Advances in Mass Spectrometry-Based Blood Metabolomics Profiling for Non-Cancer Diseases: A Comprehensive Review
Abstract
:1. Introduction
2. Review Methodology
3. Scientometrics
4. Methods Landscape
4.1. Separation Methods
4.1.1. Gas Chromatography (GC)
4.1.2. Comprehensive Two-Dimensional Gas Chromatography (GC×GC)
4.1.3. Liquid Chromatography (LC)
4.1.4. Capillary Electrophoresis (CE)
4.2. Mass Spectrometry (MS)
4.2.1. Electron Ionization (EI)
4.2.2. Atmospheric Pressure Ionization (API)
4.2.3. Tandem Mass Spectrometry (MS/MS)
5. Research Field Landscape
5.1. Lipidomics
5.1.1. Fatty Acids
5.1.2. Oxylipins
5.1.3. Phospholipids
5.1.4. Glycerophospholipids
5.1.5. Sphingolipids
5.1.6. Sterol Lipids
5.2. Glycomics
5.3. Amino Acids
6. Disease Study Landscape
6.1. Tuberculosis
6.2. Sepsis
6.3. Human Immunodeficiency Virus (HIV)
6.4. Diabetes
6.5. Non-Alcoholic Fatty Liver Disease (NAFLD)
6.6. Rheumatoid Arthritis (RA)
6.7. Systemic Lupus Erythematosus (SLE)
6.8. Other Diseases
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| AIDS | Acquired Immunodeficiency Syndrome |
| ART | Antiretroviral therapy |
| CE | Capillary Electrophoresis |
| CE-MS | Capillary Electrophoresis–Mass Spectrometry |
| CID | Collision-Induced Dissociation |
| DKD | Diabetic Kidney Disease |
| DR | Diabetic Retinopathy |
| EI | Electron Ionization |
| ESI | Electrospray Ionization |
| ESI-MS | Electrospray Ionization Mass Spectrometry |
| ESI-MS/MS | Electrospray Ionization Tandem Mass Spectrometry |
| ESI-QTOF | Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry |
| GC | Gas Chromatography |
| GC×GC | Comprehensive Two-dimensional Gas Chromatography |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| GC-MS/MS | Gas Chromatography–Tandem Mass Spectrometry |
| GDM | Gestational Diabetes Mellitus |
| HCV | Hepatitis C Virus |
| HDL | High-Density Lipoprotein |
| HF | Heart Failure |
| HIV | Human Immunodeficiency Virus |
| HPLC | High-Performance Liquid Chromatography |
| HPLC-MS | High-Performance Liquid Chromatography–Mass Spectrometry |
| HPLC-MS/MS | High-Performance Liquid Chromatography–Tandem Mass Spectrometry |
| ICU | Intense Care Unit |
| LC | Liquid Chromatography |
| LC-MS | Liquid Chromatography–Mass Spectrometry |
| LC-MS/MS | Liquid Chromatography–Tandem Mass Spectrometry |
| LDL | Low-Density Lipoprotein |
| LN | Lupus Nephritis |
| LPC | Lysophosphatidylcholine |
| m/z | Mass-to-Charge Ratio |
| MRM | Multiple Reaction Monitoring |
| MRM-MS | Multiple Reaction Monitoring Mass Spectrometry |
| MS | Mass Spectrometry |
| MS/MS | Tandem Mass Spectrometry |
| NAFL | Nonalcoholic Fatty Liver |
| NAFLD | Non-alcoholic Fatty Liver Disease |
| NASH | Nonalcoholic steatohepatitis |
| PC | Phosphatidylcholine |
| PCOS | Polycystic Ovary Syndrome |
| PCT | Procalcitonin |
| PDR | Proliferative Diabetic Retinopathy |
| QQQ | Triple Quadrupole |
| QTOF | Quadrupole Time-of-Flight |
| RA | Rheumatoid Arthritis |
| RYGB | Roux-en-Y Gastric Bypass |
| SLE | Systemic Lupus Erythematosus |
| SM | Sphingomyelin |
| SRM | Selected Reaction Monitoring |
| T1D | Type 1 Diabetes |
| T2D | Type 2 Diabetes |
| TCA | Tricarboxylic Acid |
| TNF- | Tumour Necrosis Factor alpha |
| TOF | Time-of-Flight |
| TOF-MS | Time-of-Flight Mass Spectrometry |
| UHPLC | Ultra-High-Performance Liquid Chromatography |
| UHPLC-MS/MS | Ultra-High Performance Liquid Chromatography–Tandem Mass Spectrometry |
| UPLC | Ultra Performance Liquid Chromatography |
| UPLC-Q-TOF-MS | Ultra-Performance Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry |
| WHO | World Health Organization |
References
- Hernandes, V.V.; Barbas, C.; Dudzik, D. A review of blood sample handling and pre-processing for metabolomics studies. Electrophoresis 2017, 38, 2232–2241. [Google Scholar] [CrossRef] [PubMed]
- Serkova, N.J.; Standiford, T.J.; Stringer, K.A. The emerging field of quantitative blood metabolomics for biomarker discovery in critical illnesses. Am. J. Respir. Crit. Care Med. 2011, 184, 647–655. [Google Scholar] [CrossRef]
- Dass, C. Fundamentals of Contemporary Mass Spectrometry; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Bird, S.; Klein, E.; Loper, E. Natural Language Processing with Python: Analyzing Text with the Natural Language Toolkit; O’Reilly Media, Inc.: Newton, MA, USA, 2009. [Google Scholar]
- Qaiser, S.; Ali, R. Text mining: Use of TF-IDF to examine the relevance of words to documents. Int. J. Comput. Appl. 2018, 181, 25–29. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, S.; Lu, Z. Transfer learning in biomedical natural language processing: An evaluation of BERT and ELMo on ten benchmarking datasets. arXiv 2019, arXiv:1906.05474. [Google Scholar]
- Van der Maaten, L.; Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- McInnes, L.; Healy, J.; Melville, J. Umap: Uniform manifold approximation and projection for dimension reduction. arXiv 2018, arXiv:1802.03426. [Google Scholar]
- Moon, K.R.; van Dijk, D.; Wang, Z.; Gigante, S.; Burkhardt, D.B.; Chen, W.S.; Yim, K.; Elzen, A.v.d.; Hirn, M.J.; Coifman, R.R.; et al. Visualizing structure and transitions in high-dimensional biological data. Nat. Biotechnol. 2019, 37, 1482–1492. [Google Scholar] [CrossRef]
- Kiseleva, O.; Kurbatov, I.; Ilgisonis, E.; Poverennaya, E. Defining blood plasma and serum metabolome by GC-MS. Metabolites 2021, 12, 15. [Google Scholar] [CrossRef]
- Honour, J.W. Gas chromatography-mass spectrometry. Horm. Assays Biol. Fluids 2006, 324, 53–74. [Google Scholar]
- Bou Khalil, M.; Hou, W.; Zhou, H.; Elisma, F.; Swayne, L.A.; Blanchard, A.P.; Yao, Z.; Bennett, S.A.; Figeys, D. Lipidomics era: Accomplishments and challenges. Mass Spectrom. Rev. 2010, 29, 877–929. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics by gas chromatography–mass spectrometry: Combined targeted and untargeted profiling. Curr. Protoc. Mol. Biol. 2016, 114, 30–34. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.; Zhou, Y.; Zhao, C.; Lu, X.; Xu, G. A rapid GC method coupled with quadrupole or time of flight mass spectrometry for metabolomics analysis. J. Chromatogr. B 2020, 1160, 122355. [Google Scholar] [CrossRef]
- Kałużna-Czaplińska, J. Current medical research with the application of coupled techniques with mass spectrometry. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2011, 17, RA117. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Huang, H.; Reim, A.; Charles, P.D.; Northage, A.; Jackson, D.; Parry, I.; Kessler, B.M. Optimizing 2D gas chromatography mass spectrometry for robust tissue, serum and urine metabolite profiling. Talanta 2017, 165, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Keppler, E.A.H.; Jenkins, C.L.; Davis, T.J.; Bean, H.D. Advances in the application of comprehensive two-dimensional gas chromatography in metabolomics. TrAC Trends Anal. Chem. 2018, 109, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Melder, J.; Zinsmeister, J.; Grein, T.; Jürgens, S.; Köhler, M.; Oßwald, P. Comprehensive Two-Dimensional Gas Chromatography: A Universal Method for Composition-Based Prediction of Emission Characteristics of Complex Fuels. Energy Fuels 2023, 37, 4580–4595. [Google Scholar] [CrossRef]
- Duangkumpha, K.; Jariyasopit, N.; Wanichthanarak, K.; Dhakal, E.; Wisanpitayakorn, P.; Thotsiri, S.; Sirivatanauksorn, Y.; Kitiyakara, C.; Sathirapongsasuti, N.; Khoomrung, S. GC× GC-TOFMS metabolomics analysis identifies elevated levels of plasma sugars and sugar alcohols in diabetic mellitus patients with kidney failure. J. Biol. Chem. 2022, 298, 102445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Wang, P.; Han, Y.; Wang, X. Modern analytical techniques in metabolomics analysis. Analyst 2012, 137, 293–300. [Google Scholar] [CrossRef]
- Winnike, J.H.; Wei, X.; Knagge, K.J.; Colman, S.D.; Gregory, S.G.; Zhang, X. Comparison of GC-MS and GC×GC-MS in the analysis of human serum samples for biomarker discovery. J. Proteome Res. 2015, 14, 1810–1817. [Google Scholar] [CrossRef]
- Maryutina, T.A.; Savonina, E.Y.; Fedotov, P.S.; Smith, R.M.; Siren, H.; Hibbert, D.B. Terminology of separation methods (IUPAC Recommendations 2017). Pure Appl. Chem. 2018, 90, 181–231. [Google Scholar] [CrossRef]
- Pitt, J.J. Principles and applications of liquid chromatography-mass spectrometry in clinical biochemistry. Clin. Biochem. Rev. 2009, 30, 19. [Google Scholar]
- Nováková, L.; Matysová, L.; Solich, P. Advantages of application of UPLC in pharmaceutical analysis. Talanta 2006, 68, 908–918. [Google Scholar] [CrossRef]
- de Villiers, A.; Lestremau, F.; Szucs, R.; Gélébart, S.; David, F.; Sandra, P. Evaluation of ultra performance liquid chromatography: Part I. Possibilities and limitations. J. Chromatogr. A 2006, 1127, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhong, L. Applications of liquid chromatography-mass spectrometry based metabolomics in predictive and personalized medicine. Front. Mol. Biosci. 2022, 9, 1049016. [Google Scholar] [CrossRef] [PubMed]
- Ramautar, R.; Somsen, G.W.; de Jong, G.J. CE-MS for metabolomics: Developments and applications in the period 2016–2018. Electrophoresis 2019, 40, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Robledo, V.R.; Smyth, W.F. Review of the CE-MS platform as a powerful alternative to conventional couplings in bio-omics and target-based applications. Electrophoresis 2014, 35, 2292–2308. [Google Scholar] [CrossRef]
- Chen, D.; McCool, E.N.; Yang, Z.; Shen, X.; Lubeckyj, R.A.; Xu, T.; Wang, Q.; Sun, L. Recent advances (2019–2021) of capillary electrophoresis-mass spectrometry for multilevel proteomics. Mass Spectrom. Rev. 2023, 42, 617–642. [Google Scholar] [CrossRef] [PubMed]
- McLafferty, F.; Turecek, F. Interpretation of Mass Spectra University Science Books Mill Vally. In Interpretation of Mass Spectra; University Science Books: Mill Valley, CA, USA, 1980. [Google Scholar]
- Gross, J.H. Mass Spectrometry: A Textbook; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Zullig, T.; Kofeler, H.C. High resolution mass spectrometry in lipidomics. Mass Spectrom. Rev. 2021, 40, 162–176. [Google Scholar] [CrossRef]
- Li, D.; Yi, J.; Han, G.; Qiao, L. MALDI-TOF mass spectrometry in clinical analysis and research. ACS Meas. Sci. Au 2022, 2, 385–404. [Google Scholar] [CrossRef]
