5 (140) | 0.417 |
| ASAT (U/L) | 28.0 (5.8) | 31 (24) | 30 (20.3) | 28 (13) | 0.461 |
| ALAT (U/L) | 22.5 (10) | 24 (22) # | 19.5 (21.8) | 21.5 (14.3) | 0.475 |
| CRP (mg/dL) | 0.2 (0.03) | 3.1 (2.9) °,% | 4.3 (7.5) § | 0.84 (2.1) | <0.0001 * |
| Creatinine (mg/dL) | 0.78 (0.2) | 1.3 (0.8) ° | 1.1 (0.6) § | 1.5 (1) | <0.001 * |
| Urea (mg/dL) | 34.0 (13.3) | 52.0 (43) ° | 40.5 (46) | 45.0 (41.5) | 0.006 * |
| BNP (pg/mL) | 35.5 (31.3) | 612.0 (1104.9) °,% | 392.0 (438.2) ° | 354.7 (445.9) ° | <0.0001 * |
| Metabolite Class | Pre-LVAD | >100 Days Post-LVAD | ||
|---|---|---|---|---|
| DCM | ICM | DCM | ICM | |
| AC | 8 | 7 | 7 | 1 |
| AA | 9 | 1 | 2 | 0 |
| BA | 4 | 2 | 7 | 1 |
| PC | 29 | 18 | 19 | 15 |
| SM | 8 | 10 | 6 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilse, M.S.; Kretzschmar, T.; Pistulli, R.; Franz, M.; Bekfani, T.; Haase, D.; Neugebauer, S.; Kiehntopf, M.; Gummert, J.F.; Milting, H.; et al. Analysis of Metabolic Markers in Patients with Chronic Heart Failure before and after LVAD Implantation. Metabolites 2021, 11, 615. https://doi.org/10.3390/metabo11090615
Hilse MS, Kretzschmar T, Pistulli R, Franz M, Bekfani T, Haase D, Neugebauer S, Kiehntopf M, Gummert JF, Milting H, et al. Analysis of Metabolic Markers in Patients with Chronic Heart Failure before and after LVAD Implantation. Metabolites. 2021; 11(9):615. https://doi.org/10.3390/metabo11090615
Chicago/Turabian StyleHilse, Marion S., Tom Kretzschmar, Rudin Pistulli, Marcus Franz, Tarek Bekfani, Daniela Haase, Sophie Neugebauer, Michael Kiehntopf, Jan F. Gummert, Hendrik Milting, and et al. 2021. "Analysis of Metabolic Markers in Patients with Chronic Heart Failure before and after LVAD Implantation" Metabolites 11, no. 9: 615. https://doi.org/10.3390/metabo11090615