- Han, X.; Yang, K.; Gross, R.W. Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrom. Rev. 2012, 31, 134–178. [Google Scholar] [CrossRef]
- Trbojevic-Akmacic, I.; Lageveen-Kammeijer, G.S.; Heijs, B.; Petrovic, T.; Deris, H.; Wuhrer, M.; Lauc, G. High-throughput glycomic methods. Chem. Rev. 2022, 122, 15865–15913. [Google Scholar] [CrossRef]
- Konz, T.; Migliavacca, E.; Dayon, L.; Bowman, G.; Oikonomidi, A.; Popp, J.; Rezzi, S. ICP-MS/MS-based ionomics: A validated methodology to investigate the biological variability of the human ionome. J. Proteome Res. 2017, 16, 2080–2090. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Verma, K. Metals from cell to environment: Connecting metallomics with other omics. Open J. Plant Sci. 2018, 3, 1–14. [Google Scholar]
- Giraudeau, P. NMR-based metabolomics and fluxomics: Developments and future prospects. Analyst 2020, 145, 2457–2472. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.H.; Szczepski, K.; Al-Younis, I.; Lachowicz, J.I.; Jaremko, M. Fluxomics-new metabolomics approaches to monitor metabolic pathways. Front. Pharmacol. 2022, 13, 299. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Jiang, H.; Maness, P. Dynamic Flux Analysis: An Experimental Approach of Fluxomics. Metab. Pathw. Eng. 2020, 2096, 179–196. [Google Scholar]
- Swinnen, J.V.; Dehairs, J. A beginner’s guide to lipidomics. Biochemist 2022, 44, 20–24. [Google Scholar] [CrossRef]
- Yang, L.; Li, M.; Shan, Y.; Shen, S.; Bai, Y.; Liu, H. Recent advances in lipidomics for disease research. J. Sep. Sci. 2016, 39, 38–50. [Google Scholar] [CrossRef]
- Anthonymuthu, T.S.; Kim-Campbell, N.; Bayır, H. Oxidative lipidomics: Applications in critical care. Curr. Opin. Crit. Care 2017, 23, 251. [Google Scholar] [CrossRef]
- Pisarska, A.; Wąsowicz, W.; Gromadzińska, J. Lipidomic profiles as a tool to search for new biomarkers. Int. J. Occup. Med. Environ. Health 2022, 35, 111–126. [Google Scholar] [CrossRef]
- Vvedenskaya, O.; Holčapek, M.; Vogeser, M.; Ekroos, K.; Meikle, P.J.; Bendt, A.K. Clinical lipidomics—A community-driven roadmap to translate research into clinical applications. J. Mass Spectrom. Adv. Clin. Lab 2022, 24, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Meikle, T.G.; Huynh, K.; Giles, C.; Meikle, P.J. Clinical lipidomics: Realizing the potential of lipid profiling. J. Lipid Res. 2021, 62, 100127. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Cheng, X.L.; Lin, R.C.; Wei, F. Lipidomics applications for disease biomarker discovery in mammal models. Biomarkers Med. 2015, 9, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Sarajlic, P.; Vigor, C.; Avignon, A.; Zhou, B.; Oger, C.; Galano, J.M.; Durand, T.; Sultan, A.; Bäck, M. Omega-3 to omega-6 fatty acid oxidation ratio as a novel inflammation resolution marker for metabolic complications in obesity. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Sun, B.; Zheng, P.; Li, N.; Wu, J.L. Derivatization enhanced separation and sensitivity of long chain-free fatty acids: Application to asthma using targeted and non-targeted liquid chromatography-mass spectrometry approach. Anal. Chim. Acta 2017, 989, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Szczuko, M.; Kotlęga, D.; Palma, J.; Zembroń-Łacny, A.; Tylutka, A.; Gołąb-Janowska, M.; Drozd, A. Lipoxins, RevD1 and 9, 13 HODE as the most important derivatives after an early incident of ischemic stroke. Sci. Rep. 2020, 10, 12849. [Google Scholar] [CrossRef] [PubMed]
- Szczuko, M.; Kaczkan, M.; Małgorzewicz, S.; Rutkowski, P.; Dębska-Ślizień, A.; Stachowska, E. The C18:3n6/C22:4n6 ratio is a good lipid marker of chronic kidney disease (CKD) progression. Lipids Health Dis. 2020, 19, 77. [Google Scholar] [CrossRef]
- Gautam, A.; Muhie, S.; Chakraborty, N.; Hoke, A.; Donohue, D.; Miller, S.A.; Hammamieh, R.; Jett, M. Metabolomic analyses reveal lipid abnormalities and hepatic dysfunction in non-human primate model for Yersinia pestis. Metabolomics 2019, 15, 2. [Google Scholar] [CrossRef]
- Johnson, M.; Pace, R.D.; McElhenney, W.H. Green leafy vegetables in diets with a 25: 1 omega-6/omega-3 fatty acid ratio modify the erythrocyte fatty acid profile of spontaneously hypertensive rats. Lipids Health Dis. 2018, 17, 140. [Google Scholar] [CrossRef]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef]
- Caligiuri, S.P.; Aukema, H.M.; Ravandi, A.; Pierce, G.N. Elevated levels of pro-inflammatory oxylipins in older subjects are normalized by flaxseed consumption. Exp. Gerontol. 2014, 59, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tang, X.; Wang, Y.; Chen, W.; Xue, Y.; Cao, H.; Zhang, B.; Pan, J.; Zhou, Q.; Wang, D.; et al. Serum Oxylipin Profiles Identify Potential Biomarkers in Patients with Acute Aortic Dissection. Metabolites 2022, 12, 587. [Google Scholar] [CrossRef] [PubMed]
- Nayeem, M.A. Role of oxylipins in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1142–1154. [Google Scholar] [CrossRef]
- Sun, Y.; Koh, H.W.; Choi, H.; Koh, W.P.; Yuan, J.M.; Newman, J.W.; Su, J.; Fang, J.; Ong, C.N.; van Dam, R.M. Plasma fatty acids, oxylipins, and risk of myocardial infarction: The Singapore Chinese Health Study. J. Lipid Res. 2016, 57, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Solati, Z.; Ravandi, A. Lipidomics of bioactive lipids in acute coronary syndromes. Int. J. Mol. Sci. 2019, 20, 1051. [Google Scholar] [CrossRef]
- Mastrogiovanni, M.; Trostchansky, A.; Naya, H.; Dominguez, R.; Marco, C.; Povedano, M.; López-Vales, R.; Rubbo, H. HPLC-MS/MS Oxylipin Analysis of Plasma from Amyotrophic Lateral Sclerosis Patients. Biomedicines 2022, 10, 674. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Mohan, M.; Brennan, E.P.; Woodman, O.L.; Godson, C.; Kantharidis, P.; Ritchie, R.H.; Qin, C.X. Therapeutic potential of lipoxin A4 in chronic inflammation: Focus on cardiometabolic disease. ACS Pharmacol. Transl. Sci. 2020, 3, 43–55. [Google Scholar] [CrossRef]
- Jaén, R.I.; Sánchez-García, S.; Fernández-Velasco, M.; Boscá, L.; Prieto, P. Resolution-based therapies: The potential of lipoxins to treat human diseases. Front. Immunol. 2021, 12, 658840. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Fan, M.; Jin, W. Lipoxins in the nervous system: Brighter prospects for neuroprotection. Front. Pharmacol. 2022, 13, 781889. [Google Scholar] [CrossRef]
- Anton, P.E.; Rutt, L.N.; Capper, C.; Orlicky, D.J.; McCullough, R.L. Profiling the oxylipidome in aged mice after chronic ethanol feeding: Identifying lipid metabolites as drivers of hepatocyte stress. Alcohol 2023, 107, 119–135. [Google Scholar] [CrossRef]
- Liakh, I.; Pakiet, A.; Sledzinski, T.; Mika, A. Modern methods of sample preparation for the analysis of oxylipins in biological samples. Molecules 2019, 24, 1639. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Hu, J.; Li, J.; Zhang, Y.; Shui, B.; Ding, Z.; Yao, L.; Fan, Y. Metabolomics analysis of collagen-induced arthritis in rats and interventional effects of oral tolerance. Anal. Biochem. 2014, 458, 49–57. [Google Scholar] [CrossRef]
- Li, J.; Che, N.; Xu, L.; Zhang, Q.; Wang, Q.; Tan, W.; Zhang, M. LC-MS-based serum metabolomics reveals a distinctive signature in patients with rheumatoid arthritis. Clin. Rheumatol. 2018, 37, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Surowiec, I.; Ärlestig, L.; Rantapää-Dahlqvist, S.; Trygg, J. Metabolite and lipid profiling of biobank plasma samples collected prior to onset of rheumatoid arthritis. PLoS ONE 2016, 11, e0164196. [Google Scholar] [CrossRef] [PubMed]
- Luczaj, W.; Moniuszko-Malinowska, A.; Domingues, P.; Domingues, M.R.; Gindzienska-Sieskiewicz, E.; Skrzydlewska, E. Plasma lipidomic profile signature of rheumatoid arthritis versus Lyme arthritis patients. Arch. Biochem. Biophys. 2018, 654, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Zheng, K.I.; Wang, X.D.; Qiao, J.; Li, Y.Y.; Zhang, L.; Zheng, M.H.; Wu, J. LC-MS-based lipidomic analysis in distinguishing patients with nonalcoholic steatohepatitis from nonalcoholic fatty liver. Hepatobiliary Pancreat. Dis. Int. 2021, 20, 452–459. [Google Scholar] [CrossRef]
- Dajani, A.I.; Popovic, B. Essential phospholipids for nonalcoholic fatty liver disease associated with metabolic syndrome: A systematic review and network meta-analysis. World J. Clin. Cases 2020, 8, 5235. [Google Scholar] [CrossRef]
- Hishikawa, D.; Hashidate, T.; Shimizu, T.; Shindou, H. Diversity and function of membrane glycerophospholipids generated by the remodeling pathway in mammalian cells. J. Lipid Res. 2014, 55, 799–807. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, M.; Fairn, G.D. Phospholipid subcellular localization and dynamics. J. Biol. Chem. 2018, 293, 6230–6240. [Google Scholar] [CrossRef]
- Liang, J.; Li, J.; Zhang, J.; Rong, J.; Wang, X.; Zhao, C.; Zhang, H.; Shi, H.; Wu, W. UHPLC-MS/MS-based untargeted lipidomics analysis of septic patients. Clin. Chim. Acta 2023, 544, 117336. [Google Scholar] [CrossRef]
- Mecatti, G.C.; Fernandes Messias, M.C.; Sant’Anna Paiola, R.M.; Figueiredo Angolini, C.F.; da Silva Cunha, I.B.; Eberlin, M.N.; de Oliveira Carvalho, P. Lipidomic profiling of plasma and erythrocytes from septic patients reveals potential biomarker candidates. Biomark. Insights 2018, 13, 1177271918765137. [Google Scholar] [CrossRef] [PubMed]
- Thomaidou, A.; Deda, O.; Begou, O.; Lioupi, A.; Kontou, A.; Gika, H.; Agakidou, E.; Theodoridis, G.; Sarafidis, K. A Prospective, Case-Control Study of Serum Metabolomics in Neonates with Late-Onset Sepsis and Necrotizing Enterocolitis. J. Clin. Med. 2022, 11, 5270. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, M.; Cambiaghi, A.; Brunelli, L.; Giordano, S.; Caironi, P.; Guatteri, L.; Raimondi, F.; Gattinoni, L.; Latini, R.; Masson, S.; et al. Mortality prediction in patients with severe septic shock: A pilot study using a target metabolomics approach. Sci. Rep. 2016, 6, 20391. [Google Scholar] [CrossRef] [PubMed]
- Fahrmann, J.; Grapov, D.; Yang, J.; Hammock, B.; Fiehn, O.; Bell, G.I.; Hara, M. Systemic alterations in the metabolome of diabetic NOD mice delineate increased oxidative stress accompanied by reduced inflammation and hypertriglyceremia. Am. J. Physiol.-Endocrinol. Metab. 2015, 308, E978–E989. [Google Scholar] [CrossRef]
- López-Hernández, Y.; Lara-Ramírez, E.E.; Salgado-Bustamante, M.; López, J.A.; Oropeza-Valdez, J.J.; Jaime-Sánchez, E.; Castañeda-Delgado, J.E.; Magaña-Aquino, M.; Murgu, M.; Enciso-Moreno, J.A. Glycerophospholipid metabolism alterations in patients with type 2 diabetes mellitus and tuberculosis comorbidity. Arch. Med. Res. 2019, 50, 71–78. [Google Scholar] [CrossRef] [PubMed]
- uczaj, W.; Domingues, P.; Domingues, M.; Pancewicz, S.; Skrzydlewska, E. Phospholipidomic analysis reveals changes in sphingomyelin and lysophosphatidylcholine profiles in plasma from patients with neuroborreliosis. Lipids 2017, 52, 93–98. [Google Scholar] [CrossRef]
- O’Neal, A.J.; Butler, L.R.; Rolandelli, A.; Gilk, S.D.; Pedra, J.H. Lipid hijacking: A unifying theme in vector-borne diseases. eLife 2020, 9, e61675. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.; Lee, K.C.; Lo, G.C.; Ding, V.S.; Chow, W.N.; Ke, T.Y.; Curreem, S.O.; To, K.K.; Ho, D.T.; Sridhar, S.; et al. Metabolomic profiling of plasma from melioidosis patients using UHPLC-QTOF MS reveals novel biomarkers for diagnosis. Int. J. Mol. Sci. 2016, 17, 307. [Google Scholar] [CrossRef]
- Zheng, W.; Wu, X.; Goudarzi, M.; Shi, J.; Song, W.; Li, C.; Liu, J.; Chen, H.; Zhang, X.; Zeng, X.; et al. Metabolomic alterations associated with Behcet’s disease. Arthritis Res. Ther. 2018, 20, 214. [Google Scholar] [CrossRef]
- Saito, K.; Gemma, A.; Tatsumi, K.; Hattori, N.; Ushiki, A.; Tsushima, K.; Saito, Y.; Abe, M.; Horimasu, Y.; Kashiwada, T.; et al. Identification and characterization of lysophosphatidylcholine 14: 0 as a biomarker for drug-induced lung disease. Sci. Rep. 2022, 12, 19819. [Google Scholar] [CrossRef]
- van Kruining, D.; Luo, Q.; van Echten-Deckert, G.; Mielke, M.M.; Bowman, A.; Ellis, S.; Oliveira, T.G.; Martinez-Martinez, P. Sphingolipids as prognostic biomarkers of neurodegeneration, neuroinflammation, and psychiatric diseases and their emerging role in lipidomic investigation methods. Adv. Drug Deliv. Rev. 2020, 159, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Zheng, S.J.; Wu, C.S.; Jia, Z.X.; Zhang, J.L.; Duan, Z.P. Lipidomic profiling of plasma in patients with chronic hepatitis C infection. Anal. Bioanal. Chem. 2014, 406, 555–564. [Google Scholar] [PubMed]
- Lu, H.; George, J.; Eslam, M.; Villanueva, A.; Bolondi, L.; Reeves, H.L.; McCain, M.; Chambers, E.; Ward, C.; Sartika, D.; et al. Discriminatory Changes in Circulating Metabolites as a Predictor of Hepatocellular Cancer in Patients with Metabolic (Dysfunction) Associated Fatty Liver Disease. Liver Cancer 2023, 12, 19–31. [Google Scholar] [PubMed]
- Zhang, K.K.; Chen, L.J.; Li, J.H.; Liu, J.L.; Wang, L.B.; Xu, L.L.; Yang, J.Z.; Li, X.W.; Xie, X.L.; Wang, Q. Methamphetamine disturbs gut homeostasis and reshapes serum metabolome, inducing neurotoxicity and abnormal behaviors in mice. Front. Microbiol. 2022, 13, 755189. [Google Scholar]
- Frajerman, A.; Kebir, O.; Chaumette, B.; Tessier, C.; Lamaziere, A.; Nuss, P.; Krebs, M.O. Membrane lipids in schizophrenia and early phases of psychosis: Potential biomarkers and therapeutic targets? L’encephale 2020, 46, 209–216. [Google Scholar] [CrossRef]
- Kayser, B.D.; Lhomme, M.; Dao, M.C.; Ichou, F.; Bouillot, J.L.; Prifti, E.; Kontush, A.; Chevallier, J.M.; Aron-Wisnewsky, J.; Dugail, I.; et al. Serum lipidomics reveals early differential effects of gastric bypass compared with banding on phospholipids and sphingolipids independent of differences in weight loss. Int. J. Obes. 2017, 41, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Diray-Arce, J.; Angelidou, A.; Jensen, K.J.; Conti, M.G.; Kelly, R.S.; Pettengill, M.A.; Liu, M.; van Haren, S.D.; McCulloch, S.D.; Michelloti, G.; et al. Bacille Calmette-Guerin vaccine reprograms human neonatal lipid metabolism in vivo and in vitro. Cell Rep. 2022, 39, 110772. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, X.F.; Pan, A. Lipidomics in gestational diabetes mellitus. Curr. Opin. Lipidol. 2023, 34, 1–11. [Google Scholar]
- Cao, Z.; Wang, J.; Weng, Z.; Tao, X.; Xu, Y.; Li, X.; Tan, X.; Liu, Z.; Qu, C. Metabolomic analysis of serum from pure coronary artery ectasia patients based on UPLC-QE/MS technique. Clin. Chim. Acta 2022, 534, 93–105. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Wang, Y. Sterolomics in biology, biochemistry, medicine. TrAC Trends Anal. Chem. 2019, 120, 115280. [Google Scholar]
- Rosso, L.G.; Lhomme, M.; Merono, T.; Sorroche, P.; Catoggio, L.; Soriano, E.; Saucedo, C.; Malah, V.; Dauteuille, C.; Boero, L.; et al. Altered lipidome and antioxidative activity of small, dense HDL in normolipidemic rheumatoid arthritis: Relevance of inflammation. Atherosclerosis 2014, 237, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Yassine, H.N.; Jackson, A.M.; Borges, C.R.; Billheimer, D.; Koh, H.; Smith, D.; Reaven, P.; Lau, S.S.; Borchers, C.H. The application of multiple reaction monitoring and multi-analyte profiling to HDL proteins. Lipids Health Dis. 2014, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K. Introduction to Lipids and Lipoproteins. In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2015. [Google Scholar]
- Voet, D.; Voet, J.G. Biochemistry; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Jeong, S.M.; Choi, S.; Kim, K.; Kim, S.M.; Lee, G.; Park, S.Y.; Kim, Y.Y.; Son, J.S.; Yun, J.M.; Park, S.M. Effect of change in total cholesterol levels on cardiovascular disease among young adults. J. Am. Heart Assoc. 2018, 7, e008819. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Moon, J.Y.; Lim, B.Y.; Kim, S.M.; Park, C.W.; Kim, B.J.; Jun, J.K.; Norwitz, E.R.; Choi, M.H.; Park, J.S. Increased biosynthesis and accumulation of cholesterol in maternal plasma, but not amniotic fluid in pre-eclampsia. Sci. Rep. 2019, 9, 1550. [Google Scholar] [CrossRef]
- Plomp, R.; Ruhaak, L.R.; Uh, H.W.; Reiding, K.R.; Selman, M.; Houwing-Duistermaat, J.J.; Slagboom, P.E.; Beekman, M.; Wuhrer, M. Subclass-specific IgG glycosylation is associated with markers of inflammation and metabolic health. Sci. Rep. 2017, 7, 12325. [Google Scholar] [CrossRef]
- Feingold, K.R.; Grunfeld, C. Effect of inflammation on HDL structure and function. Curr. Opin. Lipidol. 2016, 27, 521–530. [Google Scholar] [CrossRef]
- Giraud, C.; Tournadre, A.; Pereira, B.; Dutheil, F.; Soubrier, M.; Lhomme, M.; Kontush, A.; Sébédio, J.L.; Capel, F. Alterations of HDL particle phospholipid composition and role of inflammation in rheumatoid arthritis. J. Physiol. Biochem. 2019, 75, 453–462. [Google Scholar] [CrossRef]
- Charles-Schoeman, C.; Gugiu, G.; Ge, H.; Shahbazian, A.; Lee, Y.; Wang, X.; Furst, D.; Ranganath, V.; Maldonado, M.; Lee, T.; et al. Remodeling of the HDL proteome with treatment response to abatacept or adalimumab in the AMPLE trial of patients with rheumatoid arthritis. Atherosclerosis 2018, 275, 107–114. [Google Scholar] [CrossRef]
- Ikegami, T.; Honda, A.; Miyazaki, T.; Kohjima, M.; Nakamuta, M.; Matsuzaki, Y. Increased serum oxysterol concentrations in patients with chronic hepatitis C virus infection. Biochem. Biophys. Res. Commun. 2014, 446, 736–740. [Google Scholar] [CrossRef]
- Cassol, E.; Misra, V.; Holman, A.; Kamat, A.; Morgello, S.; Gabuzda, D. Plasma metabolomics identifies lipid abnormalities linked to markers of inflammation, microbial translocation, and hepatic function in HIV patients receiving protease inhibitors. BMC Infect. Dis. 2013, 13, 203. [Google Scholar] [CrossRef]
- Mukerji, S.S.; Misra, V.; Lorenz, D.R.; Chettimada, S.; Keller, K.; Letendre, S.; Ellis, R.J.; Morgello, S.; Parker, R.A.; Gabuzda, D. Low neuroactive steroids identifies a biological subtype of depression in adults with human immunodeficiency virus on suppressive antiretroviral therapy. J. Infect. Dis. 2021, 223, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Usmani, S.A.; Arya, K.; Bhardwaj, N. Analysis of Sterols by Gas Chromatography–Mass Spectrometry. In Analysis of Membrane Lipids; Springer: New York, NY, USA, 2020; pp. 83–101. [Google Scholar]
- John, C.; Werner, P.; Worthmann, A.; Wegner, K.; Tödter, K.; Scheja, L.; Rohn, S.; Heeren, J.; Fischer, M. A liquid chromatography-tandem mass spectrometry-based method for the simultaneous determination of hydroxy sterols and bile acids. J. Chromatogr. A 2014, 1371, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Borah, K.; Rickman, O.J.; Voutsina, N.; Ampong, I.; Gao, D.; Baple, E.L.; Dias, I.H.; Crosby, A.H.; Griffiths, H.R. A quantitative LC-MS/MS method for analysis of mitochondrial-specific oxysterol metabolism. Redox Biol. 2020, 36, 101595. [Google Scholar] [CrossRef]
- Esmail, S.; Manolson, M.F. Advances in understanding N-glycosylation structure, function, and regulation in health and disease. Eur. J. Cell Biol. 2021, 100, 151186. [Google Scholar] [CrossRef]
- Maverakis, E.; Kim, K.; Shimoda, M.; Gershwin, M.E.; Patel, F.; Wilken, R.; Raychaudhuri, S.; Ruhaak, L.R.; Lebrilla, C.B. Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: A critical review. J. Autoimmun. 2015, 57, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, Y.; Qin, X.; Li, H.; Guo, Y.; Wang, Y.; Liu, H.; Wang, X.; Song, G.; Li, F.; et al. Change in I g G 1 F c N-linked glycosylation in human lung cancer: Age-and sex-related diagnostic potential. Electrophoresis 2013, 34, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Dědová, T.; Grunow, D.; Kappert, K.; Flach, D.; Tauber, R.; Blanchard, V. The effect of blood sampling and preanalytical processing on human N-glycome. PLoS ONE 2018, 13, e0200507. [Google Scholar] [CrossRef]
- Zauner, G.; Selman, M.H.; Bondt, A.; Rombouts, Y.; Blank, D.; Deelder, A.M.; Wuhrer, M. Glycoproteomic analysis of antibodies. Mol. Cell. Proteom. 2013, 12, 856–865. [Google Scholar] [CrossRef]
- Haslund-Gourley, B.S.; Wigdahl, B.; Comunale, M.A. IgG N-glycan Signatures as Potential Diagnostic and Prognostic Biomarkers. Diagnostics 2023, 13, 1016. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X. IgG N-glycans. Adv. Clin. Chem. 2021, 105, 1–47. [Google Scholar]
- Goonatilleke, E.; Smilowitz, J.T.; Marino, K.V.; German, B.J.; Lebrilla, C.B.; Barboza, M. Immunoglobulin A N-glycosylation presents important body fluid-specific variations in lactating mothers. Mol. Cell. Proteom. 2019, 18, 2165–2177. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, X.; Liu, C.; Li, W.; Zeng, W.; Li, B.; Chi, H.; Liu, M.; Qin, X.; Tang, L.; et al. N-glycopeptide signatures of IgA2 in serum from patients with hepatitis B virus-related liver diseases. Mol. Cell. Proteom. 2019, 18, 2262–2272. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, A.; Seo, N.; Oh, J.; Kweon, O.K.; An, H.J.; Kim, J. Discovery of N-glycan Biomarkers for the Canine Osteoarthritis. Life 2020, 10, 199. [Google Scholar]
- Briggs, M.T.; Condina, M.R.; Klingler-Hoffmann, M.; Arentz, G.; Everest-Dass, A.V.; Kaur, G.; Oehler, M.K.; Packer, N.H.; Hoffmann, P. Translating N-glycan analytical applications into clinical strategies for ovarian cancer. PROTEOMICS-Clin. Appl. 2019, 13, 1800099. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Shu, H.; Yang, M.; Yan, G.; Zhang, L.; Wang, L.; Wang, W.; Lu, H. Fast Discrimination of Sialylated N-Glycan Linkage Isomers with One-Step Derivatization by Microfluidic Capillary Electrophoresis–Mass Spectrometry. Anal. Chem. 2022, 94, 4666–4676. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Shu, H.; Peng, Y.; Feng, X.; Yan, G.; Zhang, L.; Yao, J.; Bao, H.; Lu, H. Specific Analysis of α-2, 3-Sialylated N-Glycan Linkage Isomers by Microchip Capillary Electrophoresis–Mass Spectrometry. Anal. Chem. 2021, 93, 5537–5546. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Du, X.; Peng, Y.; Cai, Y.; Wei, L.; Zhang, Y.; Lu, H. Integrated pipeline of isotopic labeling and selective enriching for quantitative analysis of N-glycome by mass spectrometry. Anal. Chem. 2018, 91, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Biskup, K.; Braicu, E.I.; Sehouli, J.; Fotopoulou, C.; Tauber, R.; Berger, M.; Blanchard, V. Serum glycome profiling: A biomarker for diagnosis of ovarian cancer. J. Proteome Res. 2013, 12, 4056–4063. [Google Scholar]
- Wei, L.; Cai, Y.; Yang, L.; Zhang, Y.; Lu, H. Duplex stable isotope labeling (DuSIL) for simultaneous quantitation and distinction of sialylated and neutral N-glycans by MALDI-MS. Anal. Chem. 2018, 90, 10442–10449. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.; Zhang, Y.; Lu, H. Dual isotopic labeling combined with fluorous solid-phase extraction for simultaneous discovery of neutral/sialylated N-glycans as biomarkers for gastric cancer. Anal. Chim. Acta 2020, 1104, 87–94. [Google Scholar] [CrossRef]
- Thompson, N.; Wakarchuk, W. O-glycosylation and its role in therapeutic proteins. Biosci. Rep. 2022, 42, BSR20220094. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, Y.; Renfrow, M.B.; Novak, J.; Takahashi, K. Aberrantly glycosylated IgA1 in IgA nephropathy: What we know and what we don’t know. J. Clin. Med. 2021, 10, 3467. [Google Scholar] [CrossRef] [PubMed]
- Schedin-Weiss, S.; Winblad, B.; Tjernberg, L.O. The role of protein glycosylation in Alzheimer disease. FEBS J. 2014, 281, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Lehoux, S.; Mi, R.; Aryal, R.P.; Wang, Y.; Katrine, T.B.S.; Clausen, H.; van Die, I.; Han, Y.; Chapman, A.B.; Cummings, R.D.; et al. Identification of distinct glycoforms of IgA1 in plasma from patients with immunoglobulin A (IgA) nephropathy and healthy individuals. Mol. Cell. Proteom. 2014, 13, 3097–3113. [Google Scholar] [CrossRef] [PubMed]
- Takagi, J.; Aoki, K.; Turner, B.S.; Lamont, S.; Lehoux, S.; Kavanaugh, N.; Gulati, M.; Valle Arevalo, A.; Lawrence, T.J.; Kim, C.Y.; et al. Mucin O-glycans are natural inhibitors of Candida albicans pathogenicity. Nat. Chem. Biol. 2022, 18, 762–773. [Google Scholar] [CrossRef]
- Chia, J.; Goh, G.; Bard, F. Short O-GalNAc glycans: Regulation and role in tumor development and clinical perspectives. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 1623–1639. [Google Scholar] [CrossRef]
- Wang, D.; Kong, J.; Wu, J.; Wang, X.; Lai, M. GC–MS-based metabolomics identifies an amino acid signature of acute ischemic stroke. Neurosci. Lett. 2017, 642, 7–13. [Google Scholar] [CrossRef]
- Hakuno, D.; Hamba, Y.; Toya, T.; Adachi, T. Plasma amino acid profiling identifies specific amino acid associations with cardiovascular function in patients with systolic heart failure. PLoS ONE 2015, 10, e0117325. [Google Scholar] [CrossRef]
- Moskaleva, N.E.; Shestakova, K.M.; Kukharenko, A.V.; Markin, P.A.; Kozhevnikova, M.V.; Korobkova, E.O.; Brito, A.; Baskhanova, S.N.; Mesonzhnik, N.V.; Belenkov, Y.N.; et al. Target Metabolome Profiling-Based Machine Learning as a Diagnostic Approach for Cardiovascular Diseases in Adults. Metabolites 2022, 12, 1185. [Google Scholar] [CrossRef]
- Douris, N.; Melman, T.; Pecherer, J.M.; Pissios, P.; Flier, J.S.; Cantley, L.C.; Locasale, J.W.; Maratos-Flier, E. Adaptive changes in amino acid metabolism permit normal longevity in mice consuming a low-carbohydrate ketogenic diet. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 2056–2065. [Google Scholar] [CrossRef]
- Lustgarten, M.S.; Price, L.L.; Chale, A.; Phillips, E.M.; Fielding, R.A. Branched chain amino acids are associated with muscle mass in functionally limited older adults. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Mednova, I.A.; Chernonosov, A.A.; Kasakin, M.F.; Kornetova, E.G.; Semke, A.V.; Bokhan, N.A.; Koval, V.V.; Ivanova, S.A. Amino acid and acylcarnitine levels in chronic patients with schizophrenia: A preliminary study. Metabolites 2021, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Smolenska, Z.; Zabielska-Kaczorowska, M.; Wojteczek, A.; Kutryb-Zajac, B.; Zdrojewski, Z. Metabolic pattern of systemic sclerosis: Association of changes in plasma concentrations of amino acid-related compounds with disease presentation. Front. Mol. Biosci. 2020, 7, 585161. [Google Scholar] [CrossRef] [PubMed]
- Whiley, L.; Chappell, K.E.; D’Hondt, E.; Lewis, M.R.; Jiménez, B.; Snowden, S.G.; Soininen, H.; Kłoszewska, I.; Mecocci, P.; Tsolaki, M.; et al. Metabolic phenotyping reveals a reduction in the bioavailability of serotonin and kynurenine pathway metabolites in both the urine and serum of individuals living with Alzheimer’s disease. Alzheimer’s Res. Ther. 2021, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Liu, Y.; Li, H.; Wang, L.P.; Xue, L.F.; Yin, G.S.; Wu, X.S. Identification of psoriasis vulgaris biomarkers in human plasma by non-targeted metabolomics based on UPLC-Q-TOF/MS. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3940–3950. [Google Scholar]
- Su, J.; Zhu, Y.; Jiang, Y.; Zou, L.; Liu, X.; Xu, Y. Study of Plasma amino acid related metabolites of septic rats using gas chromatography-mass spectrometry. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2017, 29, 332–336. [Google Scholar]
- Binici, I.; Alp, H.H.; Karsen, H.; Koyuncu, I.; Gonel, A.; Çelik, H.; Karahocagil, M.K. Plasma Free Amino Acid Profile in HIV-Positive Cases. Curr. HIV Res. 2022, 20, 228–235. [Google Scholar] [CrossRef]
- Liao, W.; Jin, Q.; Liu, J.; Ruan, Y.; Li, X.; Shen, Y.; Zhang, Z.; Wang, Y.; Wu, S.; Zhang, J.; et al. Mahuang decoction antagonizes acute liver failure via modulating tricarboxylic acid cycle and amino acids metabolism. Front. Pharmacol. 2021, 12, 599180. [Google Scholar] [CrossRef]
- Smolenska, Z.; Smolenski, R.T.; Zdrojewski, Z. Plasma concentrations of amino acid and nicotinamide metabolites in rheumatoid arthritis–potential biomarkers of disease activity and drug treatment. Biomarkers 2016, 21, 218–224. [Google Scholar] [CrossRef]
- Chatterjee, P.; Cheong, Y.J.; Bhatnagar, A.; Goozee, K.; Wu, Y.; McKay, M.; Martins, I.J.; Lim, W.L.; Pedrini, S.; Tegg, M.; et al. Plasma metabolites associated with biomarker evidence of neurodegeneration in cognitively normal older adults. J. Neurochem. 2021, 159, 389–402. [Google Scholar] [CrossRef]
- Phipps, W.S.; Crossley, E.; Boriack, R.; Jones, P.M.; Patel, K. Quantitative amino acid analysis by liquid chromatography-tandem mass spectrometry using low cost derivatization and an automated liquid handler. JIMD Rep. 2020, 51, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Bagcchi, S. WHO’s Global Tuberculosis Report 2022. Lancet Microbe 2023, 4, e20. [Google Scholar] [CrossRef]
- Narasimhan, P.; Wood, J.; MacIntyre, C.R.; Mathai, D. Risk factors for tuberculosis. Pulm. Med. 2013, 2013, 828939. [Google Scholar] [CrossRef] [PubMed]
- Fortún, J.; Navas, E. Latent tuberculosis infection: Approach and therapeutic schemes. Rev. Espa Nola Quimioter. 2022, 35, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Preez, I.d.; Luies, L.; Loots, D.T. Metabolomics biomarkers for tuberculosis diagnostics: Current status and future objectives. Biomarkers Med. 2017, 11, 179–194. [Google Scholar] [CrossRef]
- Lau, S.K.; Lee, K.C.; Curreem, S.O.; Chow, W.N.; To, K.K.; Hung, I.F.; Ho, D.T.; Sridhar, S.; Li, I.W.; Ding, V.S.; et al. Metabolomic profiling of plasma from patients with tuberculosis by use of untargeted mass spectrometry reveals novel biomarkers for diagnosis. J. Clin. Microbiol. 2015, 53, 3750–3759. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Shi, L.Y.; Wei, L.L.; Han, Y.S.; Yi, W.J.; Pan, Z.W.; Jiang, T.T.; Chen, J.; Tu, H.H.; Li, Z.B.; et al. Plasma metabolites Xanthine, 4-Pyridoxate, and d-glutamic acid as novel potential biomarkers for pulmonary tuberculosis. Clin. Chim. Acta 2019, 498, 135–142. [Google Scholar] [CrossRef]
- Li, Y.X.; Zheng, K.D.; Duan, Y.; Liu, H.J.; Tang, Y.Q.; Wu, J.; Lin, D.Z.; Zhang, Z. Mass spectrometry-based identification of new serum biomarkers in patients with latent infection pulmonary tuberculosis. Medicine 2022, 101, e32153. [Google Scholar] [CrossRef]
- Cho, Y.; Park, Y.; Sim, B.; Kim, J.; Lee, H.; Cho, S.N.; Kang, Y.A.; Lee, S.G. Identification of serum biomarkers for active pulmonary tuberculosis using a targeted metabolomics approach. Sci. Rep. 2020, 10, 3825. [Google Scholar] [CrossRef]
- Magdalena, D.; Michal, S.; Marta, S.; Magdalena, K.P.; Anna, P.; Magdalena, G.; Rafał, S. Targeted metabolomics analysis of serum and Mycobacterium tuberculosis antigen-stimulated blood cultures of pediatric patients with active and latent tuberculosis. Sci. Rep. 2022, 12, 4131. [Google Scholar] [CrossRef]
- Chen, X.; Ye, J.; Lei, H.; Wang, C. Novel Potential Diagnostic Serum Biomarkers of Metabolomics in Osteoarticular Tuberculosis Patients: A Preliminary Study. Front. Cell. Infect. Microbiol. 2022, 12, 827528. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Liu, F.; Yin, Q.; Jiang, T.; Fang, M.; Duan, L.; Quan, S.; Tian, X.; Shen, A.; Mi, K.; et al. Exploration of Lipid Metabolism Alterations in Children with Active Tuberculosis Using UHPLC-MS/MS. J. Immunol. Res. 2023, 2023, 8111355. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.J.; Han, Y.S.; Wei, L.L.; Shi, L.Y.; Huang, H.; Jiang, T.T.; Li, Z.B.; Chen, J.; Hu, Y.T.; Tu, H.H.; et al. l-Histidine, arachidonic acid, biliverdin, and l-cysteine-glutathione disulfide as potential biomarkers for cured pulmonary tuberculosis. Biomed. Pharmacother. 2019, 116, 108980. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Han, Y.S.; Chen, J.; Shi, L.Y.; Wei, L.L.; Jiang, T.T.; Yi, W.J.; Yu, Y.; Li, Z.B.; Li, J.C. The novel potential biomarkers for multidrug-resistance tuberculosis using UPLC-Q-TOF-MS. Exp. Biol. Med. 2020, 245, 501–511. [Google Scholar] [CrossRef]
- Dutta, N.K.; Tornheim, J.A.; Fukutani, K.F.; Paradkar, M.; Tiburcio, R.T.; Kinikar, A.; Valvi, C.; Kulkarni, V.; Pradhan, N.; Shivakumar, S.V.B.Y.; et al. Integration of metabolomics and transcriptomics reveals novel biomarkers in the blood for tuberculosis diagnosis in children. Sci. Rep. 2020, 10, 19527. [Google Scholar] [CrossRef]
- Bauman, J.S.; Pizzey, R.; Beckmann, M.; Villarreal-Ramos, B.; King, J.; Hopkins, B.; Rooke, D.; Hewinson, G.; Mur, L.A. Untargeted metabolomic analysis of thoracic blood from badgers indicate changes linked to infection with bovine tuberculosis (Mycobacterium bovis): A pilot study. Metabolomics 2022, 18, 61. [Google Scholar] [CrossRef]
- Caraballo, C.; Jaimes, F. Focus: Death: Organ dysfunction in sepsis: An ominous trajectory from infection to death. Yale J. Biol. Med. 2019, 92, 629. [Google Scholar]
- Chiu, C.; Legrand, M. Epidemiology of sepsis and septic shock. Curr. Opin. Anesthesiol. 2021, 34, 71–76. [Google Scholar] [CrossRef]
- Vijayan, A.L.; Vanimaya; Ravindran, S.; Saikant, R.; Lakshmi, S.; Kartik, R.; G, M. Procalcitonin: A promising diagnostic marker for sepsis and antibiotic therapy. J. Intensive Care 2017, 5, 51. [Google Scholar] [CrossRef]
- Huynh, H.H.; Delatour, V.; Derbez-Morin, M.; Liu, Q.; Boeuf, A.; Vinh, J. Candidate high-resolution mass spectrometry-based reference method for the quantification of procalcitonin in human serum using a characterized recombinant protein as a primary calibrator. Anal. Chem. 2022, 94, 4146–4154. [Google Scholar] [CrossRef]
- Guan, S.; Liu, K.; Liu, Z.; Zhou, L.; Jia, B.; Wang, Z.; Nie, Y.; Zhang, X. UPLC–Q-TOF/MS-Based Plasma and Urine Metabolomics Contribute to the Diagnosis of Sepsis. J. Proteome Res. 2021, 21, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Tong, R.; Song, H.; Sun, G.; Wang, D.; Liang, H.; Sun, J.; Cui, Y.; Zhang, X.; Liu, S.; et al. Identification of metabolomics-based prognostic prediction models for ICU septic patients. Int. Immunopharmacol. 2022, 108, 108841. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gimenez, J.L.; Garcia-Lopez, E.; Mena-Molla, S.; Beltran-Garcia, J.; Osca-Verdegal, R.; Nacher-Sendra, E.; Aguado-Velasco, C.; Casabo-Valles, G.; Roma-Mateo, C.; Rodriguez-Gimillo, M.; et al. Validation of circulating histone detection by mass spectrometry for early diagnosis, prognosis, and management of critically ill septic patients. J. Transl. Med. 2023, 21, 344. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Xu, S.; Zhou, B.; Zhou, R.; Liu, P.; Hui, X.; Long, Y.; Su, L. Dynamic Plasma Lipidomic Analysis Revealed Cholesterol Ester and Amides Associated with Sepsis Development in Critically Ill Patients after Cardiovascular Surgery with Cardiopulmonary Bypass. J. Pers. Med. 2022, 12, 1838. [Google Scholar] [CrossRef]
- Li, Z.; Luo, Z.; Shi, X.; Pang, B.; Ma, Y.; Jin, J. The levels of oxidized phospholipids in high-density lipoprotein during the course of sepsis and their prognostic value. Front. Immunol. 2022, 13, 893929. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, J.; Shi, H.; Xia, Y.; Li, J.; Wu, W.; Wang, H. Nontargeted lipidomic analysis of sera from sepsis patients based on ultra-high performance liquid chromatography-mass spectrometry/mass spectrometry. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2022, 34, 346–351. [Google Scholar] [PubMed]
- Kauppi, A.M.; Edin, A.; Ziegler, I.; Molling, P.; Sjostedt, A.; Gylfe, A.; Straalin, K.; Johansson, A. Metabolites in blood for prediction of bacteremic sepsis in the emergency room. PLoS ONE 2016, 11, e0147670. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Y.; Ji, X.; Huang, M.; Xie, B. Metabonomic Analysis of Metabolites Produced by Escherichia coli in Patients With and Without Sepsis. Infect. Drug Resist. 2022, 15, 7339–7350. [Google Scholar] [CrossRef]
- de Haan, N.; Boeddha, N.P.; Ekinci, E.; Reiding, K.R.; Emonts, M.; Hazelzet, J.A.; Wuhrer, M.; Driessen, G.J. Differences in IgG Fc glycosylation are associated with outcome of pediatric meningococcal sepsis. MBio 2018, 9, 10–1128. [Google Scholar] [CrossRef]
- Kosyakovsky, L.B.; Somerset, E.; Rogers, A.J.; Sklar, M.; Mayers, J.R.; Toma, A.; Szekely, Y.; Soussi, S.; Wang, B.; Fan, C.P.S.; et al. Machine learning approaches to the human metabolome in sepsis identify metabolic links with survival. Intensive Care Med. Exp. 2022, 10, 24. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, P.; Amathieu, R.; Savarin, P.; Xu, G. Application of LC-MS-based metabolomics method in differentiating septic survivors from non-survivors. Anal. Bioanal. Chem. 2016, 408, 7641–7649. [Google Scholar] [CrossRef] [PubMed]
- Mardegan, V.; Giordano, G.; Stocchero, M.; Pirillo, P.; Poloniato, G.; Donadel, E.; Salvadori, S.; Giaquinto, C.; Priante, E.; Baraldi, E. Untargeted and targeted metabolomic profiling of preterm newborns with earlyonset sepsis: A case-control study. Metabolites 2021, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Duess, J.W.; Sampah, M.E.; Lopez, C.M.; Tsuboi, K.; Scheese, D.J.; Sodhi, C.P.; Hackam, D.J. Necrotizing enterocolitis, gut microbes, and sepsis. Gut Microbes 2023, 15, 2221470. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Nelson, A.; Treumann, A.; Skeath, T.; Cummings, S.P.; Embleton, N.D.; Berrington, J.E. Metabolomic and proteomic analysis of serum from preterm infants with necrotising entercolitis and late-onset sepsis. Pediatr. Res. 2016, 79, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Tong, P.; Huang, F.R.; Xu, J.; Wu, Z.Q.; Hu, X.; Ling, M.; Wang, D.; Wu, B.F.; Yang, D.J.; Zhang, A.M. Metabolomic changes of neonatal sepsis: An exploratory clinical study. Zhongguo Dang Dai Er Ke Za Zhi Chin. J. Contemp. Pediatr. 2022, 24, 675–680. [Google Scholar]
- Cao, Y.; Liu, Z.; Ma, W.; Fang, C.; Pei, Y.; Jing, Y.; Huang, J.; Han, X.; Xiao, W. Untargeted metabolomic profiling of sepsis-induced cardiac dysfunction. Front. Endocrinol. 2023, 14, 1060470. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, M.; Han, T.; Wu, H.; Xiao, Z.; Lin, S.; Wang, C.; Xu, F. Exploring the biomarkers of sepsis-associated encephalopathy (SAE): Metabolomics evidence from gas chromatography-mass spectrometry. BioMed Res. Int. 2019, 2019, 2612849. [Google Scholar] [CrossRef]
- Sharma, N.K.; Ferreira, B.L.; Tashima, A.K.; Brunialti, M.K.C.; Torquato, R.J.S.; Bafi, A.; Assuncao, M.; Azevedo, L.C.P.; Salomao, R. Lipid metabolism impairment in patients with sepsis secondary to hospital acquired pneumonia, a proteomic analysis. Clin. Proteom. 2019, 16, 29. [Google Scholar] [CrossRef]
- Elmassry, M.M.; Mudaliar, N.S.; Colmer-Hamood, J.A.; San Francisco, M.J.; Griswold, J.A.; Dissanaike, S.; Hamood, A.N. New markers for sepsis caused by Pseudomonas aeruginosa during burn infection. Metabolomics 2020, 16, 40. [Google Scholar] [CrossRef]
- Eberle, J.; Gürtler, L. HIV types, groups, subtypes and recombinant forms: Errors in replication, selection pressure and quasispecies. Intervirology 2012, 55, 79–83. [Google Scholar] [CrossRef]
- Mehraeen, E.; Safdari, R.; SeyedAlinaghi, S.; Noori, T.; Kahouei, M.; Soltani-Kermanshahi, M. A mobile-based self-management application-usability evaluation from the perspective of HIV-positive people. Health Policy Technol. 2020, 9, 294–301. [Google Scholar] [CrossRef]
- Rogando, A.C.; Weber, K.M.; Xing, J.; Xue, X.; Yohannes, T.; Morack, R.; Qi, Q.; Clish, C.; Bullock, K.; Gustafson, D.; et al. The IDOze Study: The Link Between Sleep Disruption and Tryptophan-Kynurenine Pathway Activation in Women With Human Immunodeficiency Virus. J. Infect. Dis. 2022, 226, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Pau, A.K.; George, J.M. Antiretroviral therapy: Current drugs. Infect. Dis. Clin. 2014, 28, 371–402. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.; Lewin, S.; Havlir, D. The end of AIDS: HIV infection as a chronic disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Nyström, S.; Govender, M.; Yap, S.H.; Kamarulzaman, A.; Rajasuriar, R.; Larsson, M. HIV-infected individuals on ART With impaired immune recovery have altered plasma metabolite profiles. Open Forum Infect. Dis. 2021, 8, ofab288. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, H.L.; Cheng, A.C.; Cherry, C.L.; Weir, J.M.; Meikle, P.J.; Hoy, J.F.; Crowe, S.M.; Palmer, C.S. Immunometabolic and lipidomic markers associated with the frailty index and quality of life in aging HIV+ men on antiretroviral therapy. EBioMedicine 2017, 22, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Mena, A.; Clavero, E.; Díaz-Díaz, J.L.; Castro, A. Similar plasma lipidomic profile in people living with HIV treated with a darunavir-based or an integrase inhibitor-based antiretroviral therapy. Sci. Rep. 2019, 9, 17184. [Google Scholar] [CrossRef]
- Boyd, A.; Boccara, F.; Meynard, J.L.; Ichou, F.; Bastard, J.P.; Fellahi, S.; Samri, A.; Sauce, D.; Haddour, N.; Autran, B.; et al. Serum tryptophan-derived quinolinate and indole-3-acetate are associated with carotid intima-media thickness and its evolution in HIV-infected treated adults. Open Forum Infect. Dis. 2019, 6, ofz516. [Google Scholar] [CrossRef]
- Okeke, N.L.; Craig, D.M.; Muehlbauer, M.J.; Ilkayeva, O.; Clement, M.E.; Naggie, S.; Shah, S.H. Metabolites predict cardiovascular disease events in persons living with HIV: A pilot case–control study. Metabolomics 2018, 14, 23. [Google Scholar] [CrossRef]
- Bravo, C.A.; Hua, S.; Deik, A.; Lazar, J.; Hanna, D.B.; Scott, J.; Chai, J.C.; Kaplan, R.C.; Anastos, K.; Robles, O.A.; et al. Metabolomic profiling of left ventricular diastolic dysfunction in women with or at risk for HIV infection: The Women’s interagency HIV study. J. Am. Heart Assoc. 2020, 9, e013522. [Google Scholar] [CrossRef]
- Gelpi, M.; Mikaeloff, F.; Knudsen, A.D.; Benfeitas, R.; Krishnan, S.; Akusjarvi, S.S.; Hogh, J.; Murray, D.D.; Ullum, H.; Neogi, U.; et al. The central role of the glutamate metabolism in long-term antiretroviral treated HIV-infected individuals with metabolic syndrome. Aging 2021, 13, 22732. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, X.; Duan, S.; Ye, R.; Yang, Y.; Wang, J.; He, N. Untargeted Plasma Metabolomics Reveals Extensive Metabolic Alterations Among Treatment-Naive Human Immunodeficiency Virus/Hepatitis C Virus Co-Infected Patients with Liver Disease Progression. AIDS Res. Hum. Retroviruses 2022, 38, 378–393. [Google Scholar] [CrossRef]
- Hodgson, S.; Griffin, T.J.; Reilly, C.; Harvey, S.; Witthuhn, B.A.; Sandri, B.J.; Kunisaki, K.M.; Wendt, C.H. Plasma sphingolipids in HIV-associated chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2017, 4, e000180. [Google Scholar] [CrossRef]
- Masip, J.; Rallon, N.; Yeregui, E.; Olona, M.; Resino, S.; Benito, J.M.; Vilades, C.; Garcia-Pardo, G.; Alcami, J.; Ruiz-Mateos, E.; et al. Elevated α-ketoglutaric acid concentrations and a lipid-balanced signature are the key factors in long-term HIV control. Front. Immunol. 2022, 13, 822272. [Google Scholar] [CrossRef] [PubMed]
- Atlas, I.D. IDF Atlas, 10th ed.; IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Classification of Diabetes Mellitus; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Kononenko, I.V.; Smirnova, O.M.; Mayorov, A.Y.; Shestakova, M.V. Classification of diabetes. World Health Organization 2019. What’s new? Diabetes Mellit. 2020, 23, 329–339. [Google Scholar] [CrossRef]
- Solis-Herrera, C.; Triplitt, C.; Reasner, C.; DeFronzo, R.A.; Cersosimo, E. Classification of diabetes mellitus. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2015. [Google Scholar]
- Punthakee, Z.; Goldenberg, R.; Katz, P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can. J. Diabetes 2018, 42, S10–S15. [Google Scholar] [CrossRef]
- Balasubramanyam, A. Classification of Diabetes Mellitus and Genetic Diabetic Síndromes; Nathan, D.M., Wolfsdorf, J.I., Mulder, B.J.E., Eds.; UpToDate: Waltham, MA, USA, 2022. [Google Scholar]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and diagnosis of diabetes: Standards of care in diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef]
- Van Eupen, M.G.; Schram, M.T.; Colhoun, H.M.; Scheijen, J.L.; Stehouwer, C.D.; Schalkwijk, C.G. Plasma levels of advanced glycation endproducts are associated with type 1 diabetes and coronary artery calcification. Cardiovasc. Diabetol. 2013, 12, 149. [Google Scholar] [CrossRef]
- Orešič, M.; Gopalacharyulu, P.; Mykkänen, J.; Lietzen, N.; Mäkinen, M.; Nygren, H.; Simell, S.; Simell, V.; Hyöty, H.; Veijola, R.; et al. Cord serum lipidome in prediction of islet autoimmunity and type 1 diabetes. Diabetes 2013, 62, 3268–3274. [Google Scholar] [CrossRef]
- La Torre, D.; Seppänen-Laakso, T.; Larsson, H.E.; Hyötyläinen, T.; Ivarsson, S.A.; Lernmark, Å.; Orešič, M.; Group, D.S. Decreased cord-blood phospholipids in young age–at–onset type 1 diabetes. Diabetes 2013, 62, 3951–3956. [Google Scholar] [CrossRef] [PubMed]
- Tuomainen, M.; Lindström, J.; Lehtonen, M.; Auriola, S.; Pihlajamäki, J.; Peltonen, M.; Tuomilehto, J.; Uusitupa, M.; de Mello, V.D.; Hanhineva, K. Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutr. Diabetes 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Spiller, S.; Li, Y.; Blüher, M.; Welch, L.; Hoffmann, R. Glycated lysine-141 in haptoglobin improves the diagnostic accuracy for type 2 diabetes mellitus in combination with glycated hemoglobin HbA 1c and fasting plasma glucose. Clin. Proteom. 2017, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Spiller, S.; Blüher, M.; Hoffmann, R. Plasma levels of free fatty acids correlate with type 2 diabetes mellitus. Diabetes Obes. Metab. 2018, 20, 2661–2669. [Google Scholar] [CrossRef] [PubMed]
- Soboleva, A.; Mavropulo-Stolyarenko, G.; Karonova, T.; Thieme, D.; Hoehenwarter, W.; Ihling, C.; Stefanov, V.; Grishina, T.; Frolov, A. Multiple glycation sites in blood plasma proteins as an integrated biomarker of type 2 diabetes mellitus. Int. J. Mol. Sci. 2019, 20, 2329. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; An, Z.; Li, H.; Liu, Y.; Xia, L.; Qiu, L.; Yao, A.; Ma, L.; Liu, L. UHPLC-MS/MS-Based Metabolomics and Clinical Phenotypes Analysis Reveal Broad-Scale Perturbations in Early Pregnancy Related to Gestational Diabetes Mellitus. Dis. Markers 2022, 2022, 4231031. [Google Scholar] [CrossRef] [PubMed]
- McMichael, L.E.; Heath, H.; Johnson, C.M.; Fanter, R.; Alarcon, N.; Quintana-Diaz, A.; Pilolla, K.; Schaffner, A.; Jelalian, E.; Wing, R.R.; et al. Metabolites involved in purine degradation, insulin resistance, and fatty acid oxidation are associated with prediction of Gestational diabetes in plasma. Metabolomics 2021, 17, 105. [Google Scholar] [CrossRef]
- Razo-Azamar, M.; Nambo-Venegas, R.; Meraz-Cruz, N.; Guevara-Cruz, M.; Ibarra-González, I.; Vela-Amieva, M.; Delgadillo-Velázquez, J.; Santiago, X.C.; Escobar, R.F.; Vadillo-Ortega, F.; et al. An early prediction model for gestational diabetes mellitus based on metabolomic biomarkers. Diabetol. Metab. Syndr. 2023, 15, 116. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.; Chung, A.C.K.; Xiang, L.; Li, X.; Zheng, Y.; Luan, H.; Zhu, L.; Liu, W.; Peng, Y.; et al. Large-scale longitudinal metabolomics study reveals different trimester-specific alterations of metabolites in relation to gestational diabetes mellitus. J. Proteome Res. 2018, 18, 292–300. [Google Scholar] [CrossRef]
- Zhan, Y.; Wang, J.; He, X.; Huang, M.; Yang, X.; He, L.; Qiu, Y.; Lou, Y. Plasma metabolites, especially lipid metabolites, are altered in pregnant women with gestational diabetes mellitus. Clin. Chim. Acta 2021, 517, 139–148. [Google Scholar] [CrossRef]
- Diboun, I.; Ramanjaneya, M.; Majeed, Y.; Ahmed, L.; Bashir, M.; Butler, A.E.; Abou-Samra, A.B.; Atkin, S.L.; Mazloum, N.A.; Elrayess, M.A. Metabolic profiling of pre-gestational and gestational diabetes mellitus identifies novel predictors of pre-term delivery. J. Transl. Med. 2020, 18, 366. [Google Scholar] [CrossRef] [PubMed]
- Fuller, H.; Iles, M.; Moore, J.B.; Zulyniak, M.A. Unique metabolic profiles associate with gestational diabetes and ethnicity in low-and high-risk women living in the UK. J. Nutr. 2022, 152, 2186–2197. [Google Scholar] [CrossRef] [PubMed]
- Taschereau-Charron, A.; Bilodeau, J.F.; Larose, J.; Greffard, K.; Berthiaume, L.; Audibert, F.; Fraser, W.D.; Julien, P.; Rudkowska, I. F2-isoprostanes and fatty acids profile in early pregnancy complicated by pre-existing diabetes. Prostaglandins Leukot. Essent. Fat. Acids 2018, 135, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Yu, H.; Zhao, X.; Bao, Y.; Hong, C.S.; Zhang, P.; Tu, Y.; Yin, P.; Gao, P.; Wei, L.; et al. Metabolomics study of roux-en-Y gastric bypass surgery (RYGB) to treat type 2 diabetes patients based on ultraperformance liquid chromatography—Mass spectrometry. J. Proteome Res. 2016, 15, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.S.; Dempsey, P.C.; Sethi, P.; Mundra, P.A.; Mellett, N.A.; Weir, J.M.; Owen, N.; Dunstan, D.W.; Meikle, P.J.; Kingwell, B.A. Breaking up prolonged sitting alters the postprandial plasma lipidomic profile of adults with type 2 diabetes. J. Clin. Endocrinol. Metab. 2017, 102, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.; Naderpoor, N.; Mellett, N.; Wilson, K.; Plebanski, M.; Meikle, P.J.; de Courten, B. Lipidomic profiling reveals early-stage metabolic dysfunction in overweight or obese humans. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 335–343. [Google Scholar]
- Hu, T.; Zhang, W.; Han, F.; Zhao, R.; Liu, L.; An, Z. Plasma fingerprint of free fatty acids and their correlations with the traditional cardiac biomarkers in patients with type 2 diabetes complicated by coronary heart disease. Front. Cardiovasc. Med. 2022, 9, 903412. [Google Scholar] [CrossRef]
- Alshehry, Z.H.; Mundra, P.A.; Barlow, C.K.; Mellett, N.A.; Wong, G.; McConville, M.J.; Simes, J.; Tonkin, A.M.; Sullivan, D.R.; Barnes, E.H.; et al. Plasma lipidomic profiles improve on traditional risk factors for the prediction of cardiovascular events in type 2 diabetes mellitus. Circulation 2016, 134, 1637–1650. [Google Scholar] [CrossRef]
- Welsh, P.; Rankin, N.; Li, Q.; Mark, P.B.; Würtz, P.; Ala-Korpela, M.; Marre, M.; Poulter, N.; Hamet, P.; Chalmers, J.; et al. Circulating amino acids and the risk of macrovascular, microvascular and mortality outcomes in individuals with type 2 diabetes: Results from the ADVANCE trial. Diabetologia 2018, 61, 1581–1591. [Google Scholar] [CrossRef]
- Tomofuji, Y.; Suzuki, K.; Kishikawa, T.; Shojima, N.; Hosoe, J.; Inagaki, K.; Matsubayashi, S.; Ishihara, H.; Watada, H.; Ishigaki, Y.; et al. Identification of serum metabolome signatures associated with retinal and renal complications of type 2 diabetes. Commun. Med. 2023, 3, 5. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Jung, E.S.; Park, H.M.; Jeong, S.J.; Kim, K.; Chon, S.; Yu, S.Y.; Woo, J.T.; Lee, C.H. Plasma glutamine and glutamic acid are potential biomarkers for predicting diabetic retinopathy. Metabolomics 2018, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Sumarriva, K.; Uppal, K.; Ma, C.; Herren, D.J.; Wang, Y.; Chocron, I.M.; Warden, C.; Mitchell, S.L.; Burgess, L.G.; Goodale, M.P.; et al. Arginine and carnitine metabolites are altered in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3119–3126. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.S.; Rivera, E.; Warden, C.; Harlow, P.A.; Mitchell, S.L.; Calcutt, M.W.; Samuels, D.C.; Brantley, M.A., Jr. Plasma arginine and citrulline are elevated in diabetic retinopathy. Am. J. Ophthalmol. 2022, 235, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zou, H.; Li, X.; Xu, S.; Liu, C. Plasma metabolomics reveals metabolic profiling for diabetic retinopathy and disease progression. Front. Endocrinol. 2021, 12, 757088. [Google Scholar] [CrossRef]
- Zuo, J.; Lan, Y.; Hu, H.; Hou, X.; Li, J.; Wang, T.; Zhang, H.; Zhang, N.; Guo, C.; Peng, F.; et al. Metabolomics-based multidimensional network biomarkers for diabetic retinopathy identification in patients with type 2 diabetes mellitus. BMJ Open Diabetes Res. Care 2021, 9, e001443. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Dai, M.; Ai, J.; Li, Y.; Mahon, B.; Dai, S.; Deng, Y. Plasma lipidomics profiling identified lipid biomarkers in distinguishing early-stage breast cancer from benign lesions. Oncotarget 2016, 7, 36622. [Google Scholar] [CrossRef]
- Yun, J.H.; Kim, J.M.; Jeon, H.J.; Oh, T.; Choi, H.J.; Kim, B.J. Metabolomics profiles associated with diabetic retinopathy in type 2 diabetes patients. PLoS ONE 2020, 15, e0241365. [Google Scholar] [CrossRef]
- Zhu, X.R.; Yang, F.y.; Lu, J.; Zhang, H.r.; Sun, R.; Zhou, J.B.; Yang, J.K. Plasma metabolomic profiling of proliferative diabetic retinopathy. Nutr. Metab. 2019, 16, 37. [Google Scholar] [CrossRef]
- Curovic, V.R.; Suvitaival, T.; Mattila, I.; Ahonen, L.; Trošt, K.; Theilade, S.; Hansen, T.W.; Legido-Quigley, C.; Rossing, P. Circulating metabolites and lipids are associated to diabetic retinopathy in individuals with type 1 diabetes. Diabetes 2020, 69, 2217–2226. [Google Scholar] [CrossRef]
- Trifonova, O.P.; Maslov, D.L.; Balashova, E.E.; Lichtenberg, S.; Lokhov, P.G. Potential Plasma Metabolite Biomarkers of Diabetic Nephropathy: Untargeted Metabolomics Study. J. Pers. Med. 2022, 12, 1889. [Google Scholar] [CrossRef]
- Haukka, J.K.; Sandholm, N.; Forsblom, C.; Cobb, J.E.; Groop, P.H.; Ferrannini, E. Metabolomic profile predicts development of microalbuminuria in individuals with type 1 diabetes. Sci. Rep. 2018, 8, 13853. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, X.; Shao, X.; Wang, Y.; Feng, S.; Wang, C.; Ye, C.; Chen, J.; Jiang, H. Serum metabolomics benefits discrimination kidney disease development in type 2 diabetes patients. Front. Med. 2022, 9, 819311. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gui, Y.; Wang, M.S.; Zhang, L.; Xu, T.; Pan, Y.; Zhang, K.; Yu, Y.; Xiao, L.; Qiao, Y.; et al. Serum integrative omics reveals the landscape of human diabetic kidney disease. Mol. Metab. 2021, 54, 101367. [Google Scholar] [CrossRef] [PubMed]
- Solini, A.; Manca, M.L.; Penno, G.; Pugliese, G.; Cobb, J.E.; Ferrannini, E. Prediction of declining renal function and albuminuria in patients with type 2 diabetes by metabolomics. J. Clin. Endocrinol. Metab. 2016, 101, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, Q.; Lu, L.; Wang, J.; Liu, D.; Liu, Z. Metabolomic profiling of amino acids in human plasma distinguishes diabetic kidney disease from type 2 diabetes mellitus. Front. Med. 2021, 8, 765873. [Google Scholar] [CrossRef] [PubMed]
- Tofte, N.; Suvitaival, T.; Ahonen, L.; Winther, S.A.; Theilade, S.; Frimodt-Møller, M.; Ahluwalia, T.S.; Rossing, P. Lipidomic analysis reveals sphingomyelin and phosphatidylcholine species associated with renal impairment and all-cause mortality in type 1 diabetes. Sci. Rep. 2019, 9, 16398. [Google Scholar] [CrossRef]
- Lai, M.; Liu, Y.; Ronnett, G.V.; Wu, A.; Cox, B.J.; Dai, F.F.; Röst, H.L.; Gunderson, E.P.; Wheeler, M.B. Amino acid and lipid metabolism in post-gestational diabetes and progression to type 2 diabetes: A metabolic profiling study. PLoS Med. 2020, 17, e1003112. [Google Scholar] [CrossRef] [PubMed]
- Shokry, E.; Marchioro, L.; Uhl, O.; Bermúdez, M.G.; García-Santos, J.A.; Segura, M.T.; Campoy, C.; Koletzko, B. Impact of maternal BMI and gestational diabetes mellitus on maternal and cord blood metabolome: Results from the PREOBE cohort study. Acta Diabetol. 2019, 56, 421–430. [Google Scholar] [CrossRef]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M. Nonalcoholic fatty liver disease: Pathologic patterns and biopsy evaluation in clinical research. In Seminars in Liver Disease; Thieme Medical Publishers: New York, NY, USA, 2012; Volume 32, pp. 3–13. [Google Scholar]
- Alkhouri, N.; Berk, M.; Yerian, L.; Lopez, R.; Chung, Y.M.; Zhang, R.; McIntyre, T.M.; Feldstein, A.E.; Hazen, S.L. OxNASH score correlates with histologic features and severity of nonalcoholic fatty liver disease. Dig. Dis. Sci. 2014, 59, 1617–1624. [Google Scholar] [CrossRef]
- Caldwell, S.; Lackner, C. Perspectives on NASH histology: Cellular ballooning. Ann. Hepatol. 2017, 16, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Kobayashi, T.; Honda, Y.; Kessoku, T.; Tomeno, W.; Imajo, K.; Nakahara, T.; Oeda, S.; Nagaoki, Y.; Amano, Y.; et al. Metabolomic/lipidomic-based analysis of plasma to diagnose hepatocellular ballooning in patients with non-alcoholic fatty liver disease: A multicenter study. Hepatol. Res. 2020, 50, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Kordy, K.; Li, F.; Lee, D.J.; Kinchen, J.M.; Jew, M.H.; La Rocque, M.E.; Zabih, S.; Saavedra, M.; Woodward, C.; Cunningham, N.J.; et al. Metabolomic predictors of non-alcoholic steatohepatitis and advanced fibrosis in children. Front. Microbiol. 2021, 12, 713234. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Caruso, M.G.; Tutino, V.; Bonfiglio, C.; Cozzolongo, R.; Giannuzzi, V.; De Nunzio, V.; De Leonardis, G.; Abbrescia, D.I.; Franco, I.; et al. Significant decrease of saturation index in erythrocytes membrane from subjects with non-alcoholic fatty liver disease (NAFLD). Lipids Health Dis. 2017, 16, 160. [Google Scholar] [CrossRef]
- Pastore, A.; Alisi, A.; Di Giovamberardino, G.; Crudele, A.; Ceccarelli, S.; Panera, N.; Dionisi-Vici, C.; Nobili, V. Plasma levels of homocysteine and cysteine increased in pediatric NAFLD and strongly correlated with severity of liver damage. Int. J. Mol. Sci. 2014, 15, 21202–21214. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, A.E.; Lopez, R.; Tamimi, T.A.R.; Yerian, L.; Chung, Y.M.; Berk, M.; Zhang, R.; McIntyre, T.M.; Hazen, S.L. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J. Lipid Res. 2010, 51, 3046–3054. [Google Scholar] [CrossRef]
- Montefusco, D.J.; Allegood, J.C.; Spiegel, S.; Cowart, L.A. Non-alcoholic fatty liver disease: Insights from sphingolipidomics. Biochem. Biophys. Res. Commun. 2018, 504, 608–616. [Google Scholar] [CrossRef]
- Serviddio, G.; Bellanti, F.; Villani, R.; Tamborra, R.; Zerbinati, C.; Blonda, M.; Ciacciarelli, M.; Poli, G.; Vendemiale, G.; Iuliano, L. Effects of dietary fatty acids and cholesterol excess on liver injury: A lipidomic approach. Redox Biol. 2016, 9, 296–305. [Google Scholar] [CrossRef]
- González-Fernández, R.; Grigoruţă, M.; Chávez-Martínez, S.; Ruiz-May, E.; Elizalde-Contreras, J.M.; Valero-Galván, J.; Martínez-Martínez, A. Liver proteome alterations in psychologically distressed rats and a nootropic drug. PeerJ 2021, 9, e11483. [Google Scholar] [CrossRef]
- Jin, R.; McConnell, R.; Catherine, C.; Xu, S.; Walker, D.I.; Stratakis, N.; Jones, D.P.; Miller, G.W.; Peng, C.; Conti, D.V.; et al. Perfluoroalkyl substances and severity of nonalcoholic fatty liver in children: An untargeted metabolomics approach. Environ. Int. 2020, 134, 105220. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xiang, B.; Jin, Y.; Li, C.; Ren, S.; Wu, Y.; Li, J.; Luo, Q. Hepatotoxic effects of inhalation exposure to polycyclic aromatic hydrocarbons on lipid metabolism of C57BL/6 mice. Environ. Int. 2020, 134, 105000. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Bai, J.; Li, G.; Liu, Y.; Deng, S.; Zhou, R.; Tao, K.; Xia, Z. Increased plasma genistein after bariatric surgery could promote remission of NAFLD in patients with obesity. Front. Endocrinol. 2022, 13, 1024769. [Google Scholar] [CrossRef]
- Głuszyńska, P.; Lemancewicz, D.; Dzięcioł, J.B.; Razak Hady, H. Non-alcoholic fatty liver disease (NAFLD) and bariatric/metabolic surgery as its treatment option: A review. J. Clin. Med. 2021, 10, 5721. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.k.; Ma, W.j.; Zhang, W.; Li, H.; Zhai, J.; Zhao, T.; Guo, X.f.; Sinclair, A.J.; Li, D. Elevated serum phosphatidylcholine (16: 1/22: 6) levels promoted by fish oil and vitamin D 3 are highly correlated with biomarkers of non-alcoholic fatty liver disease in Chinese subjects. Food Funct. 2022, 13, 11705–11714. [Google Scholar] [CrossRef] [PubMed]
- Wijarnpreecha, K.; Thongprayoon, C.; Ungprasert, P. Coffee consumption and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2017, 29, e8–e12. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Milic, N.; Luzza, F.; Boccuto, L.; De Lorenzo, A. Polyphenols treatment in patients with nonalcoholic fatty liver disease. J. Transl. Intern. Med. 2017, 5, 144–147. [Google Scholar] [CrossRef]
- Ghosal, S.; Datta, D.; Sinha, B. A meta-analysis of the effects of glucagon-like-peptide 1 receptor agonist (GLP1-RA) in nonalcoholic fatty liver disease (NAFLD) with type 2 diabetes (T2D). Sci. Rep. 2021, 11, 22063. [Google Scholar] [CrossRef]
- Senthelal, S.; Li, J.; Ardeshirzadeh, S.; Thomas, M.A. Arthritis; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Jang, S.; Kwon, E.J.; Lee, J.J. Rheumatoid arthritis: Pathogenic roles of diverse immune cells. Int. J. Mol. Sci. 2022, 23, 905. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, J.; Hu, C.; Xie, Z.; Li, H.; Wei, S.; Wang, D.; Wen, C.; Xu, G. Exploration of the serum metabolite signature in patients with rheumatoid arthritis using gas chromatography—Mass spectrometry. J. Pharm. Biomed. Anal. 2016, 127, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Carrio, J.; Alperi-Lopez, M.; Lopez, P.; Ballina-García, F.J.; Suarez, A. Non-esterified fatty acids profiling in rheumatoid arthritis: Associations with clinical features and Th1 response. PLoS ONE 2016, 11, e0159573. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Dai, R.; Wang, W.; Peng, Y.; Zhao, X.; Bi, K. Simultaneous profiling of eicosanoid metabolome in plasma by UPLC–MS/MS method: Application to identify potential makers for rheumatoid arthritis. Talanta 2016, 161, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chen, T.; Feng, H.; Zhang, Y.; Li, L.; Zhao, A.; Niu, X.; Liang, F.; Wang, M.; Zhan, J.; et al. Serum metabolic signatures of four types of human arthritis. J. Proteome Res. 2013, 12, 3769–3779. [Google Scholar] [CrossRef] [PubMed]
- Koubar, S.; Kort, J.; Kawtharani, S.; Chaaya, M.; Makki, M.; Uthman, I. Characteristics of lupus and lupus nephritis at a tertiary care center in Lebanon. Lupus 2019, 28, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.N.; Drenkard, C.; Lim, S.S. The impact of social determinants of health on the presentation, management and outcomes of systemic lupus erythematosus. Rheumatology 2023, 62, i10–i14. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.T.; Chien, H.J.; Chang, Y.H.; Liao, T.L.; Chen, D.Y.; Lai, C.C. Metabolic disturbances in systemic lupus erythematosus evaluated with UPLC-MS/MS. Clin. Exp. Rheumatol. 2021; Online ahead of print. [Google Scholar] [CrossRef]
- Shin, T.H.; Kim, H.A.; Jung, J.Y.; Baek, W.Y.; Lee, H.S.; Park, H.J.; Min, J.; Paik, M.J.; Lee, G.; Suh, C.H. Analysis of the free fatty acid metabolome in the plasma of patients with systemic lupus erythematosus and fever. Metabolomics 2018, 14, 14. [Google Scholar] [CrossRef]
- Saegusa, J.; Kawano, S.; Morinobu, A. Metabolomics for Biomarker Discovery in Systemic Lupus Erythematosus. Rinsho Byori. Jpn. J. Clin. Pathol. 2015, 63, 498–505. [Google Scholar]
- Liu, J.; Zhang, D.; Wang, K.; Li, Z.; He, Z.; Wu, D.; Xu, Z.; Zhou, J. Time Course of Metabolic Alterations Associated with the Progression of Systemic Lupus Erythematosus in MRL/lpr Mice Based on GC/MS. J. Proteome Res. 2020, 20, 1243–1251. [Google Scholar] [CrossRef]
- Pryor, K.P.; Barbhaiya, M.; Costenbader, K.H.; Feldman, C.H. Disparities in lupus and lupus nephritis care and outcomes among US Medicaid beneficiaries. Rheum. Dis. Clin. 2021, 47, 41–53. [Google Scholar] [CrossRef]
- He, J.; Ma, C.; Tang, D.; Zhong, S.; Yuan, X.; Zheng, F.; Zeng, Z.; Chen, Y.; Liu, D.; Hong, X.; et al. Absolute quantification and characterization of oxylipins in lupus nephritis and systemic lupus erythematosus. Front. Immunol. 2022, 13, 964901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gan, L.; Tang, J.; Liu, D.; Chen, G.; Xu, B. Metabolic profiling reveals new serum signatures to discriminate lupus nephritis from systemic lupus erythematosus. Front. Immunol. 2022, 13, 967371. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, X.; Zhou, H.; Wang, B.; Zhang, M.; Tang, F. Metabolic profiling reveals new serum biomarkers of lupus nephritis. Lupus 2017, 26, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.C.; Tsai, C.Y.; Yu, C.L. Potential serum and urine biomarkers in patients with lupus nephritis and the unsolved problems. Open Access Rheumatol. Res. Rev. 2016, 8, 81–91. [Google Scholar]
- Zhang, T.; Mohan, C. Caution in studying and interpreting the lupus metabolome. Arthritis Res. Ther. 2020, 22, 172. [Google Scholar] [CrossRef]
- Fazzari, M.J.; Guerra, M.M.; Salmon, J.; Kim, M.Y. Adverse pregnancy outcomes in women with systemic lupus erythematosus: Can we improve predictions with machine learning? Lupus Sci. Med. 2022, 9, e000769. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, E.M.; Park, J.K.; Jeon, H.S.; Oh, S.; Hong, S.; Jung, Y.M.; Kim, B.J.; Kim, S.M.; Norwitz, E.R.; et al. Metabolic biomarkers in midtrimester maternal plasma can accurately predict adverse pregnancy outcome in patients with SLE. Sci. Rep. 2019, 9, 15169. [Google Scholar] [CrossRef]
- Pasquali, R.; Zanotti, L.; Fanelli, F.; Mezzullo, M.; Fazzini, A.; Morselli Labate, A.M.; Repaci, A.; Ribichini, D.; Gambineri, A. Defining hyperandrogenism in women with polycystic ovary syndrome: A challenging perspective. J. Clin. Endocrinol. Metab. 2016, 101, 2013–2022. [Google Scholar] [CrossRef]
- Ge, J.; Yang, N.; Zhang, X.; Li, M.; Zhang, W.; He, J.; Zhu, H.; Cheng, X.; Shen, S.; Ge, W. Steroid Hormone Profiling in Hyperandrogenism and Non-hyperandrogenism Women with Polycystic Ovary Syndrome. Reprod. Sci. 2022, 29, 3449–3458. [Google Scholar] [CrossRef]
- Çelebier, M.; Kaplan, O.; Özel, Ş.; Engin-Üstün, Y. Polycystic ovary syndrome in adolescents: Q-TOF LC/MS analysis of human plasma metabolome. J. Pharm. Biomed. Anal. 2020, 191, 113543. [Google Scholar] [CrossRef]
- Fan, X.; Jiang, J.; Huang, Z.; Gong, J.; Wang, Y.; Xue, W.; Deng, Y.; Wang, Y.; Zheng, T.; Sun, A.; et al. UPLC/Q-TOF-MS based plasma metabolomics and clinical characteristics of polycystic ovarian syndrome. Mol. Med. Rep. 2019, 19, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xu, F.; Qi, B.; Hao, S.; Li, Y.; Li, Y.; Zou, L.; Lu, C.; Xu, G.; Hou, L. Serum metabolomics study of polycystic ovary syndrome based on liquid chromatography–mass spectrometry. J. Proteome Res. 2014, 13, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Buszewska-Forajta, M.; Rachoń, D.; Stefaniak, A.; Wawrzyniak, R.; Konieczna, A.; Kowalewska, A.; Markuszewski, M.J. Identification of the metabolic fingerprints in women with polycystic ovary syndrome using the multiplatform metabolomics technique. J. Steroid Biochem. Mol. Biol. 2019, 186, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Deng, D.; Chen, H.; Cheng, W.; Li, Q.; Luo, R.; Ding, S. Serum metabolomics study of polycystic ovary syndrome based on UPLC-QTOF-MS coupled with a pattern recognition approach. Anal. Bioanal. Chem. 2015, 407, 4683–4695. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, L.M.; Song, J.L.; Yau, L.F.; Mi, J.N.; Zhang, C.R.; Wu, W.T.; Lai, M.H.; Jiang, Z.H.; Wang, J.R.; et al. Alterations of sphingolipid metabolism in different types of polycystic ovary syndrome. Sci. Rep. 2019, 9, 3204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cai, Y.; Su, X.; Jing, Q.; Liu, H.; Na, K.; Qiu, M.; Tian, X.; Liu, D.; Wu, T.; et al. Untargeted metabolomics identified kynurenine as a predictive prognostic biomarker in acute myocardial infarction. Front. Immunol. 2022, 13, 950441. [Google Scholar] [CrossRef] [PubMed]
- Chorell, E.; Olsson, T.; Jansson, J.H.; Wennberg, P. Lysophospholipids as predictive markers of ST-elevation myocardial infarction (STEMI) and non-ST-elevation myocardial infarction (NSTEMI). Metabolites 2020, 11, 25. [Google Scholar] [CrossRef]
- Cheng, C.W.; Liu, M.H.; Tang, H.Y.; Cheng, M.L.; Wang, C.H. Factors associated with elevated plasma phenylalanine in patients with heart failure. Amino Acids 2021, 53, 149–157. [Google Scholar] [CrossRef]
- Chan, C.X.; Khan, A.A.; Choi, J.H.; Ng, C.D.; Cadeiras, M.; Deng, M.; Ping, P. Technology platform development for targeted plasma metabolites in human heart failure. Clin. Proteom. 2013, 10, 7. [Google Scholar] [CrossRef]
- Orešič, M.; Anderson, G.; Mattila, I.; Manoucheri, M.; Soininen, H.; Hyötyläinen, T.; Basignani, C. Targeted serum metabolite profiling identifies metabolic signatures in patients with Alzheimer’s disease, Normal pressure hydrocephalus and brain tumor. Front. Neurosci. 2018, 11, 747. [Google Scholar] [CrossRef]
- Pomilio, A.B.; Vitale, A.A.; Lazarowski, A.J. Neuroproteomics Chip-Based Mass Spectrometry and Other Techniques for Alzheimer’s Disease Biomarkers–Update. Curr. Pharm. Des. 2022, 28, 1124–1151. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, A.; Riedinger, J.M.; Ahmed, S.H.; Hammami, S.; Chaabane, W.; Debbabi, M.; Ben Ammou, S.; Rouaud, O.; Frih, M.; Lizard, G.; et al. Fatty acid profiles in demented patients: Identification of hexacosanoic acid (C26: 0) as a blood lipid biomarker of dementia. J. Alzheimer’s Dis. 2015, 44, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Frajerman, A.; Kebir, O.; Chaumette, B.; Tessier, C.; Lamazière, A.; Nuss, P.; Krebs, M.O. Lipides membranaires dans la schizophrénie et la psychose débutante: De potentiels biomarqueurs et pistes thérapeutiques? L’Encéphale 2020, 46, 209–216. [Google Scholar] [CrossRef] [PubMed]

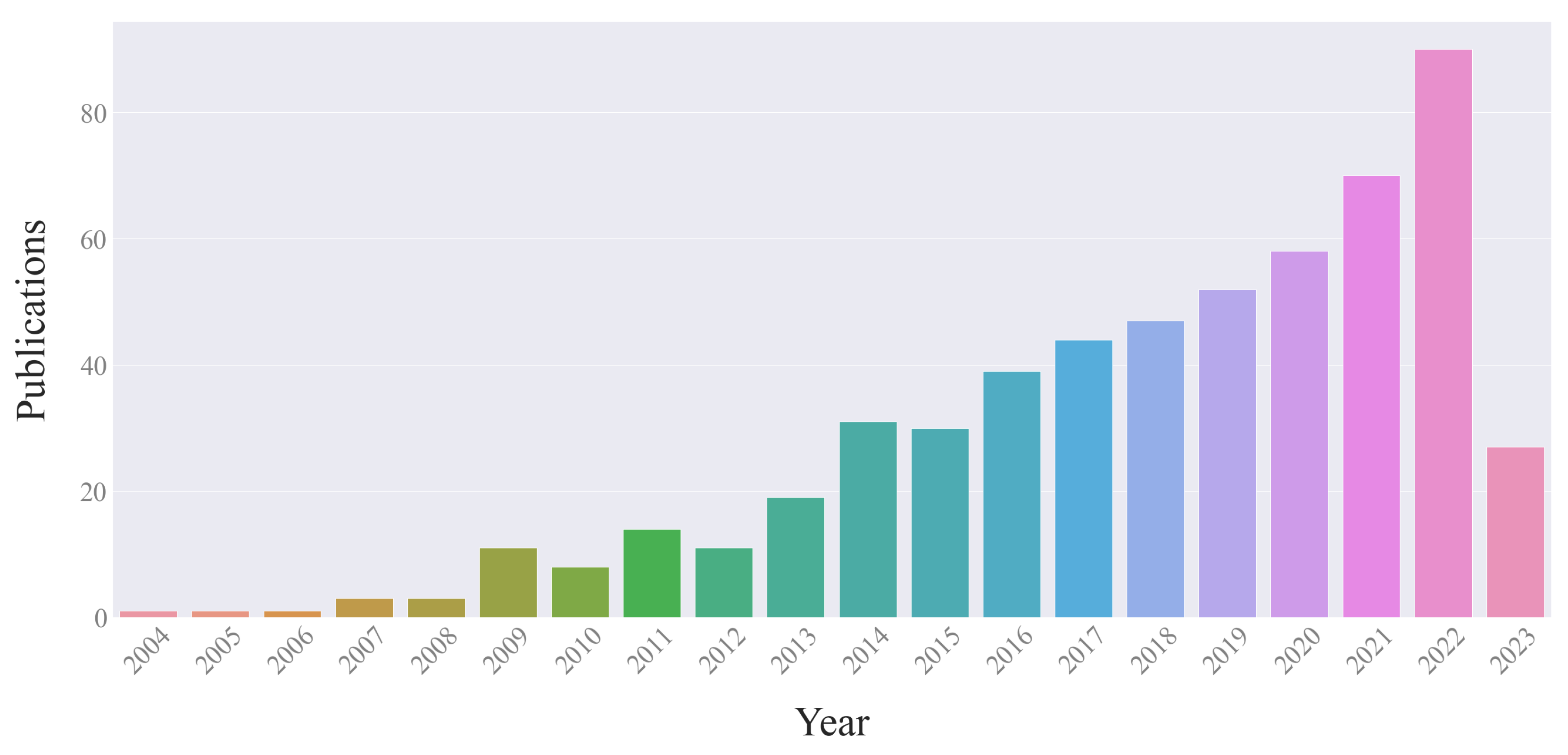
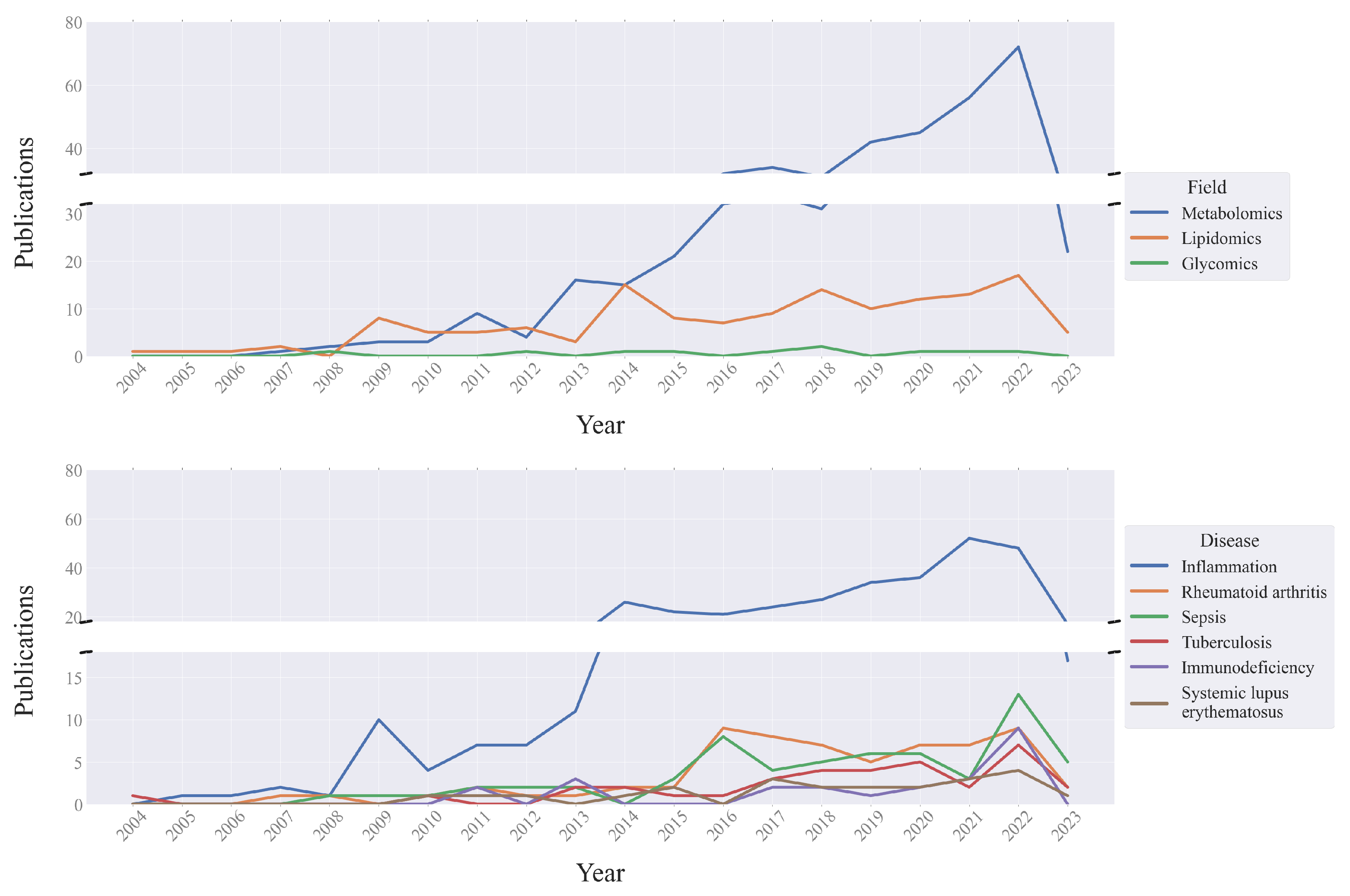
| Fields | Field Tags | Diseases | Biomaterial | Methodology | Exception Tags |
|---|---|---|---|---|---|
| Metabolomics | metabolomics, metabonomics, metabolic profiling, metabolomics profile | Systemic lupus erythematosus Sepsis Rheumatoid arthritis Tuberculosis Immunodeficiency Immunodeficient state Inflammation | blood serum plasma | liquid chromatography gas chromatography mass spectrometry LC-MS HPLC-MS HPLC-MS/MS UPLC-MS UHPLC-MS GC-MS | genomics genetics food markers pharmacology |
| Lipidomics | lipidomics, lipidome, lipid profiling | ||||
| Glycomics | glycomics, glycome |
| Metabolomics | Lipidomics | Glycomics | Total | |
|---|---|---|---|---|
| Inflammation | 223 | 91 | 4 | 318 |
| Rheumatoid arthritis (RA) | 48 | 9 | 2 | 59 |
| Sepsis | 45 | 9 | 1 | 55 |
| Tuberculosis | 32 | 0 | 1 | 33 |
| Immunodeficiency | 19 | 3 | 0 | 22 |
| Systemic lupus erythematosus (SLE) | 19 | 1 | 0 | 20 |
| Total | 386 | 113 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demicheva, E.; Dordiuk, V.; Polanco Espino, F.; Ushenin, K.; Aboushanab, S.; Shevyrin, V.; Buhler, A.; Mukhlynina, E.; Solovyova, O.; Danilova, I.; et al. Advances in Mass Spectrometry-Based Blood Metabolomics Profiling for Non-Cancer Diseases: A Comprehensive Review. Metabolites 2024, 14, 54. https://doi.org/10.3390/metabo14010054
Demicheva E, Dordiuk V, Polanco Espino F, Ushenin K, Aboushanab S, Shevyrin V, Buhler A, Mukhlynina E, Solovyova O, Danilova I, et al. Advances in Mass Spectrometry-Based Blood Metabolomics Profiling for Non-Cancer Diseases: A Comprehensive Review. Metabolites. 2024; 14(1):54. https://doi.org/10.3390/metabo14010054
Chicago/Turabian StyleDemicheva, Ekaterina, Vladislav Dordiuk, Fernando Polanco Espino, Konstantin Ushenin, Saied Aboushanab, Vadim Shevyrin, Aleksey Buhler, Elena Mukhlynina, Olga Solovyova, Irina Danilova, and et al. 2024. "Advances in Mass Spectrometry-Based Blood Metabolomics Profiling for Non-Cancer Diseases: A Comprehensive Review" Metabolites 14, no. 1: 54. https://doi.org/10.3390/metabo14010054
APA StyleDemicheva, E., Dordiuk, V., Polanco Espino, F., Ushenin, K., Aboushanab, S., Shevyrin, V., Buhler, A., Mukhlynina, E., Solovyova, O., Danilova, I., & Kovaleva, E. (2024). Advances in Mass Spectrometry-Based Blood Metabolomics Profiling for Non-Cancer Diseases: A Comprehensive Review. Metabolites, 14(1), 54. https://doi.org/10.3390/metabo14010054









